Design for Distributed Feedback Laser Biosensors Based on the Active Grating Model
Abstract
1. Introduction
2. Simulation Results and Discussion
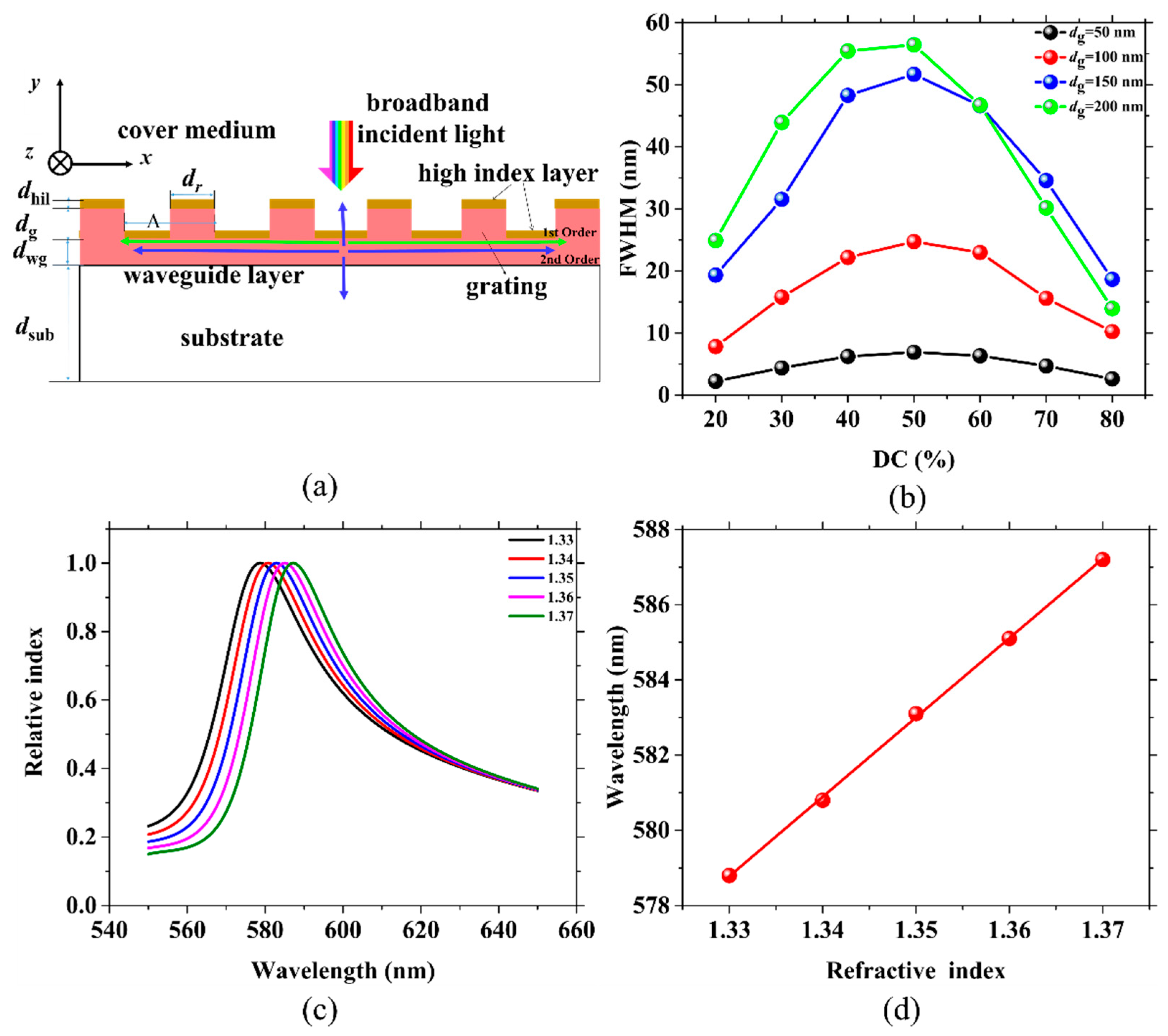
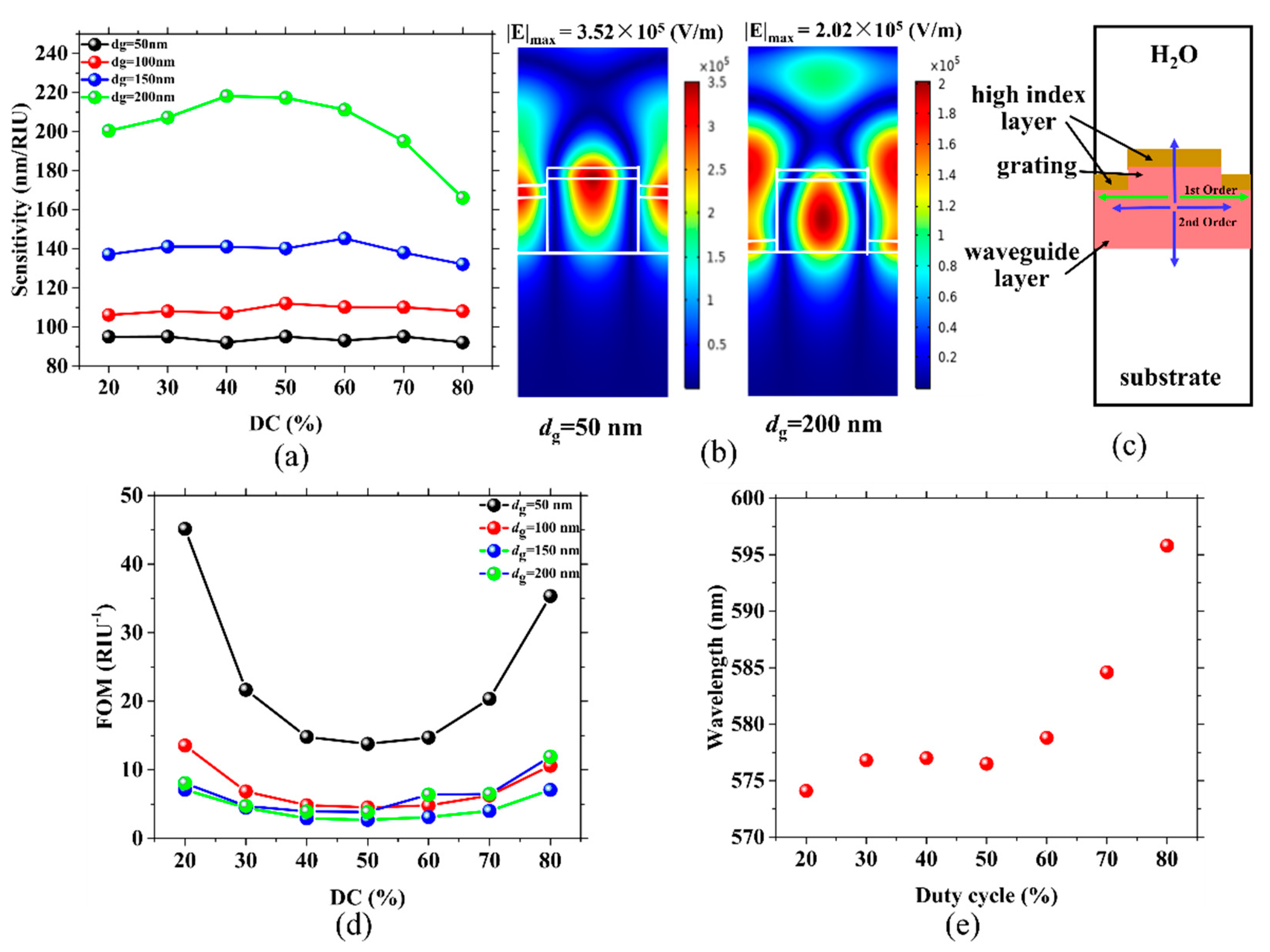
2.1. The Effect of DC and dg on Passive Grating Sensors
2.2. Active Grating for High FOMs of Sensors
2.3. Sensors with Active Gratings for Biomolecule Detection
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vannahme, C.; Smith, C.; Dufva, M. Optimized Distributed Feedback Dye Laser Sensor for Real-Time Monitoring of Small Molecule Diffusion. In Proceedings of the European Conference on Integrated Optics (ECIO 17th) and the MicroOptics Conference (MOC 19th), Nice, France, 24–27 June 2014. [Google Scholar]
- Lu, M.; Choi, S.S.; Irfan, U. Plastic Distributed Feedback Laser Biosensor. Appl. Phys. Lett. 2008, 93, 111113. [Google Scholar] [CrossRef]
- Yafang, T.; Chun, G.; Allen, C. Plastic-Based Distributed Feedback Laser Biosensors in Microplate Format. IEEE Sens. J. 2012, 12, 1174–1180. [Google Scholar]
- Chu, A.C. Instrumentation for a Distributed Feedback Laser Biosensor System. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, USA, 2011. [Google Scholar]
- Kogelnik, H.; Shank, C.V. Stimulated Emission in a Periodic Structure. Appl. Phys. Lett. 2003, 18, 152–154. [Google Scholar] [CrossRef]
- Kogelnik, H.; Shank, C.V. Coupled-Wave Theory of Distributed Feedback Lasers. J. Appl. Phys. 1972, 43, 2327–2335. [Google Scholar] [CrossRef]
- Boj, P.G.; Morales-Vidal, M.; Villalvilla, J.M. Organic Distributed Feedback Laser to Monitor Solvent Extraction upon Thermal Annealing in Solution-Processed Polymer Films. Sens. Actuators B Chem. 2016, 232, 605–610. [Google Scholar] [CrossRef]
- Gersborg-Hansen, M.; Kristensen, A. Optofluidic Dye Lasers. Ph.D. Thesis, Department of Micro- and Nanotechnology Technical University of Denmark, Kongens Lyngby, Denmark, 14 December 2007. [Google Scholar]
- Heydari, E.; Buller, J.; Wischerhoff, E. Label-Free Biosensor Based on an All-Polymer DFB Laser. Adv. Opt. Mater. 2014, 2, 137–141. [Google Scholar] [CrossRef]
- Vannahme, C.; Leung, M.C.; Richter, F. Nanoimprinted Distributed Feedback Lasers Comprising TiO2 Thin Films: Design Guidelines for High Performance Sensing. Laser Photonics Rev. 2013, 7, 1036–1042. [Google Scholar] [CrossRef]
- Gao, F.; Chen, L.; Wang, X. Design and Simulation of Label-Free Biosensor Based on the Dynamic Distributed Feedback Laser Emission. In Proceedings of the SPIE Proceedings Optical Sensors and Biophotonics III, Shanghai, China, 13 November 2011. [Google Scholar]
- Ge, C.; Lu, M.; Jian, X. Large-Area Organic Distributed Feedback Laser Fabricated by Nanoreplica Molding and Horizontal Dipping. Opt. Express 2010, 18, 12980. [Google Scholar] [CrossRef]
- Morales-Vidal, M.; Boj, P.G.; Quintana, J.A. Distributed Feedback Lasers Based on Perylenediimide Dyes for Label-Free Refractive Index Sensing. Sens. Actuators B Chem. 2015, 220, 1368–1375. [Google Scholar] [CrossRef]
- Ge, C.; Lu, M.; Zhang, W.; Cunningham, B.T. Distributed Feedback Laser Biosensor Incorporating a Titanium Dioxide Nanorod Surface. Appl. Phys. Lett. 2010, 96, 163702. [Google Scholar] [CrossRef]
- Vannahme, C.; Kristian, T.S.; Gade, C. Nanoimprinted Distributed Feedback Dye Laser Sensors for High Frame Rate Refractometric Imaging of Dissolution and Fluid Flow. In Proceedings of the SPIE Proceedings Optical Sensors 2015, Prague, Czech Republic, 13 April 2015. [Google Scholar]
- Vannahme, C.; Dufva, M.; Kristensen, A. High Frame Rate Multi-Resonance Imaging Refractometry with Distributed Feedback Dye Laser Sensor. Light Sci. Appl. 2015, 4, 269. [Google Scholar] [CrossRef]
- Lu, M.; Cunningham, B.T.; Park, S.J. Vertically Emitting, Dye-Doped Polymer Laser in the Green (λ ∼536 nm) with a Second Order Distributed Feedback Grating Fabricated by Replica Molding. Optics Commun. 2008, 281, 3159–3162. [Google Scholar] [CrossRef]
- Vannahme, C.; Smith, C.L.C.; Christiansen, M.B. Emission Wavelength of Multilayer Distributed Feedback Dye Lasers. Appl. Phys. Lett. 2012, 101, 152. [Google Scholar] [CrossRef]
- Haughey, A.M.; Guilhabert, B.; Kanibolotsky, A.L. An Organic Semiconductor Laser Based on Star-Shaped Truxene-Core Oligomers for Refractive Index Sensing. Sens. Actuators B Chem. 2013, 185, 132–139. [Google Scholar] [CrossRef]
- Haughey, A.M.; Guilhabert, B.; Kanibolotsky, A.L. An Oligofluorene Truxene Based Distributed Feedback Laser for Biosensing Applications. Biosens. Bioelectron. 2014, 54, 679–686. [Google Scholar] [CrossRef][Green Version]
- Haughey, A.M.; Mcconnell, G.; Guilhabert, B. Organic Semiconductor Laser Biosensor: Design and Performance Discussion. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 6–14. [Google Scholar] [CrossRef]
- Retolaza, A.; Martinez-Perdiguero, J.; Merino, S. Organic Distributed Feedback Laser for Label-Free Biosensing of ErbB2 Protein Biomarker. Sens. Actuators B Chem. 2016, 223, 261–265. [Google Scholar] [CrossRef]
- Haughey, A.M.; Burley, G.A.; Kanibolotsky, A.L. Organic Distributed Feedback Laser Biosensor. In Proceedings of the 2013 IEEE Photonics Conference, Bellevue, WA, USA, 8–12 September 2013; pp. 161–162. [Google Scholar]
- Chenais, S.; Forget, S. Recent Advances in Solid-State Organic Lasers. Polym. Int. 2012, 61, 390–406. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Turnbull, G.A. Explosive Sensing Using Polymer Lasers. Mol. Cryst. 2012, 554, 8. [Google Scholar] [CrossRef]
- Pitruzzello, G.; Krauss, T.F. Photonic Crystal Resonances for Sensing and Imaging. J. Opt. 2018, 20, 073004. [Google Scholar] [CrossRef]
- Huang, X.B.; Kang, W.X.; Liu, C.; Dong, J.; Zhang, S.Q. Improving the Sensitivity of Compound Waveguide Grating Biosensor via Modulated Wavevector. Appl. Phys. Express 2018, 11, 082202. [Google Scholar]
- Ju, J.; Han, Y.A.; Kim, S.M. Design Optimization of Structural Parameters for Highly Sensitive Photonic Crystal Label-Free Biosensors. Sensors 2013, 13, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Abutoama, M.; Abdulhalim, I. Self-Referenced Biosensor Based on Thin Dielectric Grating Combined with Thin Metal Film. Opt. Express 2015, 23, 28667–28682. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Krueger, N.A.; Ocier, C.R. Resonant Mode Engineering of Photonic Crystal Sensors Clad with Ultralow Refractive Index Porous Silicon Dioxide. Adv. Opt. Mater. 2017, 5, 1700605. [Google Scholar] [CrossRef]
- Huang, Q.L.; Peh, J.; Hergenrother, P.J.; Cunningham, B.T. Porous Photonic Crystal External Cavity Laser Biosensor. Appl. Phys. Lett. 2016, 109, 071103. [Google Scholar] [CrossRef] [PubMed]
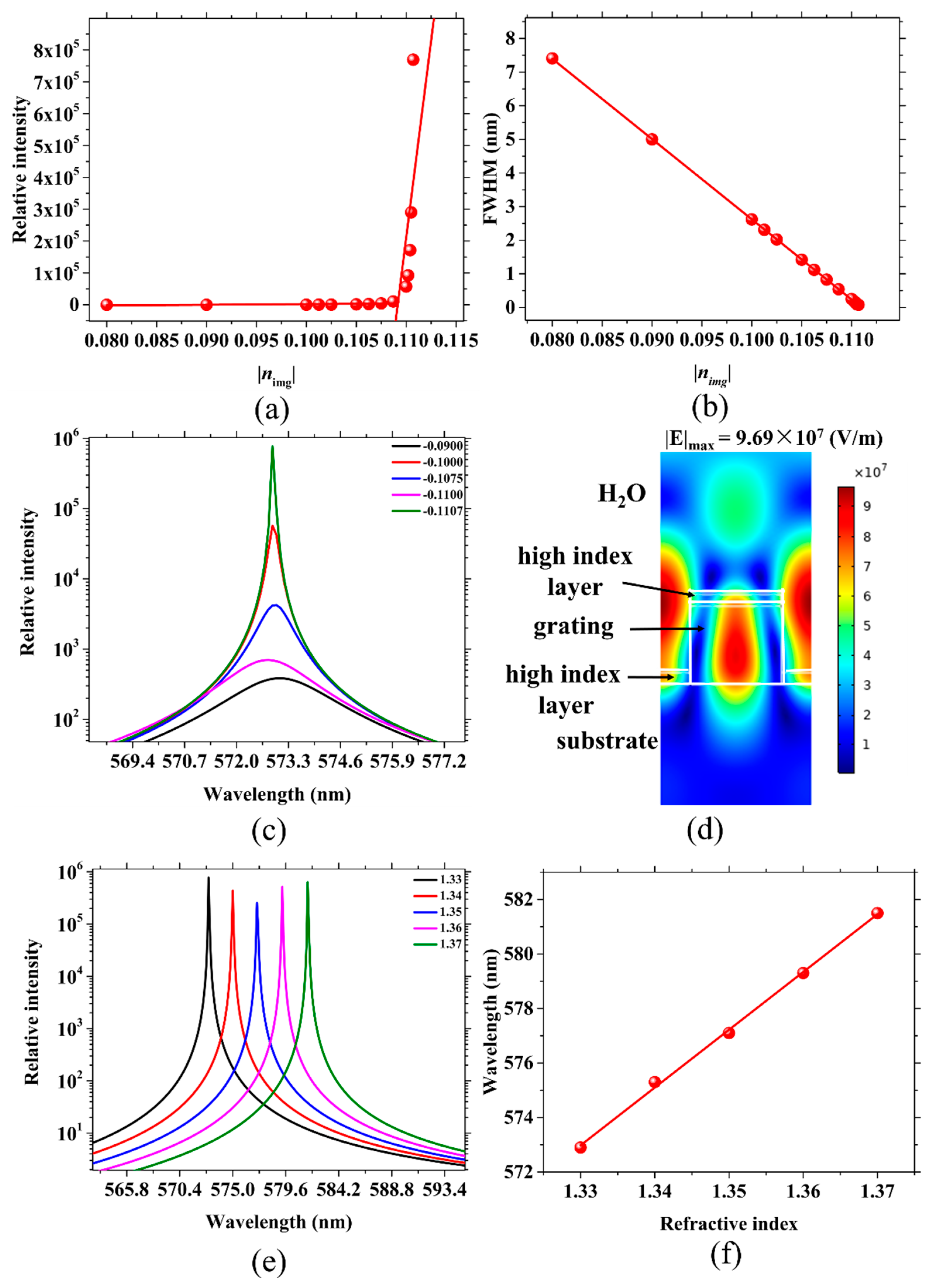

| nimg | −0.1482 | −0.1480 | −0.1475 | −0.1460 | −0.1450 | −0.1430 |
| Relative intensity | 306680 | 165010 | 61732 | 13141 | 7043 | 2968 |
| FWHM (nm) | 0.13 | 0.18 | 0.29 | 0.64 | 0.86 | 1.32 |
| nimg | −0.1400 | −0.1375 | −0.1286 | −0.1229 | −0.1100 | −0.100 |
| Relative intensity | 1251 | 746 | 216 | 123 | 48 | 28 |
| FWHM (nm) | 1.99 | 2.56 | 4.51 | 5.85 | 8.74 | 10.97 |
| nimg | −0.1410 | −0.1409 | −0.1407 | −0.1405 | −0.1400 | −0.1395 |
| Relative intensity | 770000 | 464040 | 226950 | 133970 | 52827 | 27984 |
| FWHM (nm) | 0.08 | 0.13 | 0.16 | 0.20 | 0.32 | 0.44 |
| nimg | −0.1375 | −0.1325 | −0.1271 | −0.1214 | −0.1150 | −0.1100 |
| Relative intensity | 6396 | 1437 | 423 | 203 | 109 | 73 |
| FWHM (nm) | 0.90 | 2.05 | 3.36 | 4.68 | 6.23 | 7.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhou, Y.; Guo, Z.; Wu, X. Design for Distributed Feedback Laser Biosensors Based on the Active Grating Model. Sensors 2019, 19, 2569. https://doi.org/10.3390/s19112569
Wang B, Zhou Y, Guo Z, Wu X. Design for Distributed Feedback Laser Biosensors Based on the Active Grating Model. Sensors. 2019; 19(11):2569. https://doi.org/10.3390/s19112569
Chicago/Turabian StyleWang, Bowen, Yi Zhou, Zhihe Guo, and Xiang Wu. 2019. "Design for Distributed Feedback Laser Biosensors Based on the Active Grating Model" Sensors 19, no. 11: 2569. https://doi.org/10.3390/s19112569
APA StyleWang, B., Zhou, Y., Guo, Z., & Wu, X. (2019). Design for Distributed Feedback Laser Biosensors Based on the Active Grating Model. Sensors, 19(11), 2569. https://doi.org/10.3390/s19112569






