Near Room Temperature Light-Activated WS2-Decorated rGO as NO2 Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
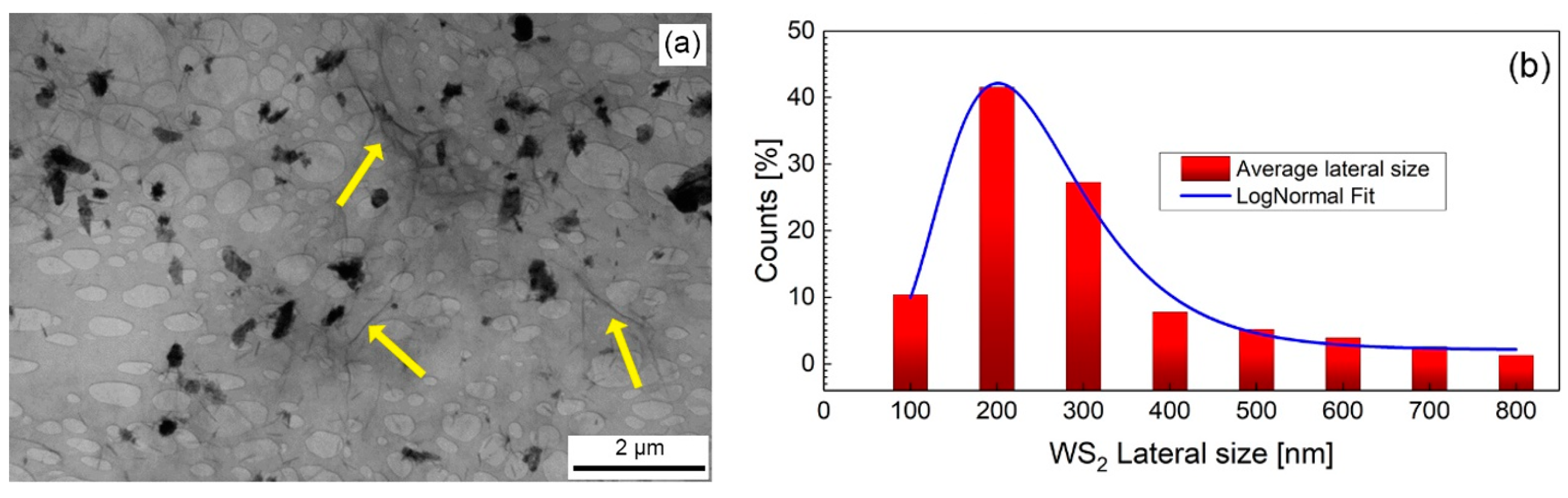
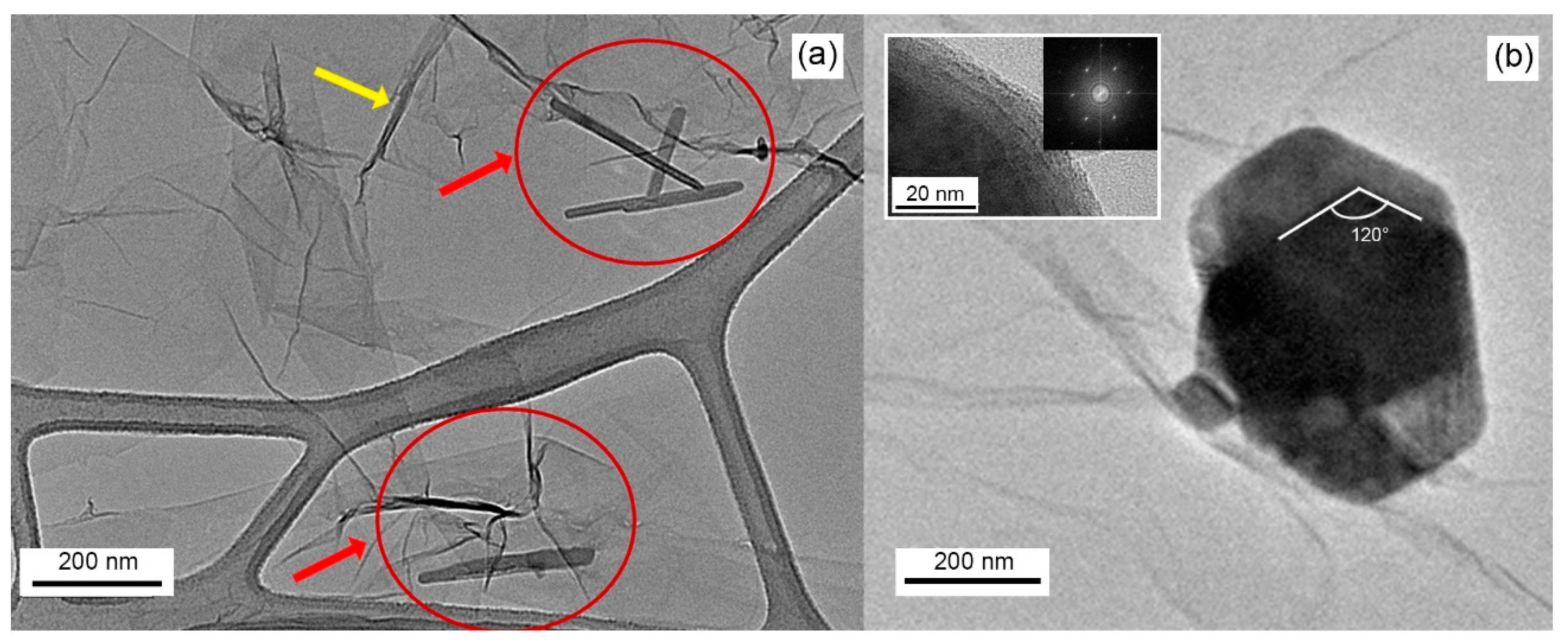
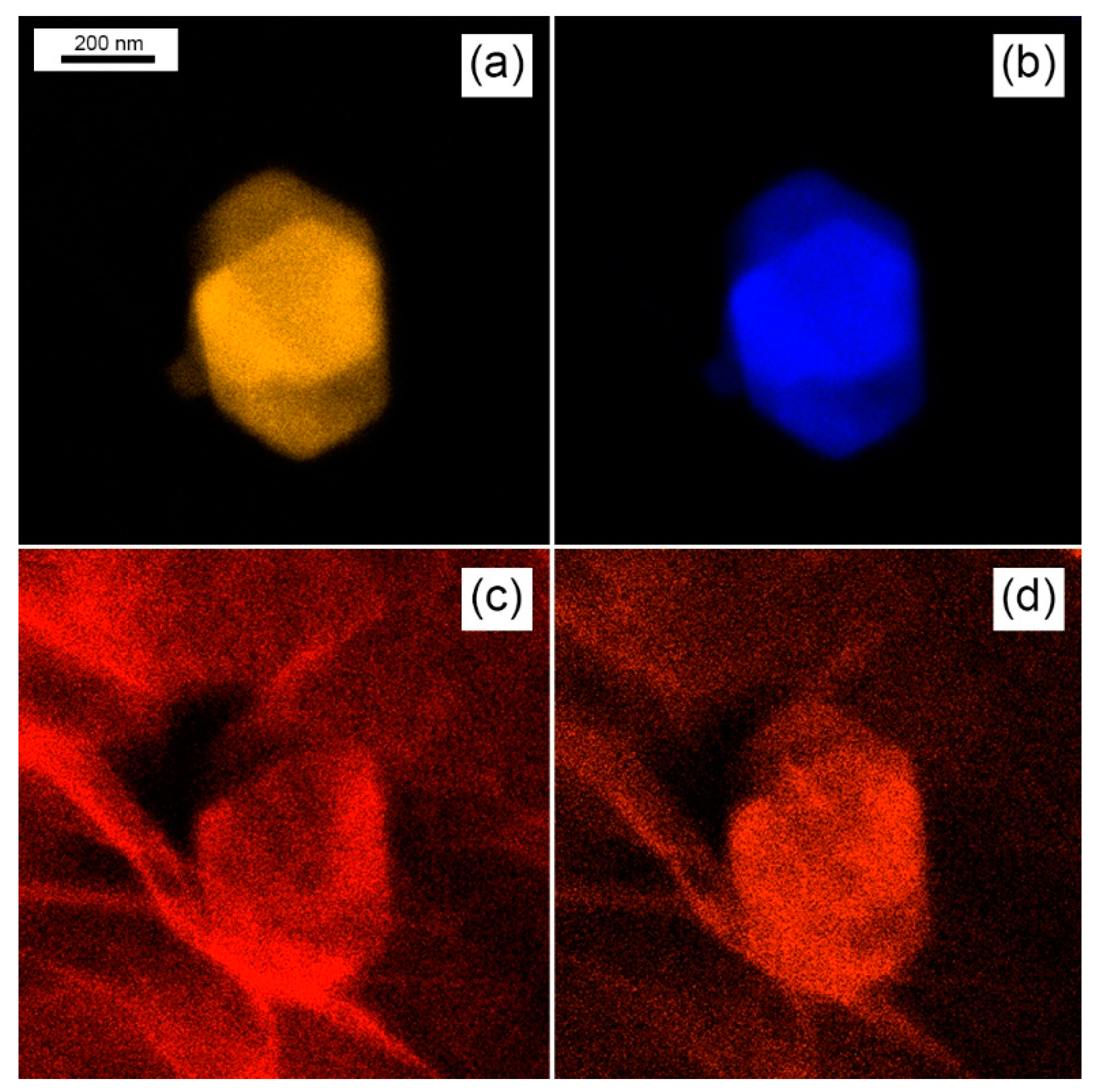
3.1. Morphological and Compositional Characterization of the WS2-Decorated rGO
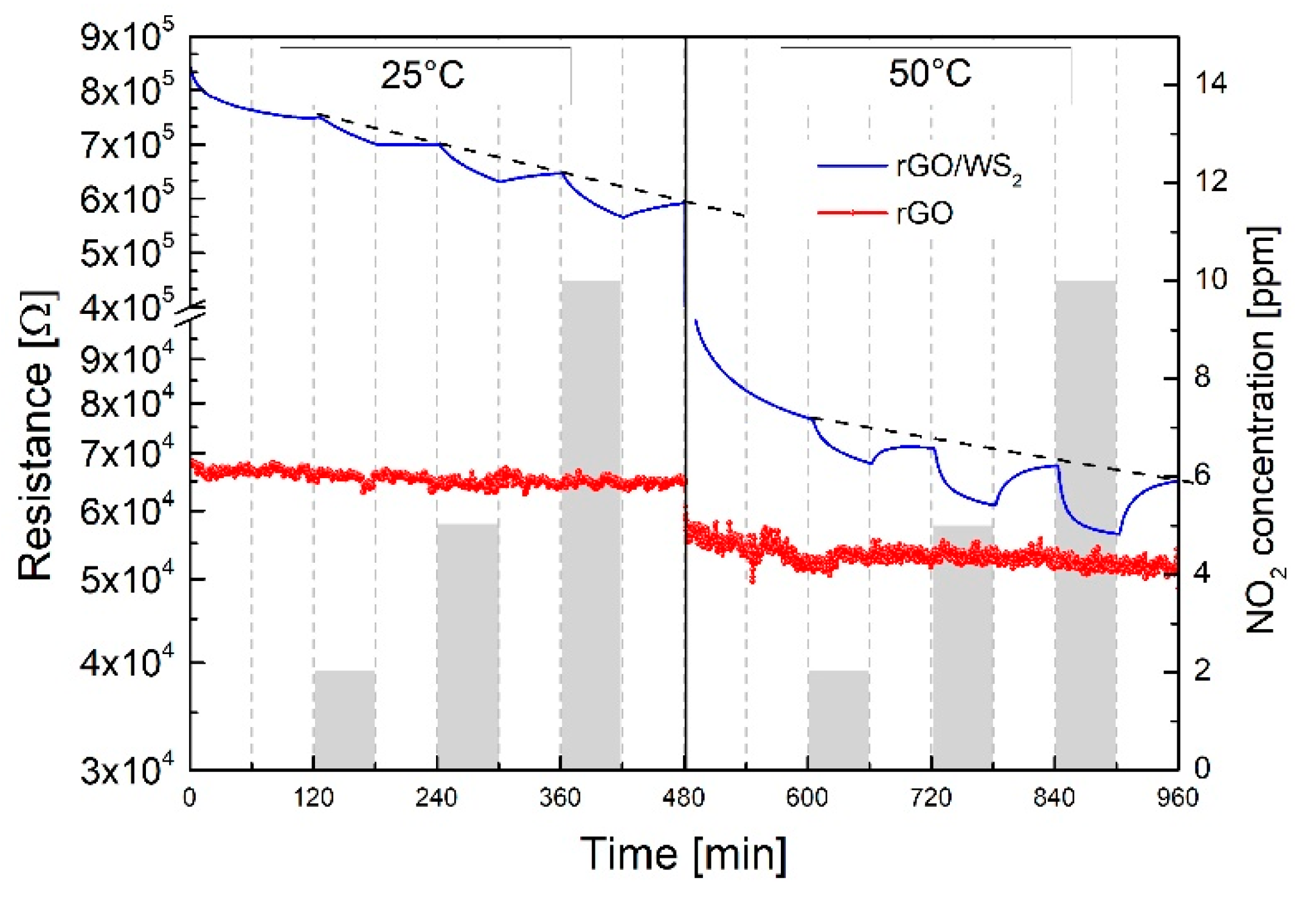
3.2. NO2 Gas Response
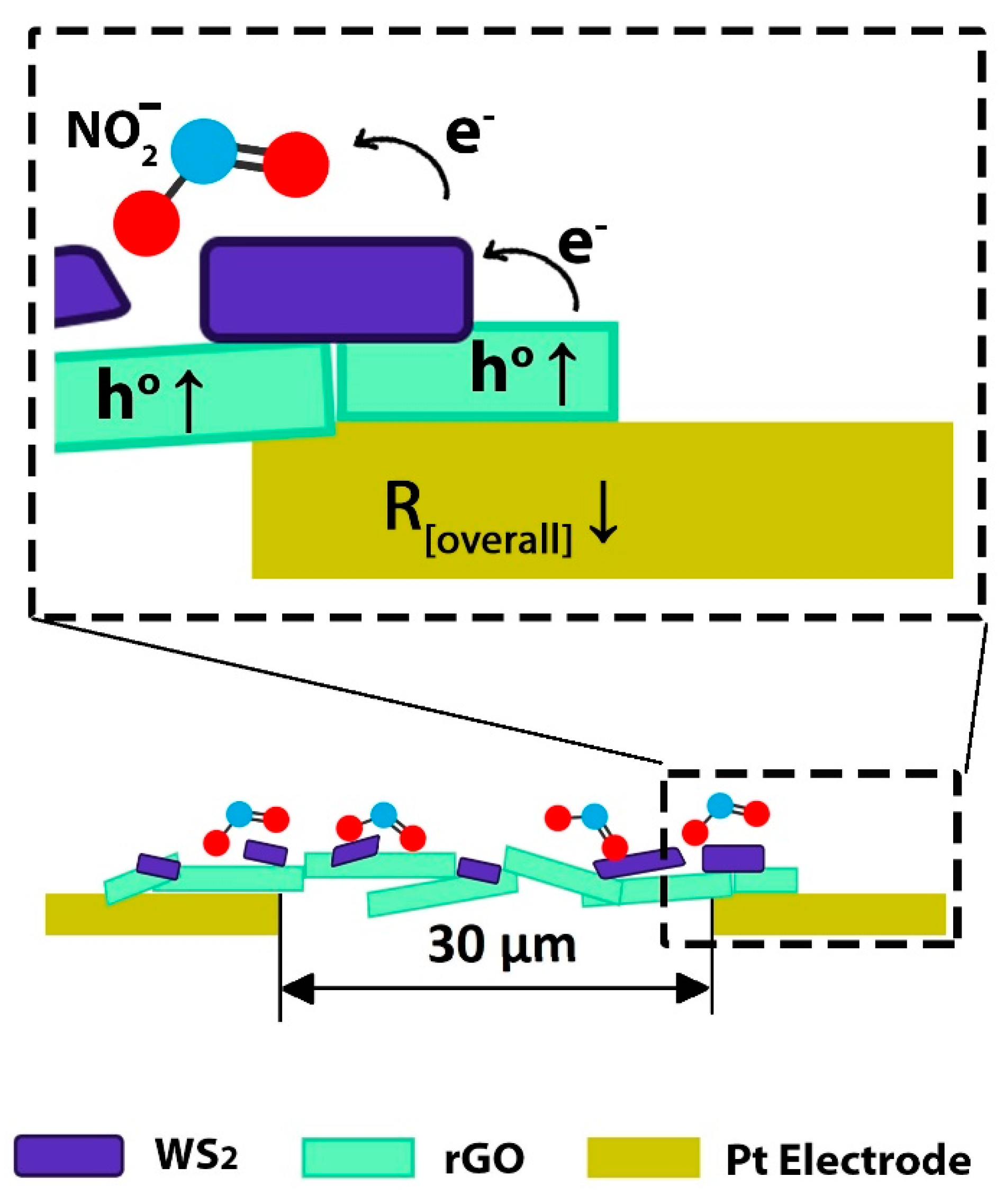
Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-dimensional layered nanomaterials for gas-sensing applications. Inorg. Chem. Front. 2016, 3, 433–451. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef]
- Late, D.J.; Huang, Y.K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing behavior of atomically thin-layered MoS2 transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Response to NO2 and other gases of resistive chemically exfoliated MoS2-based gas sensors. Sens. Actuators B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Kuru, C.; Choi, D.; Kargar, A.; Liu, C.H.; Yavuz, S.; Choi, C.; Jin, S.; Bandaru, P.R. High-performance flexible hydrogen sensor made of WS2 nanosheet–Pd nanoparticle composite film. Nanotechnology 2016, 27, 195501. [Google Scholar] [CrossRef] [PubMed]
- Perrozzi, F.; Emamjomeh, S.M.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sens. Actuators B Chem. 2017, 243, 812–822. [Google Scholar] [CrossRef]
- Baek, J.; Yin, D.; Liu, N.; Omkaram, I.; Jung, C.; Im, H.; Hong, S.; Kim, S.M.; Hong, Y.K.; Hur, J.; et al. A highly sensitive chemical gas detecting transistor based on highly crystalline CVD-grown MoSe2 films. Nano Res. 2017, 10, 1861–1871. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, Y.; Chen, J.; Yu, Y.; Zheng, S.; Zhang, R.; Li, Q.; Chen, X.; Sun, C.; Zhang, H.; et al. Highly sensitive MoTe2 chemical sensor with fast recovery rate through gate biasing. 2D Mater. 2017, 4, 025018. [Google Scholar] [CrossRef]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.-J.J.; Park, S.-G.G.; Kwon, J.-D.D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based gas sensing using atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Tan, J.; Chow, P.; Lu, T.M.; Koratkar, N. Aging of Transition Metal Dichalcogenide Monolayers. ACS Nano 2016, 10, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Giancaterini, L.; Emamjomeh, S.M.; De Marcellis, A.; Palange, E.; Resmini, A.; Anselmi-Tamburini, U.; Cantalini, C. The influence of thermal and visible light activation modes on the NO2 response of WO3 nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2016, 229, 387–395. [Google Scholar] [CrossRef]
- Geng, X.; Lahem, D.; Zhang, C.; Li, C.-J.; Olivier, M.-G.; Debliquy, M. Visible light enhanced black NiO sensors for ppb-level NO2 detection at room temperature. Ceram. Int. 2019, 45, 4253–4261. [Google Scholar] [CrossRef]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Ye, X.; Cai, B.; Kong, E.S.-W.; Yang, Z.; Zhang, Y. Light-assisted recovery for a highly-sensitive NO2 sensor based on RGO-CeO2 hybrids. Sens. Actuators B Chem. 2018, 270, 119–129. [Google Scholar] [CrossRef]
- Berholts, A.; Kahro, T.; Floren, A.; Alles, H.; Jaaniso, R. Photo-activated oxygen sensitivity of graphene at room temperature. Appl. Phys. Lett. 2014, 105, 163111. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.-S.; Xia, J.-B.; Li, J. Photoresponsive and Gas Sensing Field-Effect Transistors based on Multilayer WS2 Nanoflakes. Sci. Rep. 2015, 4, 5209. [Google Scholar] [CrossRef]
- Ottaviano, L.; Palleschi, S.; Perrozzi, F.; D’Olimpio, G.; Priante, F.; Donarelli, M.; Benassi, P.; Nardone, M.; Gonchigsuren, M.; Gombosuren, M.; et al. Mechanical exfoliation and layer number identification of MoS2 revisited. 2D Mater. 2017, 4, 045013. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.-H.; Park, S.-G.; Kwon, J.-D.; Lee, Y.-J.; Lee, K.-H.; Lee, B.H.; et al. Chemical Sensing of 2D Graphene/MoS2 Heterostructure device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High Surface Area MoS2/Graphene Hybrid Aerogel for Ultrasensitive NO2 Detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef]
- Niu, Y.; Jiao, W.C.; Wang, R.G.; Ding, G.M.; Huang, Y.F. Hybrid nanostructures combining graphene-MoS2 quantum dots for gas sensing. J. Mater. Chem. A 2016, 4, 8198–8203. [Google Scholar] [CrossRef]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 2013, 52, 13751–13754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Wang, L.; Li, X.; Huang, W. Layer-controllable WS2-reduced graphene oxide hybrid nanosheets with high electrocatalytic activity for hydrogen evolution. Nanoscale 2015, 7, 10391–10397. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Burman, D.; Santra, S.; Guha, P.K. WS2/GO Nanohybrids for Enhanced Relative Humidity Sensing at Room Temperature. IEEE Sens. J. 2017, 17, 7340–7347. [Google Scholar] [CrossRef]
- Wang, X.; Gu, D.; Li, X.; Lin, S.; Zhao, S.; Rumyantseva, M.N.; Gaskov, A.M. Reduced graphene oxide hybridized with WS2 nanoflakes based heterojunctions for selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2019, 282, 290–299. [Google Scholar] [CrossRef]
- Treossi, E.; Melucci, M.; Liscio, A.; Gazzano, M.; Samorì, P.; Palermo, V. High-Contrast Visualization of Graphene Oxide on Dye-Sensitized Glass, Quartz, and Silicon by Fluorescence Quenching. J. Am. Chem. Soc. 2009, 131, 15576–15577. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, Y.; Xue, Q.; Wang, W.; Gao, D. Ferromagnetism in exfoliated tungsten disulfide nanosheets. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.B.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Nardone, M.; Santucci, S.; Ottaviano, L. Graphene Oxide as a Practical Solution to High Sensitivity Gas Sensing. J. Phys. Chem. C 2013, 117, 10683–10690. [Google Scholar] [CrossRef]
- Di Paola, A.; Palmisano, L.; Venezia, A.M.; Augugliaro, V. Coupled Semiconductor Systems for Photocatalysis. Preparation and Characterization of Polycrystalline Mixed WO3/WS2 Powders. J. Phys. Chem. B 1999, 103, 8236–8244. [Google Scholar] [CrossRef]
- Wong, K.C.; Lu, X.; Cotter, J.; Eadie, D.T.; Wong, P.C.; Mitchell, K.A.R. Surface and friction characterization of MoS2 and WS2 third body thin films under simulated wheel/rail rolling-sliding contact. Wear 2008, 264, 526–534. [Google Scholar] [CrossRef]
- Li, H.; Huang, M.; Cao, G. Markedly different adsorption behaviors of gas molecules on defective monolayer MoS2: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 15110–15117. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wang, Q.; Li, T.; He, C.; Ma, B.; Tang, Y.; Lu, Z.; Yang, Z. Repairing sulfur vacancies in the MoS2 monolayer by using CO, NO and NO2 molecules. J. Mater. Chem. C 2016, 4, 7093–7101. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W.; Zhu, H. Mechanism of charge transfer and its impacts on Fermi-level pinning for gas molecules adsorbed on monolayer WS2. J. Chem. Phys. 2015, 142, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.Q.; Pham, T.T.; Le, D.A.; Thi, C.M.; Le, H.M. A first-principles investigation of various gas (CO, H2O, NO, and O2) absorptions on a WS2monolayer: Stability and electronic properties. J. Phys. Condens. Matter 2015, 27, 305005. [Google Scholar] [CrossRef] [PubMed]
- Bagsican, F.R.; Winchester, A.; Ghosh, S.; Zhang, X.; Ma, L.; Wang, M.; Murakami, H.; Talapatra, S.; Vajtai, R.; Ajayan, P.M.; et al. Adsorption energy of oxygen molecules on graphene and two-dimensional tungsten disulfide. Sci. Rep. 2017, 7, 1774. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jiang, H.; Shao, R.; Zhang, Y.; Xie, S. Two-beam-laser interference mediated reduction, patterning and nanostructuring of graphene oxide for the production of a flexible humidity sensing device. Carbon N. Y. 2011, 50, 1667–1673. [Google Scholar] [CrossRef]

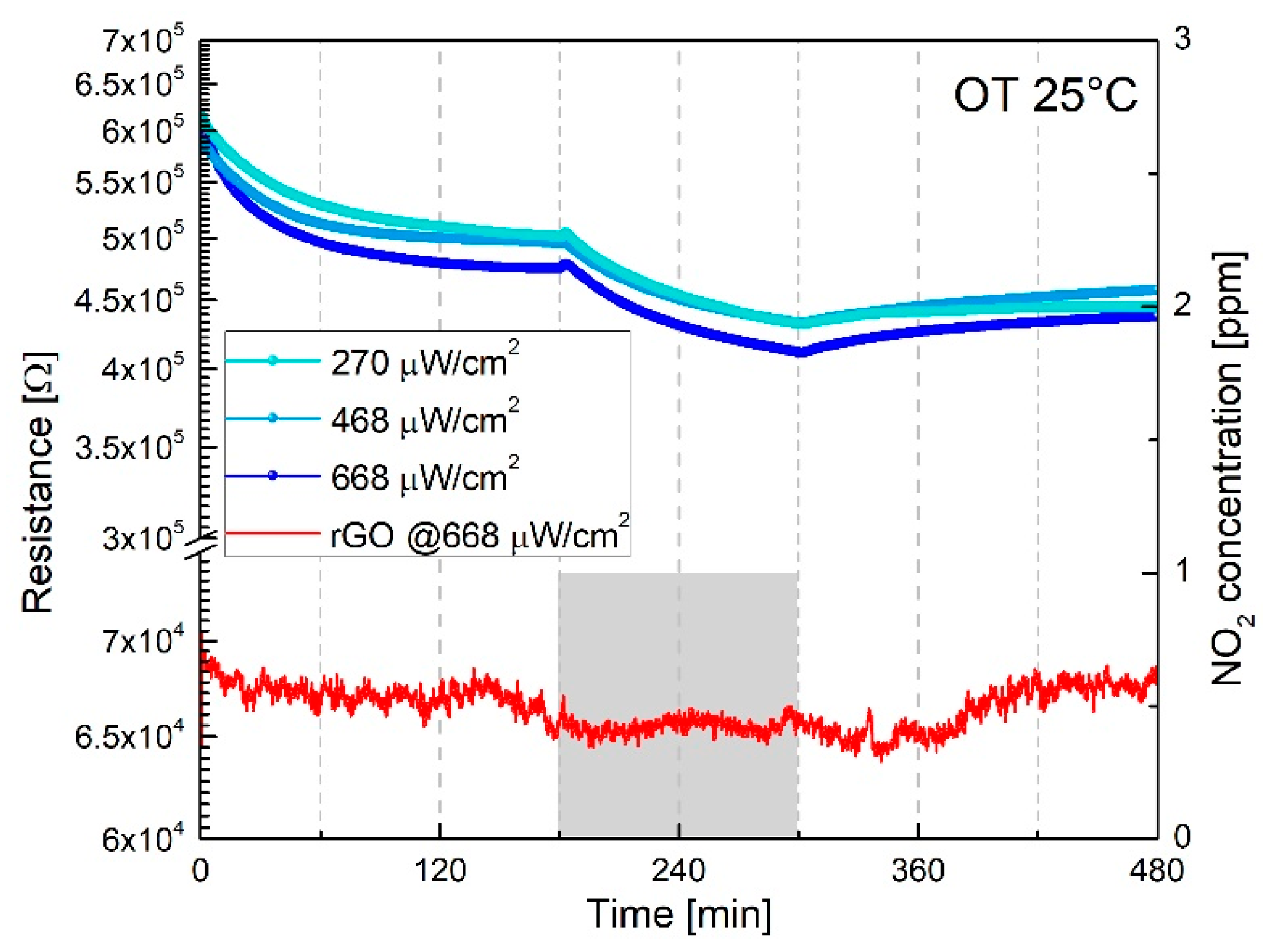
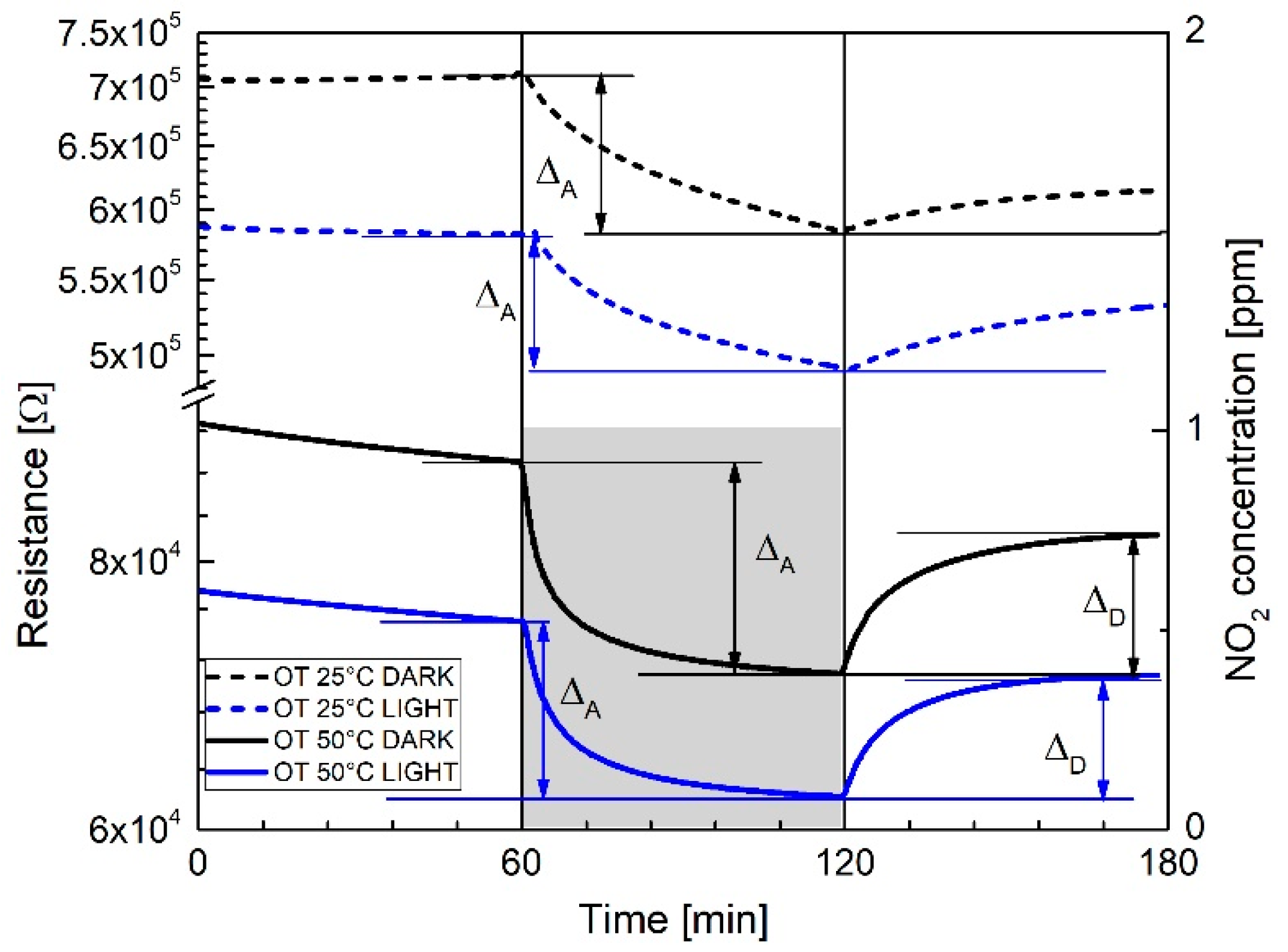
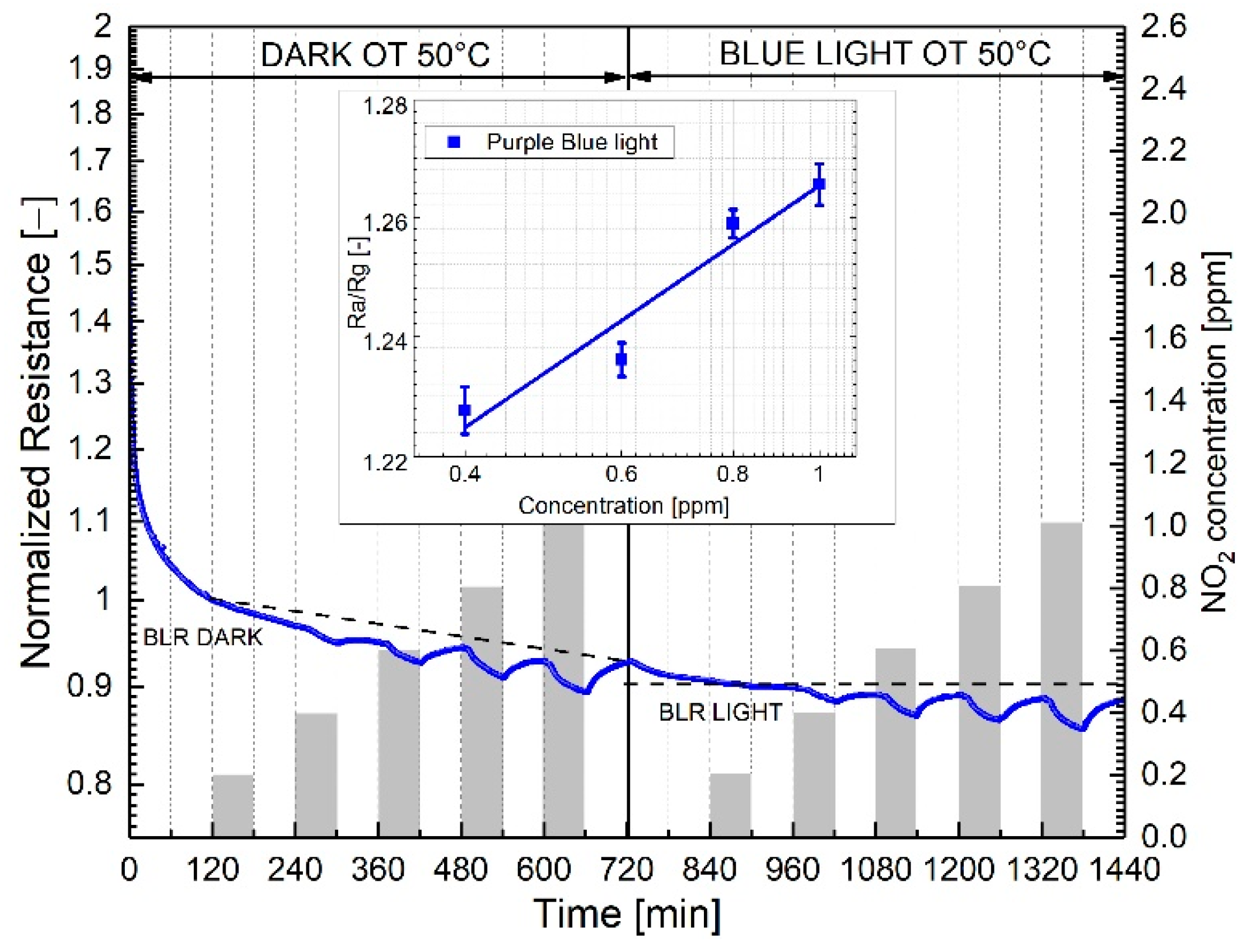
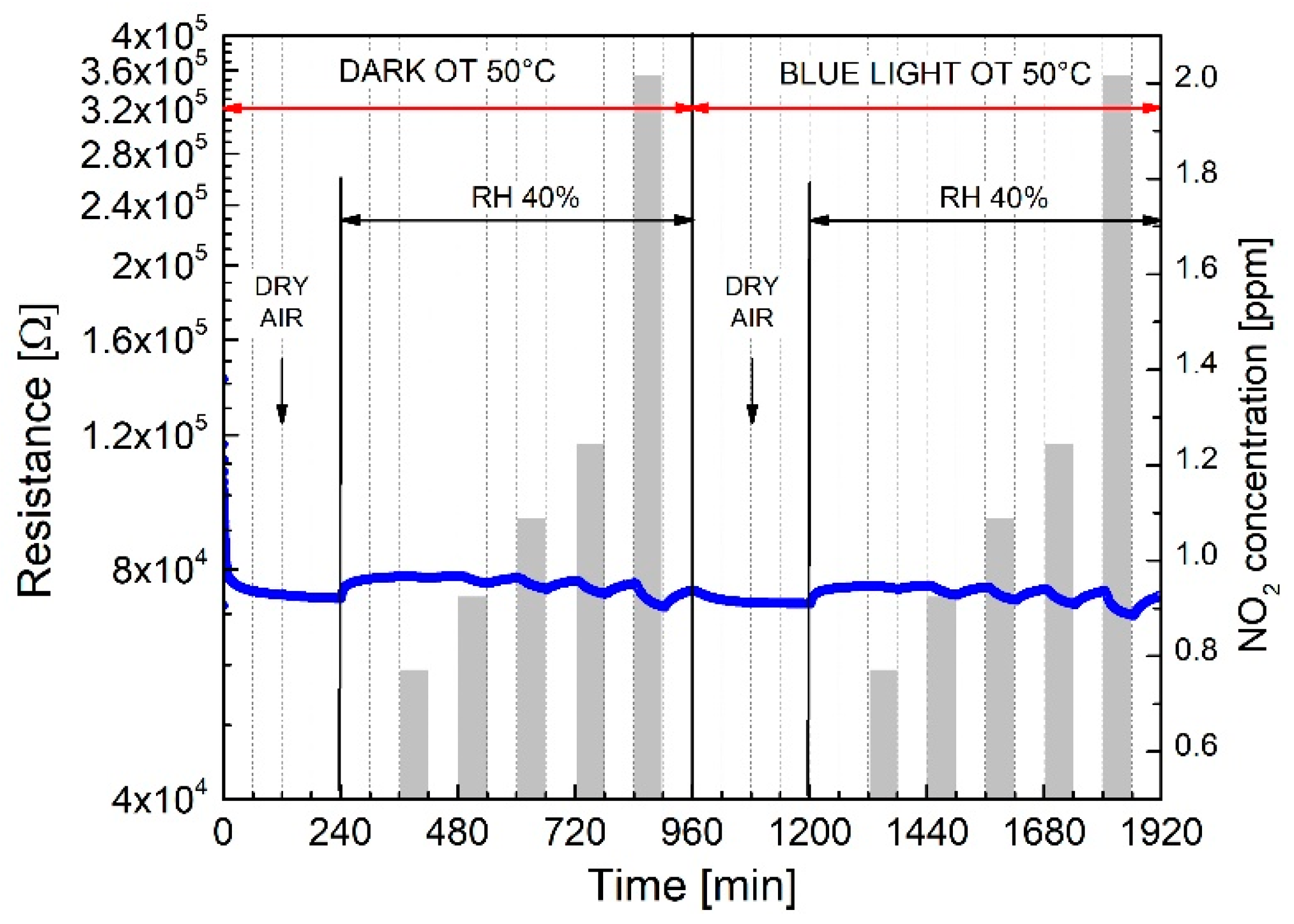
| Response TO 1 ppm NO2 | ||||
|---|---|---|---|---|
| Operating Conditions | RR Ra/Rg | RP ΔD/ΔA | τ ads | τ des |
| (–) | (%) | (min) | (min) | |
| 25 °C DARK | 1.18 ± 0.02 | 23 | - | - |
| 25 °C LIGHT | 1.21 ± 0.02 | 65 | - | - |
| 50 °C DARK | 1.20 ± 0.02 | 60 | 22 | 26 |
| 50 °C LIGHT | 1.27 ± 0.02 | 70 | 16 | 18 |
| Physisorbed Molecules | Adsorption Energy on rGO (eV) | Reference | Adsorption Energy on WS2 (eV) | Reference |
|---|---|---|---|---|
| O2 | −0.16 | [36] | −0.24 | [36] |
| NO2 | −0.80 | [32,34,35] | −0.41 | [34,35] |
| H2O | −0.04 | [37] | −0.23 | [34] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolucci, V.; Emamjomeh, S.M.; Ottaviano, L.; Cantalini, C. Near Room Temperature Light-Activated WS2-Decorated rGO as NO2 Gas Sensor. Sensors 2019, 19, 2617. https://doi.org/10.3390/s19112617
Paolucci V, Emamjomeh SM, Ottaviano L, Cantalini C. Near Room Temperature Light-Activated WS2-Decorated rGO as NO2 Gas Sensor. Sensors. 2019; 19(11):2617. https://doi.org/10.3390/s19112617
Chicago/Turabian StylePaolucci, Valentina, Seyed Mahmoud Emamjomeh, Luca Ottaviano, and Carlo Cantalini. 2019. "Near Room Temperature Light-Activated WS2-Decorated rGO as NO2 Gas Sensor" Sensors 19, no. 11: 2617. https://doi.org/10.3390/s19112617
APA StylePaolucci, V., Emamjomeh, S. M., Ottaviano, L., & Cantalini, C. (2019). Near Room Temperature Light-Activated WS2-Decorated rGO as NO2 Gas Sensor. Sensors, 19(11), 2617. https://doi.org/10.3390/s19112617






