Bilateral Tactile Feedback-Enabled Training for Stroke Survivors Using Microsoft KinectTM
Abstract
1. Introduction
1.1. Bilateral Training
1.2. Tactile Feedback
1.3. Kinect Application
1.4. Proprioception Training
2. Methods
- the absence of primary joint trauma and dislocation (elbow and shoulder);
- no history of fractures and peripheral nerve paralysis;
- normal cognitive score >25 with the Mini-Mental State Examination [51];
- ability to understand instructions in English;
- ability to sit on a chair and to perform tasks;
- mild to moderate paralysis (Fugl-Meyer assessment (FMA) > 19);
- more than 60 degrees of active range of motion for shoulder abduction, shoulder flexion, and elbow flexion.
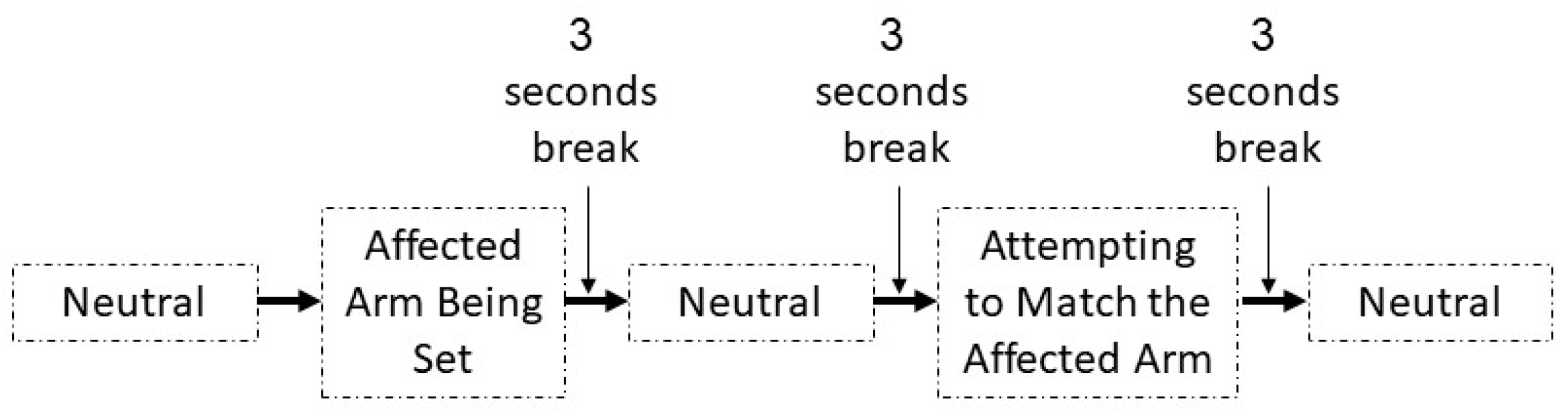
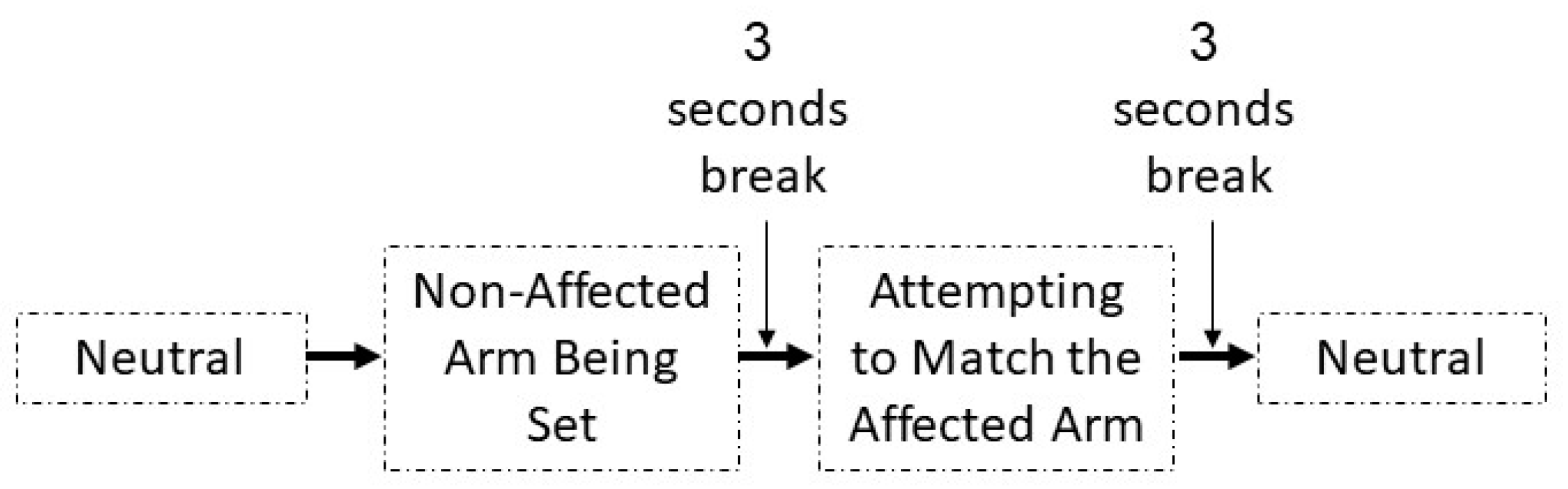
2.1. Training Protocol
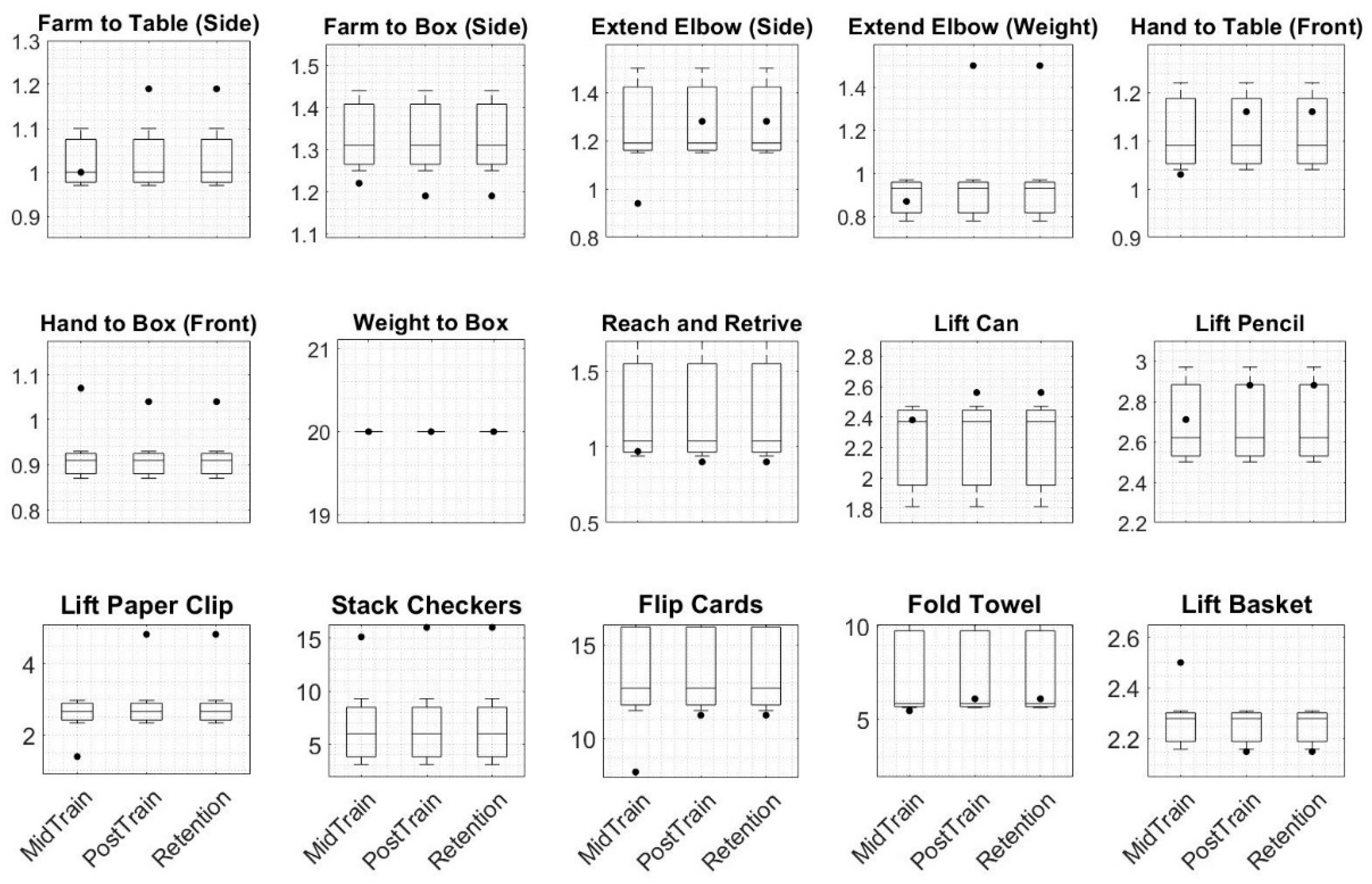
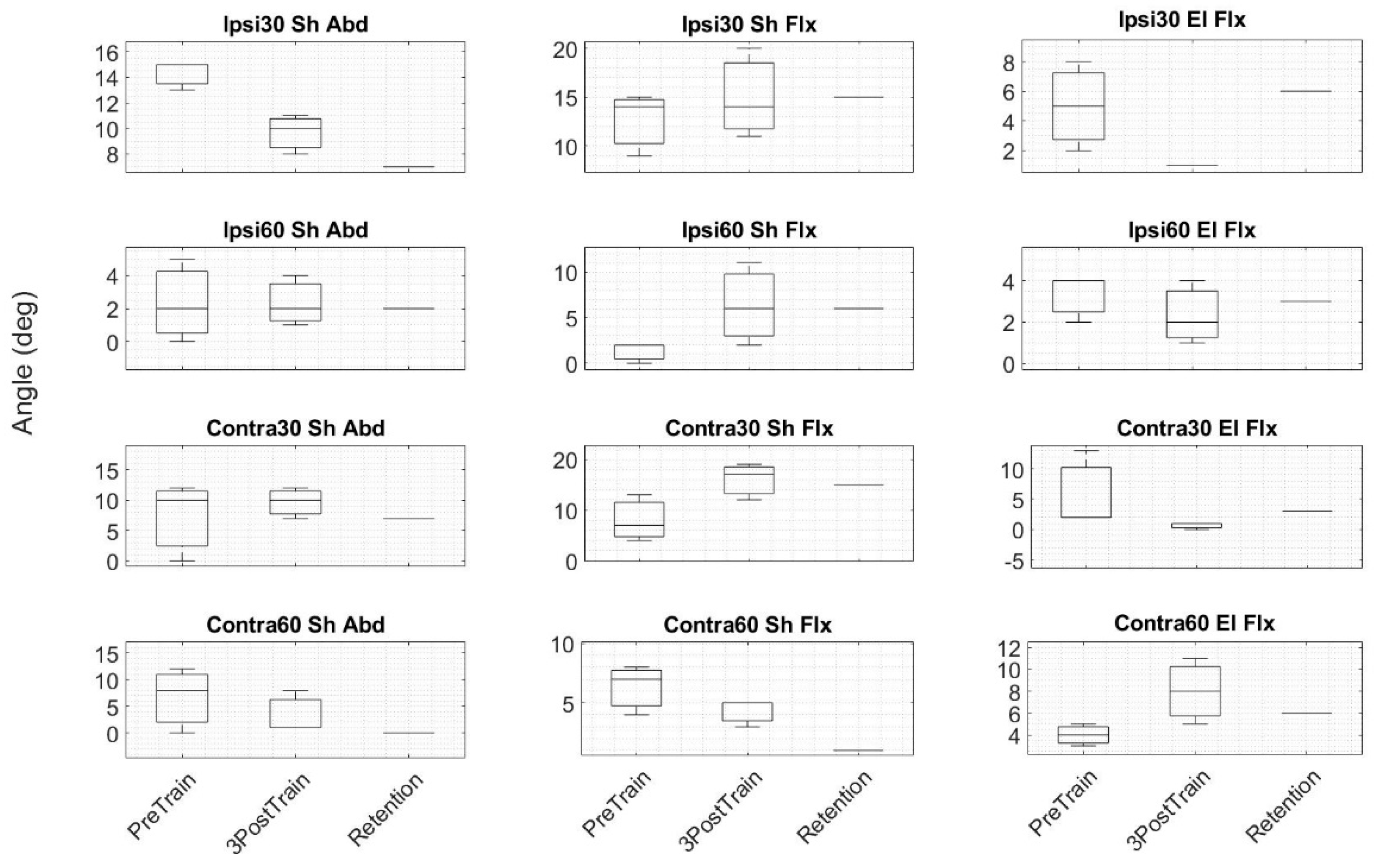
2.2. Assessment Protocol
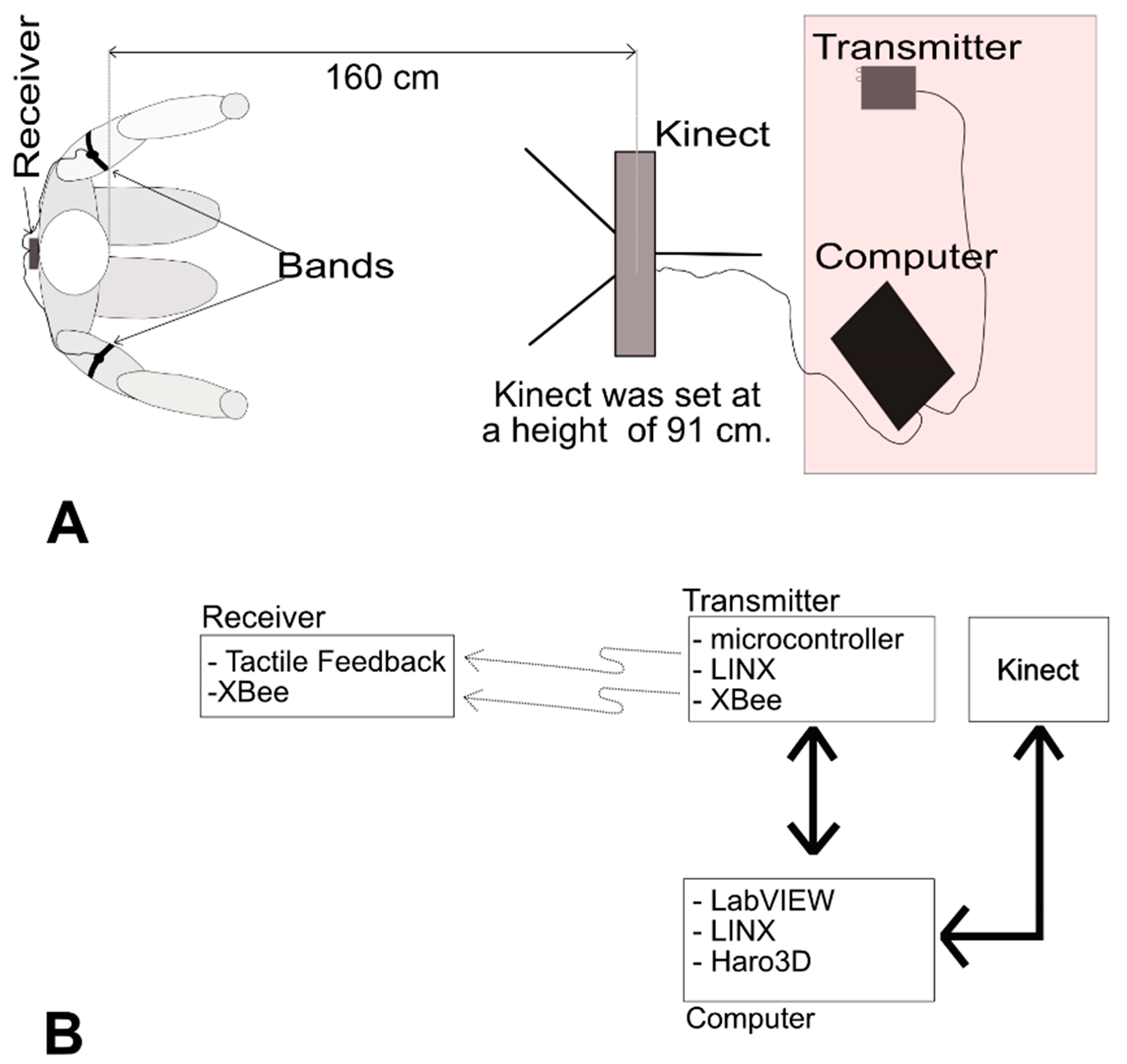
3. System
3.1. Receiver
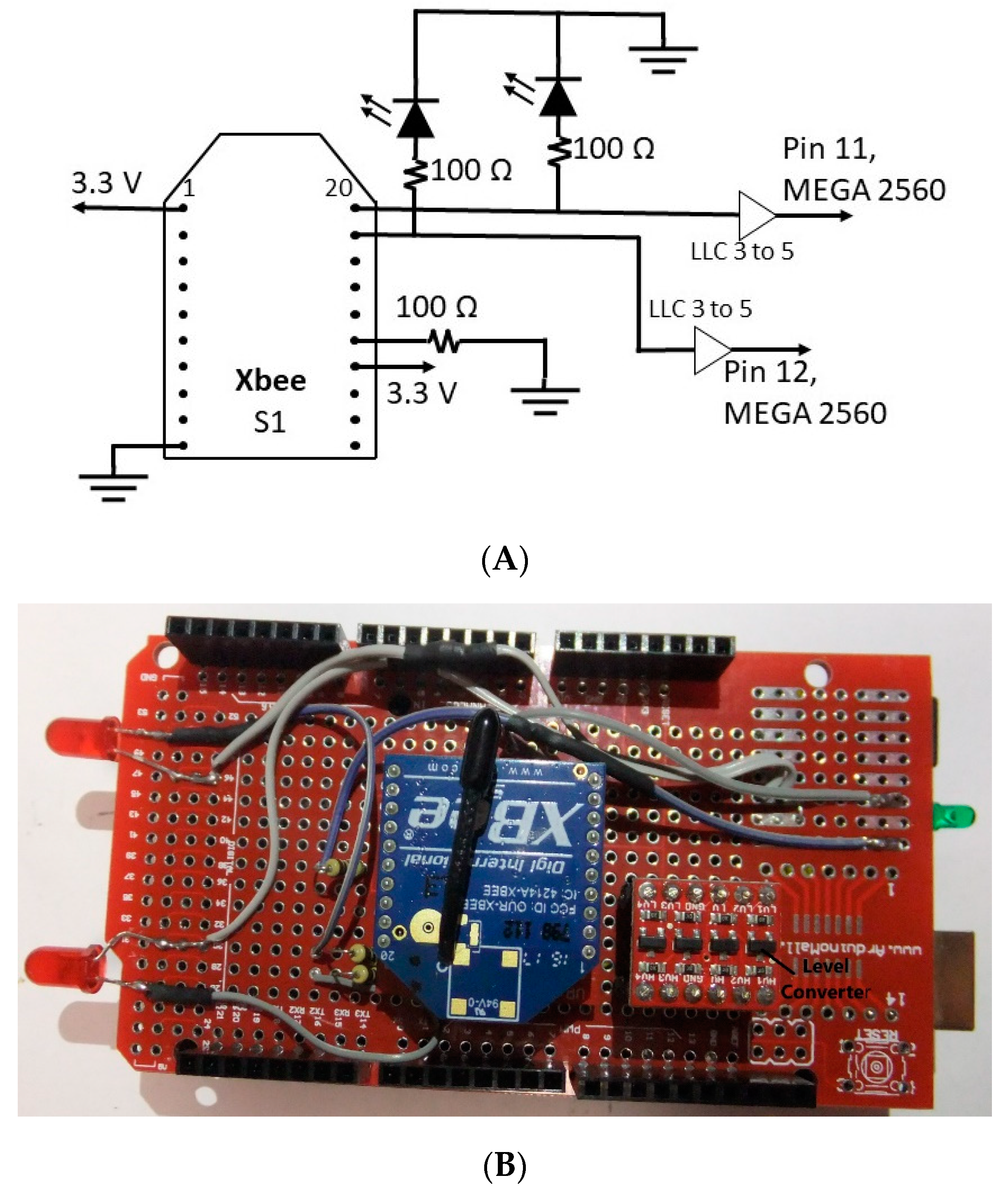
3.2. Transmitter
3.3. Software and Hardware
3.3.1. Hardware
3.3.2. LabVIEW
4. Results
4.1. Motor Function
4.2. Proprioception
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossmann, K.A. Pathophysiology and Therapy of Experimental Stroke. Cell. Mol. Neurobiol. 2006, 26, 1057–1083. [Google Scholar] [CrossRef] [PubMed]
- Warlow, C.P.; Van Gijn, J.; Dennis, M.S.; Wardlaw, J.M.; Bamford, J.M.; Hankey, G.J.; Sandercock, P.A.; Rinkel, G.; Langhorne, P.; Sudlow, C.; et al. Stroke Practical Management, 3rd ed.; Oxford Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Bonita, R.; Mendis, S.; Truelsen, T.; Bogousslavsky, J.; Toole, J.; Yatsu, F. The global stroke initiative. Lancet Neurol. 2004, 3, 391–393. [Google Scholar] [CrossRef]
- Cramer, S.C.; Nelles, G.; Benson, R.R.; Kaplan, J.D.; Parker, R.A.; Kwong, K.K.; Kennedy, D.N.; Finklestein, S.P.; Rosen, B.R. A functional mri study of subjects recovered from hemiparetic stroke. Stroke 1997, 28, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Bleyenheuft, Y.; Gordon, A.M. Precision grip in congenital and acquired hemiparesis: Similarities in impairments and implications for neurorehabilitation. Front. Hum. Neurosci. 2014, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Buschfort, R.; Brocke, J.; Heß, A.; Werner, C.; Waldner, A.; Stefan, H. Arm studio to intensify upper limb rehabilitation after stroke: Concept, acceptance, utilization and preliminary clinical results. J. Rehabil. Med. 2010, 42, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Gowland, C.; deBruin, H.; Basmajian, J.; Plews, N.; Nurcea, I. Agonist and Antagonist Activity During Voluntary Upper Limb Movement in Patients with Stroke. Phys. Ther. 1992, 72, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Gresham, G.E.; Fitzpatrick, T.E.; Wolf, P.A.; McNamara, P.M.; Kannel, W.B.; Dawber, T.R. Residual Disability in Survivors of Stroke—The Framingham Study. N. Engl. J. Med. 1975, 293, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.P.; Keeling, J.; Lin, B.; Henderson, M.; Hale, L.A. The impact of bilateral therapy on upper limb function after chronic stroke: A systematic review. Disabil. Rehabil. 2010, 32, 1221–1231. [Google Scholar] [CrossRef]
- Van Delden, A.E.Q.; Peper, C.E.; Beek, P.J.; Kwakkel, G. Unilateral versus bilateral upper limb exercise therapy after stroke: A systematic review. J. Rehabil. Med. 2012, 44, 106–117. [Google Scholar] [CrossRef]
- Stewart, K.C.; Cauraugh, J.H.; Summers, J.J. Bilateral movement training and stroke rehabilitation: A systematic review and meta-analysis. J. Neurol. Sci. 2006, 244, 89–95. [Google Scholar] [CrossRef]
- Chen, P.M.; Kwong, P.W.H.; Lai, C.K.Y.; Ng, S.S.M. Comparison of bilateral and unilateral upper limb training in people with stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0216357. [Google Scholar] [CrossRef] [PubMed]
- Coldberg, G. Supplementary motor area structure and function: Review and hypothesies. Behav. Brain Sci. 1985, 8, 567–616. [Google Scholar] [CrossRef]
- Swinnen, S.P.; Wenderoth, N. Two hands, one brain: Cognitive neuroscience of bimanual skill. Trends Cogn. Sci. 2004, 8, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.M.C.; Whitall, J. Bilateral arm training: Why and who benefits? NeuroRehabilitation 2008, 23, 29–41. [Google Scholar]
- Lee, M.J.; Lee, J.H.; Koo, H.M.; Lee, S.M. Effectiveness of Bilateral Arm Training for Improving Extremity Function and Activities of Daily Living Performance in Hemiplegic Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Cauraugh, J.H.; Lodha, N.; Naik, S.K.; Summers, J.J. Bilateral movement training and stroke motor recovery progress: A structured review and meta-analysis. Hum. Mov. Sci. 2010, 29, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Herrnstadt, G.; Alavi, N.; Randhawa, B.K.; Boyd, L.A.; Menon, C. Bimanual elbow robotic orthoses: Preliminary investigations on an impairment force-feedback rehabilitation method. Front. Hum. Neurosci. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.S.; Burgar, C.G.; Van der Loos, M.; Shor, P.C. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J. Rehabil. Res. Dev. 2006, 43, 631. [Google Scholar] [CrossRef]
- Van Delden, A.L.E.Q.; Peper, C.L.E.; Kwakkel, G.; Beek, P.J. A Systematic Review of Bilateral Upper Limb Training Devices for Post Stroke Rehabilitation. Stroke Res. Treat. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Richards, L.G.; Senesac, C.R.; Davis, S.B.; Woodbury, M.L.; Nadeau, S.E. Bilateral arm training with rhythmic auditory cueing in chronic stroke: Not always efficacious. Neurorehabilit. Neural Repair 2008, 22, 180–184. [Google Scholar] [CrossRef]
- Jones, L.A.; Sarter, N.B. Tactile Displays: Guidance for Their Design and Application. Hum. Factors 2008, 50, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Bark, K.; Wheeler, J.; Shull, P.B.; Savall, J.; Cutkosky, M. Rotational skin stretch feedback: A wearable haptic display for motion. IEEE Trans. Haptics 2010, 3, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Shull, P.B.; Damian, D.D. Haptic wearables as sensory replacement, sensory augmentation and trainer—A review. J. Neuroeng. Rehabil. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: A review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef] [PubMed]
- Stepp, C.E.; Matsuoka, Y. Object manipulation improvements due to single session training outweigh the differences among stimulation sites during vibrotactile feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, M.A.; Graczyk, E.L.; Sidik, S.M.; Dustin, D.W.T. Artificial tactile and proprioceptive feedback improves performance and confidence on object identification tasks. PLoS ONE 2018, 13, e0207659. [Google Scholar] [CrossRef]
- Held, J.P.; Klaassen, B.; van Beijnum, B.J.F.; Luft, A.R.; Veltink, P.H. Usability Evaluation of a VibroTactile Feedback System in Stroke Subjects. Bioeng. Biotechnol. 2017, 4, 98. [Google Scholar] [CrossRef]
- Diego-Mas, J.A.; Alcaide-Marzal, J. Using Kinect sensor in observational methods for assessing postures at work. Appl. Ergon. 2014, 45, 976–985. [Google Scholar] [CrossRef]
- Xu, X.; Robertson, M.; Chen, K.B.; Lin, J.H.; McGorry, R.W. Using the Microsoft KinectTM to assess 3-D shoulder kinematics during computer use. Appl. Ergon. 2017, 65, 418–423. [Google Scholar] [CrossRef]
- Galna, B.; Barry, G.; Jackson, D.; Mhiripiri, D.; Olivier, P.; Rochester, L. Accuracy of the Microsoft Kinect sensor for measuring movement in people with Parkinson’s disease. Gait Posture 2014, 39, 1062–1068. [Google Scholar] [CrossRef]
- Webster, D.; Celik, O. Systematic review of Kinect applications in elderly care and stroke rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Cho, S.; Park, S.H.; Lee, J.Y.; Kwon, S.Y.; Paik, N.J. A low cost kinect-based virtual rehabilitation system for inpatient rehabilitation of the upper limb in patients with subacute stroke: A randomized, double-blind, sham-controlled pilot trial. Medicine 2018, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Da Gama, A.; Chaves, T.; Figueiredo, L.; Teichrieb, V. Poster: Improving motor rehabilitation process through a natural interaction based system using kinect sensor. In Proceedings of the 2012 IEEE Symposium on 3D User Interfaces (3DUI), Costa Mesa, CA, USA, 4–5 March 2012; pp. 145–146. [Google Scholar]
- Pastor, I.; Hayes, H.A.; Bamberg, S.J. A feasibility study of an upper limb rehabilitation system using kinect and computer games. In Proceedings of the 2012 Annual International Conference of the Engineering inMedicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 1286–1289. [Google Scholar]
- Yeh, S.C.; Hwang, W.Y.; Huang, T.C.; Liu, W.K.; Chen, Y.T.; Hung, Y.P. A study for the application of body sensing in assisted rehabilitation training. In Proceedings of the 2012 International Symposium on Computer, Consumer and Control (IS3C), Taichung, Taiwan, 4–6 June 2012; pp. 922–925. [Google Scholar]
- Shiratuddin, M.F.; Hajnal, A.; Farkas, A.; Wong, K.W.; Legradi, G. A proposed framework for an interactive visuotactile 3D virtual environment system for visuomotor rehabilitation of stroke patients. In Proceedings of the 2012 International Conference on Computer & Information Science (ICCIS), Kuala Lumpur, Malaysia, 12–14 June 2012; pp. 1052–1057. [Google Scholar]
- Frisoli, A.; Loconsole, C.; Leonardis, D.; Banno, F.; Barsotti, M.; Chisari, C.; Bergamasco, M. A new gaze-BCI-driven control of an upper limb exoskeleton for rehabilitation in real-world tasks. IEEE Trans. Syst. Man Cybern. C 2012, 42, 1169–1179. [Google Scholar] [CrossRef]
- Tuthill, C.T.; Azim, E. Proprioception. Curr. Biol. 2018, 28, R187–R207. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Baba, A.; Agostini, M.; Turolla, A. Proprioceptive Based Training for stroke recovery. Proposal of new treatment modality for rehabilitation of upper limb in neurological diseases. Arch. Physiother. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.H.; Kim, Y.L.; Lee, S.M. Effects of phase proprioceptive training on balance in patients with chronic stroke. J. Phys. Ther. Sci. 2017, 29, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.C.; Röijezon, U.; Treleaven, J. Proprioception in musculoskeletal rehabilitation. Part 2: Clinical assessment and intervention. Man. Ther. 2015, 20, 378–387. [Google Scholar] [CrossRef]
- Rand, D. Proprioception deficits in chronic stroke–Upper extremity function and daily living. PLoS ONE 2018, 13, e0195043. [Google Scholar] [CrossRef]
- Smith, D.L.; Akhtar, A.J.; Garraway, W.M. Proprioception and spatial neglect after stroke. Age Ageing 1983, 12, 63–69. [Google Scholar] [CrossRef]
- El-Gohary, T.M.; Khaled, O.A.; Ibrahim, S.R.; Alshenqiti, A.M.; Ibrahim, M.I. Effect of proprioception cross training on repositioning accuracy and balance among healthy individuals. J. Phys. Ther. Sci. 2016, 28, 3178–3182. [Google Scholar] [CrossRef][Green Version]
- Carroll, T.J.; Herbert, R.D.; Munn, J.; Lee, M.; Gandevia, S.C. Contralateral effects of unilateral strength training: Evidence and possible mechanisms. J. Appl. Physiol. 2006, 101, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Gandevia, S.C.; Carroll, T.J. Unilateral strength training increases voluntary activation of the opposite untrained limb. Clin. Neurophysiol. 2009, 120, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Muaidi, Q.I.; Nicholson, L.L.; Refshauge, K.M.; Adams, R.D.; Roe, J.P. Effect of anterior cruciate ligament injury and reconstruction on proprioceptive acuity of knee rotation in the transverse plane. Am. J. Sports Med. 2009, 37, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Van der Loos, M.; Brugar, C.G.; Shor, P.; Leifer, L.J. Experimental Results Using Force-Feedback Cueing in Robot-Assisted Stroke Therapy. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Schreiner, A.S.; Asano, H. Caregiver burden and health-related quality of life among Japanese stroke caregivers. Age Ageing 2003, 32, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Elnady, A.M.; Randhawa, B.K.; Boyd, L.A.; Menon, C. Combining Mental Training and Physical Training with Goal-Oriented Protocols in Stroke Rehabilitation: A Feasibility Case Study. Front. Hum. Neurosci. 2018, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.A.; Moeyaert, M.; Cunha, A.B.; Babik, I. Single-case design, analysis, and quality assessment for intervention research. J. Neurol. Phys. Ther. JNPT 2017, 41, 187. [Google Scholar] [CrossRef]
- Kratochwill, T.R.; Hitchcock, J.; Horner, H.; Levin, J.R.; Odom, S.L.; Rindskopf, D.M.; Shadish, W.R. Single-Case Designs Technical Documentation. Available online: https://eric.ed.gov/?id=ED510743 (accessed on 28 July 2019).
- Dijkerman, H.C.; Webeling, M.; ter Wal, J.M.; Groet, E.; van Zanvoort, M.J. A long-lasting improvement of somatosensory function after prism adaptation, a case study. Neuropsychologia 2004, 42, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S.; et al. Fugl-Meyer Assessment of Sensorimotor Function After Stroke Standardized Training Procedure for Clinical Practice and Clinical Trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf Motor Function Test as Outcome Measure for Research in Patients After Stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.; Immink, M.; Thewlis, D. Assessing Proprioception: A Systematic Review of Possibilities. Neurorehabil. Neural Repair. 2015, 29, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair 2002, 6, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Rabadi, M.H.; Rabadi, F.M. Comparison of the action research arm test and the Fugl-Meyer assessment as measures of upper-extremity motor weakness after stroke. Arch. Phys. Med. Rehabil. 2006, 87, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, I.P.; Hsu, M.J.; Sheu, C.F.; Lee, S.; Hsieh, C.L.; Lin, J.H. Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale and 2 versions of the Stroke Rehabilitation Assessment of Movement. Neurorehabilit. Neural Repair 2008, 22, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Nijland, R.; van Wegen, E.; Verbunt, J.; van Wijk, R.; van Kordelaar, J.; Kwakkel, G. A comparison of two validated tests for upper limb function after stroke: The Wolf Motor Function Test and the Action Research Arm Test. J. Rehabil. Med. 2010, 42, 694–696. [Google Scholar] [PubMed]
- Whitall, J.; Savin, D.; Harris-Love, M.; Waller, S. Psychometric properties of a modified wolf motor function test for people with mild and moderate upper extremity hemiparesis. Arch. Phys. Med. Rehabil. 2006, 82, 750–755. [Google Scholar] [CrossRef]
- Lin, K.C.; Hsieh, Y.W.; Wu, C.Y.; Chen, C.L.; Jang, Y.; Liu, J.S. Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabilit. Neural Repair 2009, 23, 429–434. [Google Scholar] [CrossRef]
- Sabari, J.S.; Maltzev, I.; Lubarsky, D.; Liszkay, E.; Homel, P. Goniometric Assessment of Shoulder Range of Motion: Comparison of Testing in Supine and Sitting Positions. Rev. Bras. Med. Esporte. 2012, 18, 38–41. [Google Scholar] [CrossRef]
- Haro3D. 3D Visualization Library for LabVIEW, User guide version 2.2; Harotek: Keller, TX, USA, 2018. Available online: http://www.harotek.com/products (accessed on 20 April 2018).
- Huang, H.Y.; Chang, S.H. A Skeleton-occluded Repair Method from Kinect. In Proceedings of the IEEE 2014 International Symposium on Computer, Consumer and Control, Taichung, Taiwan, 10–12 June 2014. [Google Scholar]
- Moon, S.; Park, Y.; Ko, D.W.; Suh, H. Multiple Kinect Sensor Fusion for Human Skeleton Tracking Using Kalman Filtering. Int. J. Adv. Robot. Syst. 2016, 13, 65. [Google Scholar] [CrossRef]
- Titus, J. The Hands-On XBee Lab Manual, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- LabVIEW Makerhub. Available online: https://www.labviewmakerhub.com/doku.php?id=libraries:linx:start (accessed on 20 April 2018).
- Leon, S.J. Linear Algebra with Applications, 9th ed.; Pearson Education Limited: London, UK, 2015. [Google Scholar]
- Lönn, J.; Crenshaw, A.G.; Djupsjöbacka, M.; Pedersen, J.; Johansson, H. Position Sense Testing: Influence of Starting Position and Type of Displacement. Arch. Phys. Med. Rehabil. 2015, 81, 592–596. [Google Scholar] [CrossRef]
- Helson, H.; King, S.M. The tau effect: An example of pxychological relativity. J. Exp. Psychol. 1931, 14, 202–217. [Google Scholar] [CrossRef]
- Fuentes, C.T.; Bastian, A.J. Where is your arm? Variations in proprioception across space and tasks. J. Neurophysiol. 2010, 103, 164–171. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chou, L.-W.; Luo, H.-J.; Tsai, P.-Y.; Lieu, F.-K.; Chiang, S.-L.; Sung, W.H. Effects of computer-aided Interlimb force coupling training on paretic hand and arm motor control following chronic stroke: A randomized controlled trial. PLoS ONE 2015, 10, e0131048. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Miller, L.M.; Fedulow, I.; Simkins, M.; Abrams, G.M.; Byl, N.; Rosen, J. Kinematic data analysis for post-stroke patients following bilateral versus unilateral rehabilitation with an upper limb wearable robotic system. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 153–164. [Google Scholar] [CrossRef]
- Liao, W.W.; Wu, C.Y.; Hsieh, Y.W.; Lin, K.C.; Chang, W.Y. Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: A randomized controlled trial. Clin. Rehabil. 2012, 26, 111–120. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yang, C.L.; Lin, K.C.; Wu, L.L. Unilateral versus bilateral robot-assisted rehabilitation on arm-trunk control and functions post stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2013, 10, 35. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wu, C.Y.; Wang, W.E.; Lin, K.C.; Chang, K.C.; Chen, C.C.; Liu, C.T. Bilateral robotic priming before task-oriented approach in subacute stroke rehabilitation: A pilot randomized controlled trial. Clin. Rehabil. 2017, 31, 225–233. [Google Scholar] [CrossRef]
- Yang, C.L.; Lin, K.C.; Chen, H.C.; Wu, C.Y.; Chen, C.L. Pilot comparative study of unilateral and bilateral robot-assisted training on upper-extremity performance in patients with stroke. Am. J. Occup. Ther. 2012, 66, 198–206. [Google Scholar] [CrossRef]
- Luft, A.R.; McCombe-Waller, S.; Whitall, J.; Forrester, L.W.; Macko, R.; Sorkin, J.D.; Schulz, J.B.; Goldberg, A.P.; Hanley, D.F. Repetitive bilateral arm training and motor cortex activation in chronic stroke: A randomized controlled trial. JAMA 2004, 292, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Whitall, J.; Waller, S.M.; Sorkin, J.D.; Forrester, L.W.; Macko, R.F.; Hanley, D.F.; Goldberg, A.P.; Luft, A. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: A single-blinded randomized controlled trial. Neurorehabilit. Neural Repair 2011, 25, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, A.L.; Peper, C.L.; Nienhuys, K.N.; Zijp, N.I.; Beek, P.J.; Kwakkel, G. Unilateral versus bilateral upper limb training after stroke: The Upper Limb Training After Stroke clinical trial. Stroke 2013, 44, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.M.; Liu, W.; Whitall, J. Temporal and spatial control following bilateral versus unilateral training. Hum. Mov. Sci. 2008, 27, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Shahine, E.M.; Shafshak, T.S. The effect of repetitive bilateral arm training with rhythmic auditory cueing on motor performance and central motor changes in patients with chronic stroke. Egypt. Rheumatol. Rehabil. 2014, 41, 8. [Google Scholar] [CrossRef]
- Li, C.; Inoue, Y.; Liu, T.; Sun, L. Validation of bimanual-coordinated training supported by a new upper-limb rehabilitation robot: A near-infrared spectroscopy study. Disabil. Rehabil. Assist. Technol. 2013, 8, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.; Buurke, J.H.; Prange, G.B.; Amirabdollahian, F. Training modalities in robot-mediated upper limb rehabilitation in stroke: A frame work for classification based on a systematic review. J. Neuroeng. Rehabil. 2014, 11, 111. [Google Scholar] [CrossRef]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsieh, Y.W.; Lin, K.C.; Chuang, L.L.; Chang, Y.F.; Liu, H.L.; Chen, C.L.; Lin, K.H.; Wai, Y.Y. Brain Reorganization after Bilateral Arm Training and Distributed Constraint-induced Therapy in Stroke Patients: A Preliminary Functional Magnetic Resonance Imaging Study. Chang. Gung Med. J. 2010, 33, 628–637. [Google Scholar]


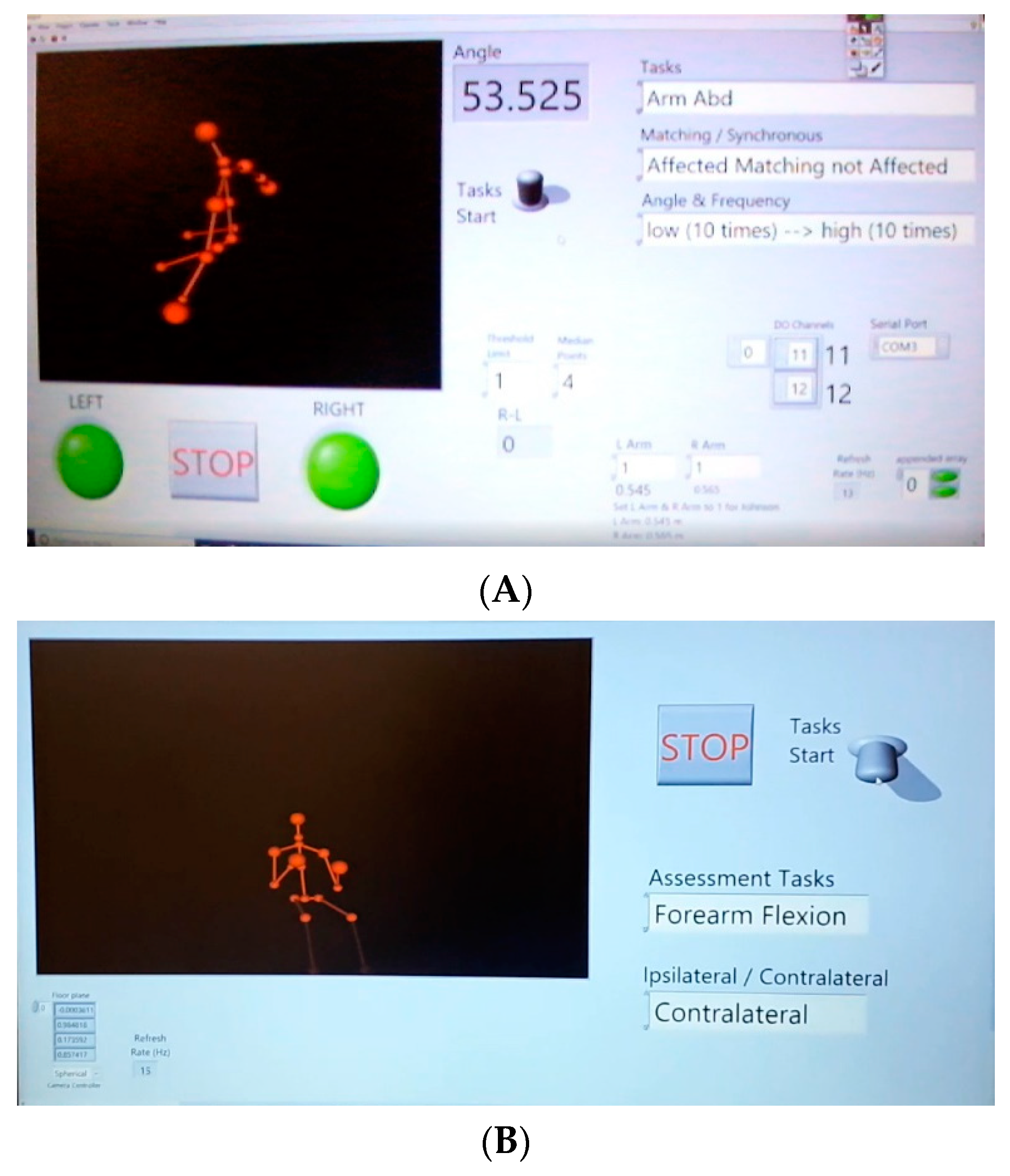

| Variables | Receiver | Transmitter |
|---|---|---|
| ID | 3456 | 3456 |
| DL | 1 | 2 |
| MY | 2 | 1 |
| D0 | DO Low [4] | DI [3] |
| D1 | DO Low [4] | DI [3] |
| D2 | DO Low [4] | DI [3] |
| IR | 64 (for 100 ms) | 64 |
| IT | - | 1 |
| IA | 1 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orand, A.; Erdal Aksoy, E.; Miyasaka, H.; Weeks Levy, C.; Zhang, X.; Menon, C. Bilateral Tactile Feedback-Enabled Training for Stroke Survivors Using Microsoft KinectTM. Sensors 2019, 19, 3474. https://doi.org/10.3390/s19163474
Orand A, Erdal Aksoy E, Miyasaka H, Weeks Levy C, Zhang X, Menon C. Bilateral Tactile Feedback-Enabled Training for Stroke Survivors Using Microsoft KinectTM. Sensors. 2019; 19(16):3474. https://doi.org/10.3390/s19163474
Chicago/Turabian StyleOrand, Abbas, Eren Erdal Aksoy, Hiroyuki Miyasaka, Carolyn Weeks Levy, Xin Zhang, and Carlo Menon. 2019. "Bilateral Tactile Feedback-Enabled Training for Stroke Survivors Using Microsoft KinectTM" Sensors 19, no. 16: 3474. https://doi.org/10.3390/s19163474
APA StyleOrand, A., Erdal Aksoy, E., Miyasaka, H., Weeks Levy, C., Zhang, X., & Menon, C. (2019). Bilateral Tactile Feedback-Enabled Training for Stroke Survivors Using Microsoft KinectTM. Sensors, 19(16), 3474. https://doi.org/10.3390/s19163474






