Highly Sensitive Micropatterned Interdigitated Electrodes for Enhancing the Concentration Effect Based on Dielectrophoresis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Simulation to Calculate Profiles of the Electric Field Between the Electrodes
2.3. Fabrication of IMEs and Immobilization of Antibody
2.4. Sysnthesis of Au Labeled Secondary Antibody
2.5. Image Measurement
2.6. Immunoassay and Signal Processing
3. Results and Discussion
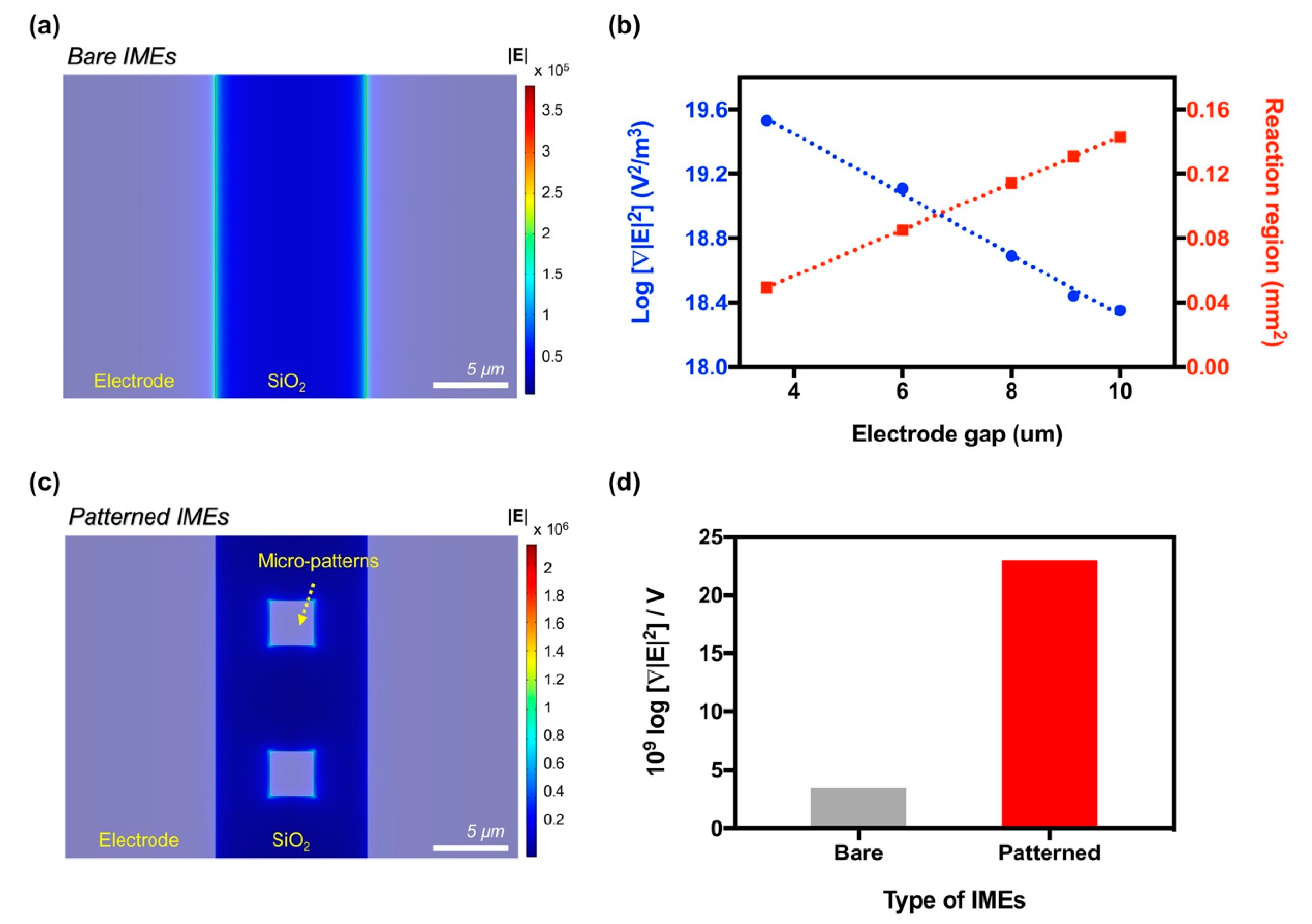
3.1. Enhancing the Intensity of the Electric Field
3.2. Optimizing the Intensity of the DEP Force
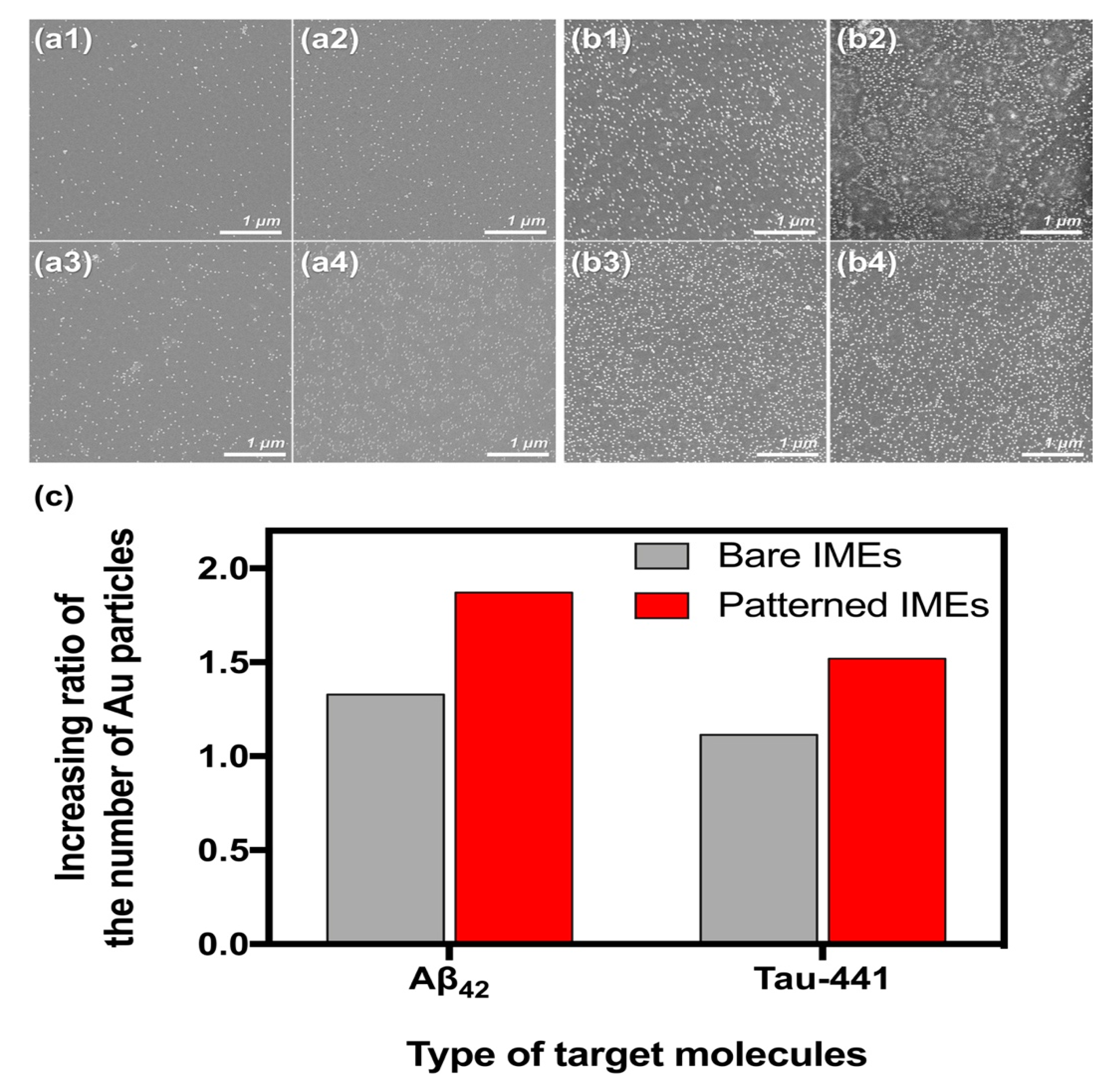
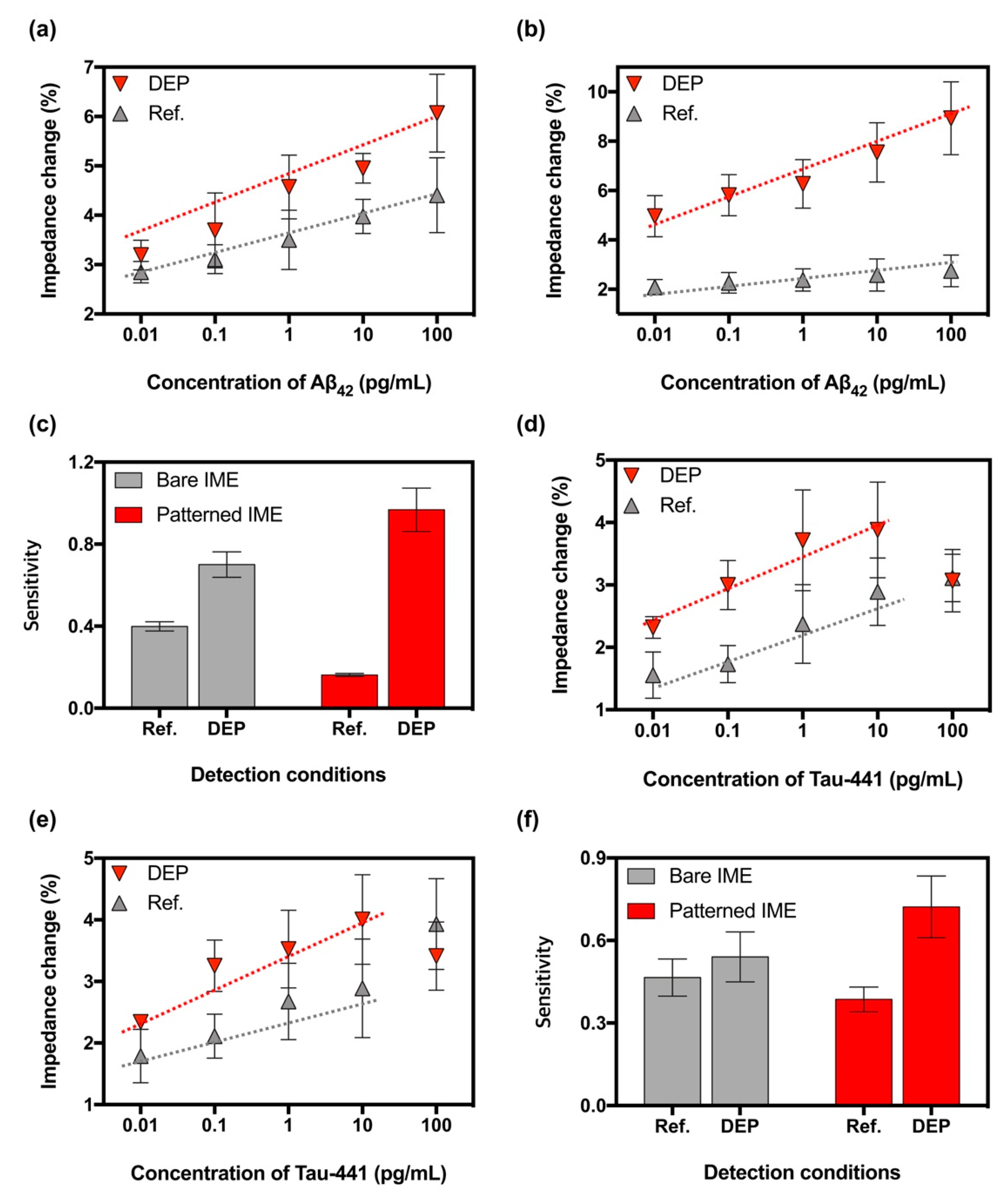
3.3. Verifying the Optimal DEP Force
3.4. Enhancing the Sensitivity in the Patterned IMEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sanjay, S.T.; Fu, G.; Dou, M.; Xu, F.; Liu, R.; Qi, H.; Li, X. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst 2015, 140, 7062–7081. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.G.; García, Y.P.; Gonzalez, J.A.S.; Bañuelos, C.V.O.; Escareño, M.P.L.; Balagurusamy, N. Biosensors for Food Quality and Safety Monitoring. In Enzymes in Food Biotechnology, 1st ed.; Mohammed, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 691–709. [Google Scholar]
- Sarkar, A.; Sarkar, K.D.; Amrutha, V.; Dutta, K. An overview of enzyme-based biosensors for environmental monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants, 1st ed.; Satinder, K.B., Krishnamoorthy, H., Vinayak, L.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–329. [Google Scholar]
- Lin, C.H.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H.; Chiu, M.J. Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Cazorla, G.; Cassaigne, A.; Déléris, G. Plasma protein contents determined by Fourier-transform infrared spectrometry. Clin. Chem. 2001, 47, 730–738. [Google Scholar] [PubMed]
- Adam, T.; Hashim, U. Highly sensitive silicon nanowire biosensor with novel liquid gate control for detection of specific single-stranded DNA molecules. Biosens. Bioelectron. 2015, 67, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.A.; Estevez, M.C.; Soler, M.; Lechuga, L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics 2017, 6, 123. [Google Scholar] [CrossRef]
- Ramanujam, J.; Verma, A.; González-Díaz, B.; Guerrero-Lemus, R.; del Canizo, C.; Garcia-Tabares, E.; Rey-Stolle, I.; Granek, F.; Korte, L.; Tucci, M.; et al. Inorganic photovoltaics—Planar and nanostructured devices. Prog. Mater. Sci. 2016, 82, 294–404. [Google Scholar] [CrossRef]
- Madou, M.J.; Cubicciotti, R. Scaling issues in chemical and biological sensors. Proc. IEEE 2003, 91, 830–838. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, Y.A.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ren, Y.; Chen, F.; Song, J.; Tao, Y.; Du, K.; Wu, Q. A microscopic physical description of electrothermal-induced flow for control of ion current transport in microfluidics interfacing nanofluidics. Electrophoresis 2019. [Google Scholar] [CrossRef] [PubMed]
- Salari, A.; Thompson, M. Recent advances in AC electrokinetic sample enrichment techniques for biosensor development. Sens. Actuators B Chem. 2018, 255, 3601–3615. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, W.; Jia, Y.; Tao, Y.; Shao, J.; Ding, Y.; Jiang, H. Induced-charge electroosmotic trapping of particles. Lab Chip 2015, 15, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shao, J.; Jia, Y.; Tao, Y.; Ding, Y.; Jiang, H.; Ren, Y. Trapping and chaining self-assembly of colloidal polystyrene particles over a floating electrode by using combined induced-charge electroosmosis and attractive dipole–dipole interactions. Soft Matter 2015, 11, 8105–8112. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, W.; Tao, Y.; Hui, M.; Wu, Q. On ac-field-induced nonlinear electroosmosis next to the sharp corner-field-singularity of leaky dielectric blocks and its application in on-chip micro-mixing. Micromachines 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Song, C.; Liu, W.; Jiang, T.; Song, J.; Wu, Q.; Jiang, H. On hybrid electroosmotic kinetics for field-effect-reconfigurable nanoparticle trapping in a four-terminal spiral microelectrode array. Electrophoresis 2019, 40, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Markx, G.H. Applications of dielectrophoresis in biotechnology. Trends Biotechnol. 1997, 15, 426–432. [Google Scholar] [CrossRef]
- Al-Ahdal, S.A.; Ahmad Kayani, A.B.; Md Ali, M.A.; Chan, J.Y.; Ali, T.; Adnan, N.; Buyoung, M.R.; Mhd Noor, E.E.; Majlis, B.Y.; Sharath, S. Dielectrophoresis of Amyloid-Beta Proteins as a Microfluidic Template for Alzheimer’s Research. Int. J. Mol. Sci. 2019, 20, 3595. [Google Scholar] [CrossRef] [PubMed]
- Nerguizian, V.; Stiharu, I.; Al-Azzam, N.; Yassine-Diab, B.; Alazzam, A. The effect of dielectrophoresis on living cells: Crossover frequencies and deregulation in gene expression. Analyst 2019, 144, 3853–3860. [Google Scholar] [CrossRef]
- Han, S.I.; Kim, H.S.; Han, A. In-droplet cell concentration using dielectrophoresis. Biosens. Bioelectron. 2017, 97, 41–45. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.; Yoo, Y.K.; Lee, J.H.; Park, J.H.; Hwang, K.S. Sensitivity improvement of an electrical sensor achieved by control of biomolecules based on the negative dielectrophoretic force. Biosens. Bioelectron. 2016, 85, 977–985. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Z.; Li, D.; Xiong, Y.; Xiong, P.; Guan, Y.; Hou, S.; Hu, Y.; Chen, S.; Liu, G.; et al. Microfluidic dielectrophoresis device for trapping, counting and detecting Shewanella oneidensis at the cell level. Biosens. Bioelectron. 2018, 99, 416–423. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Jen, C.P. Impedance detection integrated with dielectrophoresis enrichment platform for lung circulating tumor cells in a microfluidic channel. Biosens. Bioelectron. 2018, 121, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, T.; Kim, Y.; Lee, C.; Woo, K.; Seol, J.H.; Yang, S. A microfluidic device for label-free detection of Escherichia coli in drinking water using positive dielectrophoretic focusing, capturing, and impedance measurement. Biosens. Bioelectron. 2015, 74, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, D.; Baek, S.Y.; Yang, S.H.; Kim, Y.; Lim, S.M.; Kim, J.S.; Hwang, K.S. Dielectrophoresis-based filtration effect and detection of amyloid beta in plasma for Alzheimer’s disease diagnosis. Biosens. Bioelectron. 2019, 128, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Donner, L.; Fälker, K.; Gremer, L.; Klinker, S.; Pagani, G.; Ljungberg, L.U.; Lothmann, K.; Rizzi, F.; Schaller, M.; Gohlke, H.; et al. Platelets contribute to amyloid-β aggregation in cerebral vessels through integrin αIIbβ3–induced outside-in signaling and clusterin release. Sci. Signal. 2016, 9, ra52. [Google Scholar] [CrossRef] [PubMed]
- Porzig, R.; Singer, D.; Hoffmann, R. Epitope mapping of mAbs AT8 and Tau5 directed against hyperphosphorylated regions of the human tau protein. Biochem. Biophys. Res. Commun. 2007, 358, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Aho, L.; Pikkarainen, M.; Hiltunen, M.; Leinonen, V.; Alafuzoff, I. Immunohistochemical visualization of amyloid-β protein precursor and amyloid-β in extra-and intracellular compartments in the human brain. J. Alzheimers Dis. 2010, 20, 1015–1028. [Google Scholar] [CrossRef]
- Hughes, M.P. Electrokinetics. In Nanoelectromechanics in Engineering and Biology, 1st ed.; Hughes, M.P., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 28–63. [Google Scholar]
- Kayed, R.; Head, E.; Sarsoza, F.; Saing, T.; Cotman, C.W.; Necula, M.; Margol, L.; Wu, J.; Breydo, L.; Thompson, J.L.; et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2007, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Fan, J.; Smith, M.J.; Goedert, M.; Amos, L.A. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003, 22, 70–77. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Ahn, H.; Lee, D.S.; Park, D.; Kim, J.H.; Kim, J.; Yoon, D.S.; Hwang, K.S. Highly Sensitive Micropatterned Interdigitated Electrodes for Enhancing the Concentration Effect Based on Dielectrophoresis. Sensors 2019, 19, 4152. https://doi.org/10.3390/s19194152
Kim HJ, Ahn H, Lee DS, Park D, Kim JH, Kim J, Yoon DS, Hwang KS. Highly Sensitive Micropatterned Interdigitated Electrodes for Enhancing the Concentration Effect Based on Dielectrophoresis. Sensors. 2019; 19(19):4152. https://doi.org/10.3390/s19194152
Chicago/Turabian StyleKim, Hye Jin, Heeju Ahn, David S. Lee, Dongsung Park, Jae Hyun Kim, Jinsik Kim, Dae Sung Yoon, and Kyo Seon Hwang. 2019. "Highly Sensitive Micropatterned Interdigitated Electrodes for Enhancing the Concentration Effect Based on Dielectrophoresis" Sensors 19, no. 19: 4152. https://doi.org/10.3390/s19194152
APA StyleKim, H. J., Ahn, H., Lee, D. S., Park, D., Kim, J. H., Kim, J., Yoon, D. S., & Hwang, K. S. (2019). Highly Sensitive Micropatterned Interdigitated Electrodes for Enhancing the Concentration Effect Based on Dielectrophoresis. Sensors, 19(19), 4152. https://doi.org/10.3390/s19194152





