The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies
Abstract
1. Introduction
2. Materials and Methods
- Met clinical criteria for DAT (criteria positive (CP)). Typically these were PwP requiring frequent doses of levodopa with troublesome ‘off’ periods (>1–2 h/day) and or dyskinesia [16].
- Did not meet the clinical criteria for DAT (criteria negative (CN)). Typically these were PwP without a history of severe motor fluctuations or dyskinesia or if these motor fluctuations or dyskinesia had been resolved by optimization of oral therapies.
2.1. The PKG System
The Algorithms
2.2. Glossary of PKG Terms
- Median BKS. The median BKS was the 50th percentile of the BKS for all days that the PKG was worn (usually 6 days).
- Interquartile range of BKS. This was the interquartile range of the BKS of all epochs used to calculate the median BKS and was a measure of fluctuation of the BKS [29].
- Percent time in bradykinesia (PTB). Epochs whose BKS lie between 26.1 and 49.4 and whose 25th percentile of the BKS > 18.5 and the 90th percentile of BKS < 80. As well, any epoch whose BKS > 49.9 but contained tremor was included. These epochs were passed through a moving median filter and most of the epochs in a window of 30 min must be >26 for the center to be “off”.
- Median DKS: The median DKS was the 50th percentile for all the days that the PKG was worn [21]. Brisk walking introduced resonant peaks in the frequency of acceleration relevant to the DKS algorithm and thus artefactually increased the DKS. An algorithm was used to detect and remove epochs affected in this way.
- Interquartile range of DKS: This was the interquartile range of the DKS of all epochs used to calculate the median BKS and is a measure of fluctuation of the DKS [29].
- Percent Time in Dyskinesia (PTD): Those DKS used to estimate the median DKS were passed through a median filter (most of the epochs in the filer period must be in the dyskinetic range (DKS > 7) for the center to be classed as dyskinetic).
- The percent time with tremor (PTT): This was the percent of 2 min epochs estimated over all days that the PKG was worn that contained tremor [23].
- The percent time immobile (PTI): This was the percent of 2 min epochs with BKS > 80 from all days that the PKG was worn. These scores were associated with daytime sleep [22].
- Doses of levodopa/day. This was calculated from the number of reminders programmed into the logger.
3. Results
3.1. Development of DAT Classifier Scores
3.1.1. Clinical Sorting
3.1.2. Training and Re-Test Set Composition
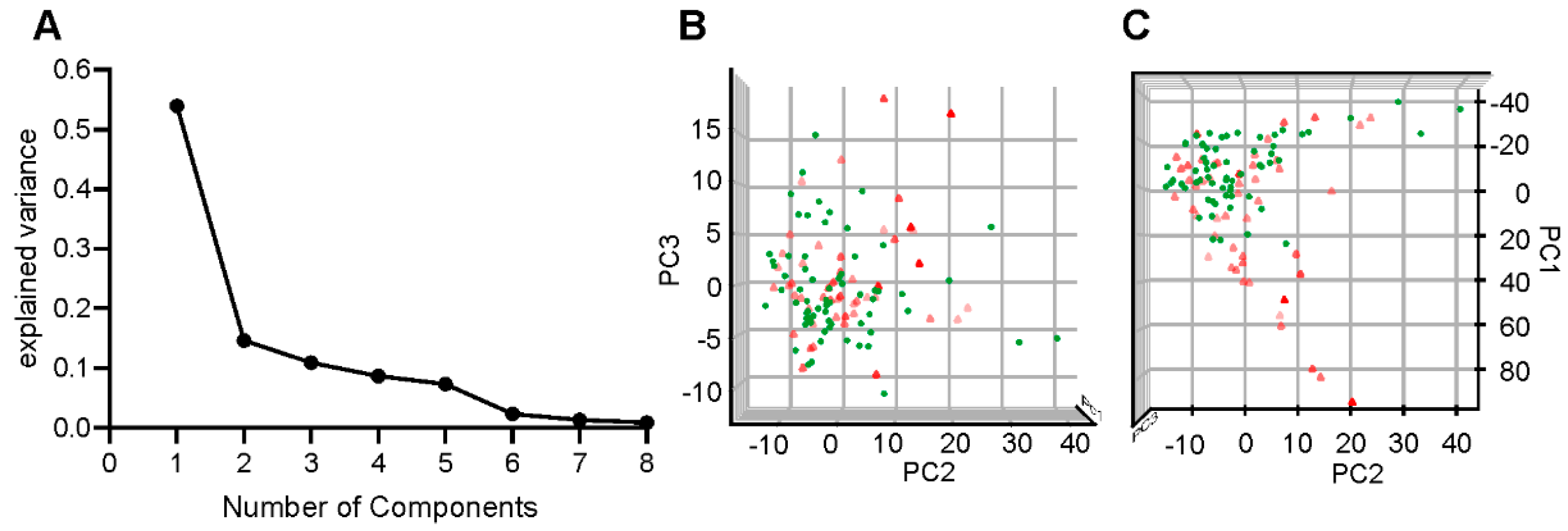
3.1.3. Parameter Selection
3.1.4. Model Building
3.1.5. Outputs of Statistical Model
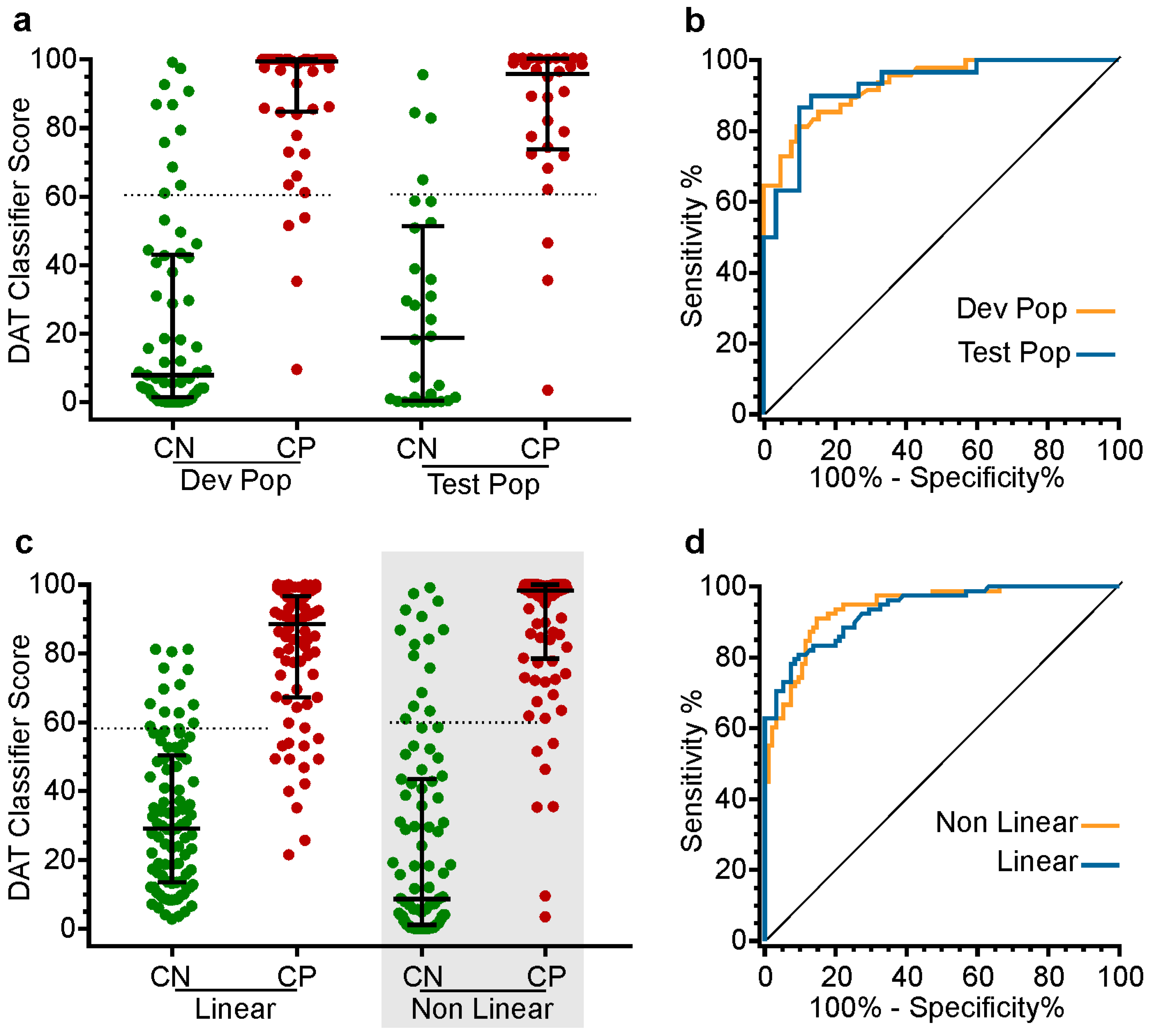
3.1.6. Outputs of the Classifiers
3.2. Performance of the DAT Classifier Scores on the Test Set
3.3. Clinical Performance of the DAT Classifier Score
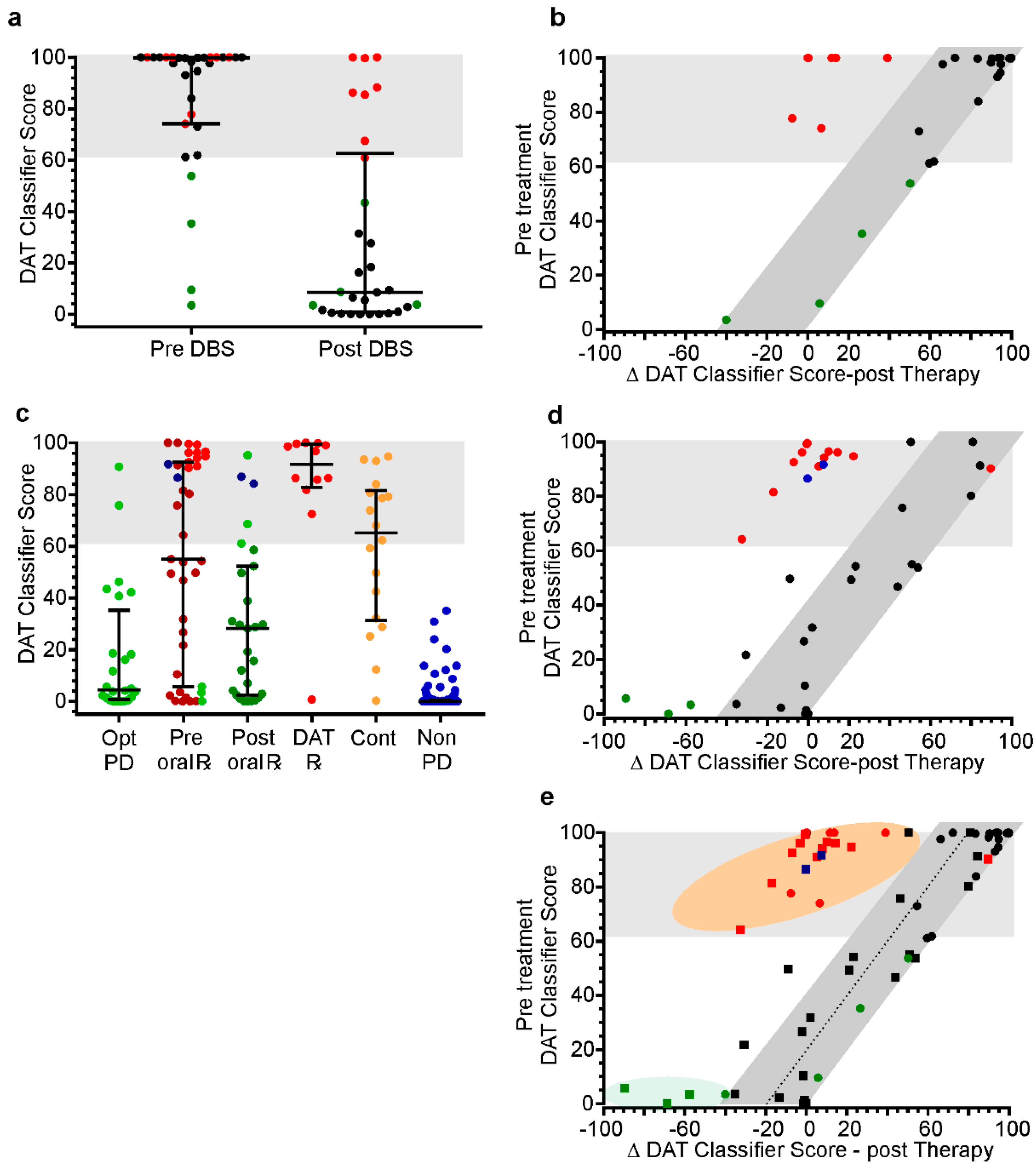
3.3.1. Testing the DAT Classifier Score on PwP Already Selected for DBS
3.3.2. Testing the Performance of the DAT Classifier Score in Identifying Potential DAT Candidates in Typical Clinic Population of PD
- Did the DAT classifier score identify all the subjects that the Movement Disorder specialist referred for DAT?
- How did the DAT classifier score change in those the Movement Disorder specialist regarded as requiring a change in oral therapies?
- It predicted those requiring DAT with high test sensitivity.
- When a change in therapy (oral or DBS) in PwP with a high DAT classifier score led to an improvement, the post-treatment score fell predictably. This is shown as the region of grey shading of Figure 3e. The better the control achieved by the change in therapy, the greater the reduction in the score along this grey zone.
- When a change of therapy (oral or DBS) in PwP with a high DAT classifier score failed to result in optimization, the score did not fall and appeared in the pink region in Figure 3e.
- When a change in therapy addressed undertreatment but uncovered fluctuations and or dyskinesia the DAT score lay in the green shaded area (in Figure 3). If the change resulted in a high DAT score, it usually revealed a need for DAT.
- Further work is required to better understand artefacts in input data (from the PKG).
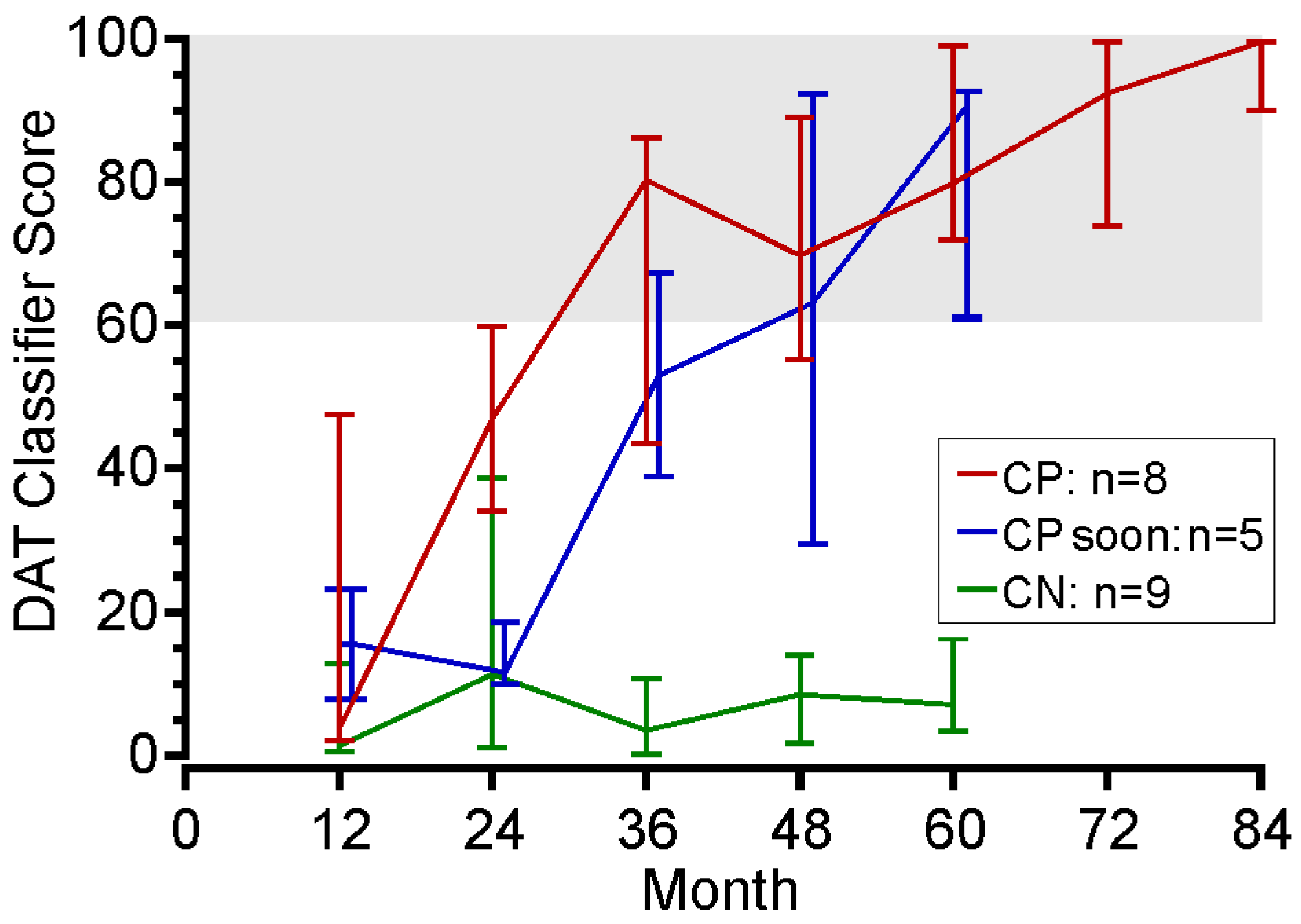
3.3.3. Testing the DAT Classifier Score on PwP Over an Extended Follow-Up from Diagnosis
4. Discussion
- The information provided by six days of accelerometry recordings from the wrist provides sufficient information to build a classifier that distinguished with high sensitivity and specificity between those subjects that did or did not meet the first clinical criterion for DAT, according to classification by specialist clinicians.
- The DAT classifier score correctly assigned subjects to DBS in cases preselected for surgery in 87% cases and the remaining miss-assigned cases may well have provoked discussion amongst specialist about whether they were indeed suitable or not.
- Cases where excessive periods of bradykinesia or dyskinesia could not be corrected by oral therapy were recognized as subjects whose DAT classifier scores remained high following changes in oral therapy directed at optimizing treatment. Thus a clinician using the DAT classifier scores score would have correctly identified these subjects as eligible for DAT.
- Serially following the change in DAT classifier scores of newly diagnosed PwP correctly identified subjects as they developed the clinical criteria for DAT.
- The change in DAT classifier scores following therapeutic intervention has led us to propose that if the change in the score does not follow a standard response then it indicates either the need for DAT or failure in its administration.
4.1. How Can Accelerometry Data Recording from One Wrist Provide Enough Information for Identifying Subjects Who Might Benefit from DAT
4.2. The Performance of the Classifier
4.3. Does the DAT Classifier Score Sort According to Its Purpose
5. Conclusions
- The information from the PKG could be used to build a classifier that identified with high sensitivity and specificity, PwP who specialist clinicians had identified as meeting the criteria for DAT from those that did not. Thus, the DAT classifier score was successful in identifying PwP who met the first criterion for DAT suitability: Having excessive periods of bradykinesia and/or dyskinesia achieves.
- The DAT classifier score correctly assigned subjects to DBS in 87% of cases who had already been preselected for surgery. The remaining miss-assigned cases may not have been considered by all specialists as suitable cases. This is in keeping with the current discussion in the movement disorder specialty around how early in the disease DBS is a suitable therapy.
- Cases where excessive periods of bradykinesia or dyskinesia could not be corrected by oral therapy were PwP who met the second criterion for DAT: That is, excessive periods of bradykinesia and/or dyskinesia could not be reduced by manipulating oral therapies. The DAT classifier met this second criterion because the scores remained high despite efforts in using oral therapy to optimize treatment. Thus, a clinician using the DAT classifier scores score would have correctly identified these subjects as eligible for DAT.
- Using an effective DAT classifier score to measure PwP from diagnosis to the onset of excessive periods of bradykinesia or dyskinesia that could not be corrected by oral therapy, which should see a commensurate change in the DAT classifier score as a movement disorder specialist was more likely to consider introducing DAT. The DAT classifier performed well under these circumstances.
- The change in DAT classifier scores following a therapeutic intervention has led us to propose that that there is a predictable change in the score if the intervention is successful. Figure 3e might suggest that the response could be Δ DAT classifier scores (before-after intervention) = DAT classifier score (before intervention) − 20. A response that does not follow this pattern indicates either the need for DAT or failure in therapeutic administration.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Fahn, S.; Oakes, D.; Shoulson, I.; Kieburtz, K.; Rudolph, A.; Lang, A.; Olanow, C.W.; Tanner, C.; Marek, K. Levodopa and the progression of parkinson’s disease. N. Engl. J. Med. 2004, 351, 2498–2508. [Google Scholar]
- Cilia, R.; Akpalu, A.; Sarfo, F.S.; Cham, M.; Amboni, M.; Cereda, E.; Fabbri, M.; Adjei, P.; Akassi, J.; Bonetti, A.; et al. The modern pre-levodopa era of parkinson’s disease: Insights into motor complications from sub-Saharan Africa. Brain J. Neurol. 2014, 137, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; Allert, N.; Eleopra, R.; Houeto, J.L.; Phan, T.M.; Stoevelaar, H.; International Study Group on Referral Criteria for DBS. A decision tool to support appropriate referral for deep brain stimulation in parkinson’s disease. J. Neurol. 2009, 256, 83–88. [Google Scholar] [CrossRef]
- Lim, S.Y.; O’Sullivan, S.S.; Kotschet, K.; Gallagher, D.A.; Lacey, C.; Lawrence, A.D.; Lees, A.L.; O’Sullivan, D.J.; Peppard, R.F.; Rodrigues, J.P.; et al. Dopamine Dysregulation Syndrome, Impulse Control Disorders and Punding after Deep Brain Stimulation Surgery for Parkinson’s Disease. J. Clin. Neurosci. 2019, 16, 1148–1152. [Google Scholar] [CrossRef]
- Katz, M.; Kilbane, C.; Rosengard, J.; Alterman, R.L.; Tagliati, M. Referring patients for deep brain stimulation: An improving practice. Arch. Neurol. 2011, 68, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.S.; Foote, K.D. Parkinson’s disease dbs: What, when, who and why? The time has come to tailor dbs targets. Expert Rev. Neurother. 2010, 10, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, W.M.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Halbig, T.D.; Hesekamp, H.; Navarro, S.M.; Meier, N.; et al. Neurostimulation for parkinson’s disease with early motor complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef]
- Okun, M.S.; Fernandez, H.H.; Pedraza, O.; Misra, M.; Lyons, K.E.; Pahwa, R.; Tarsy, D.; Scollins, L.; Corapi, K.; Friehs, G.M.; et al. Development and initial validation of a screening tool for parkinson disease surgical candidates. Neurology 2004, 63, 161–163. [Google Scholar] [CrossRef]
- Willis, A.W.; Schootman, M.; Kung, N.; Wang, X.Y.; Perlmutter, J.S.; Racette, B.A. Disparities in deep brain stimulation surgery among insured elders with parkinson disease. Neurology 2014, 82, 163–171. [Google Scholar] [CrossRef]
- Jenner, P. Wearing off, dyskinesia, and the use of continuous drug delivery in parkinson’s disease. Neurol. Clin. 2013, 31, S17–S35. [Google Scholar] [CrossRef][Green Version]
- Stacy, M.; Hauser, R.; Oertel, W.; Schapira, A.; Sethi, K.; Stocchi, F.; Tolosa, E. End-of-dose wearing off in parkinson disease: A 9-question survey assessment. Clin. Neuropharmacol. 2006, 29, 312–321. [Google Scholar] [CrossRef]
- Stocchi, F.; Antonini, A.; Barone, P.; Tinazzi, M.; Zappia, M.; Onofrj, M.; Ruggieri, S.; Morgante, L.; Bonuccelli, U.; Lopiano, L.; et al. Early detection of wearing off in parkinson disease: The deep study. Parkinsonism Relat. Disord. 2014, 20, 204–211. [Google Scholar] [CrossRef]
- Farzanehfar, P.; Braybrook, M.; Kotschet, K.; Horne, M. Objective measurement in clinical care of patients with parkinson’s disease: An rct using the pkg. In Proceedings of the 21st International Congress, Vancouver, BC, Canada, 4–8 June 2017. [Google Scholar]
- Odin, P.; Ray Chaudhuri, K.; Slevin, J.T.; Volkmann, J.; Dietrichs, E.; Martinez-Martin, P.; Krauss, J.K.; Henriksen, T.; Katzenschlager, R.; Antonini, A.; et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in parkinson’s disease: Consensus from an international survey and discussion program. Parkinsonism Relat. Disord. 2015, 21, 1133–1144. [Google Scholar] [CrossRef]
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative wearable sensors for objective assessment of parkinson’s disease. Mov. Disord. 2013, 28, 1628–1637. [Google Scholar] [CrossRef]
- Maetzler, W.; Klucken, J.; Horne, M. A clinical view on the development of technology-based tools in managing parkinson’s disease. Mov. Disord. 2016, 31, 1263–1271. [Google Scholar] [CrossRef]
- Horne, M.; Volkmann, J.; Sannelli, S.; Luyet, P.-P.; Moro, E. An evaluation of the parkinson’s kinetigraph (pkg) as a tool to support deep brain stimulation eligibility assessment in patients with parkinson’s disease. Mov. Disord. 2017, 32 (Suppl. 2). [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (mds-updrs): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Kotschet, K.; Arfon, S.; Xu, Z.M.; Johnson, W.; Drago, J.; Evans, A.; Kempster, P.; Raghav, S.; Horne, M.K. Automated assessment of bradykinesia and dyskinesia in parkinson’s disease. J. Parkinson. Dis. 2012, 2, 47–55. [Google Scholar]
- Kotschet, K.; Johnson, W.; McGregor, S.; Kettlewell, J.; Kyoong, A.; O’Driscoll, D.M.; Turton, A.R.; Griffiths, R.I.; Horne, M.K. Daytime sleep in parkinson’s disease measured by episodes of immobility. Parkinsonism Relat. Disord. 2014, 20, 578–583. [Google Scholar] [CrossRef]
- Braybrook, M.; O’Connor, S.; Churchward, P.; Perera, T.; Farzanehfar, P.; Horne, M. An ambulatory tremor score for parkinson’s disease. J. Parkinsons Dis. 2016, 6, 723–731. [Google Scholar] [CrossRef]
- Odin, P.; Chaudhuri, K.R.; Volkmann, J.; Antonini, A.; Storch, A.; Dietrichs, E.; Pirtosek, Z.; Henriksen, T.; Horne, M.; Devos, D.; et al. Viewpoint and practical recommendations from a movement disorder specialist panel on objective measurement in the clinical management of parkinson’s disease. Npj Parkinsons Dis. 2018, 4, 14. [Google Scholar] [CrossRef]
- McGregor, S.; Churchward, P.; Soja, K.; O’Driscoll, D.; Braybrook, M.; Khodakarami, H.; Evans, A.; Farzanehfar, P.; Hamilton, G.; Horne, M. The use of accelerometry as a tool to measure disturbed nocturnal sleep in parkinson’s disease. Npj Parkinsons Dis. 2018, 4, 1. [Google Scholar] [CrossRef]
- Farzanehfar, P.; Woodrow, H.; Braybrook, M.; McGregor, S.; Evans, A.; Nicklason, F.; Horne, M. Objective measurement in routine care of people with parkinson’s disease improves outcomes. Npj Parkinsons Dis. 2018, 4, 10. [Google Scholar] [CrossRef]
- Farzanehfar, P.; Horne, M. Evaluation of the parkinson’s kinetigraph in monitoring and managing parkinson’s disease. Expert Rev. Med. Devices 2017, 14, 583–591. [Google Scholar] [CrossRef]
- Horne, M.; Kotschet, K.; McGregor, S. The clinical validation of objective measurement of movement in parkinson’s disease. CNS 2016, 1, 15–22. [Google Scholar]
- Horne, M.K.; McGregor, S.; Bergquist, F. An objective fluctuation score for parkinson’s disease. PLoS ONE 2015, 10, e0124522. [Google Scholar] [CrossRef]
- Bergquist, F.; Horne, M. Can objective measurements improve treatment outcomes in parkinson’s disease? Eur. Neurol. Rev. 2014, 9, 27–30. [Google Scholar] [CrossRef]
- Bennasar, M.; Hicks, Y.; Setchi, R. Feature selection using joint mutual information maximisation. Expert Syst. Appl. 2015, 42, 8520–8532. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. Resampling methods. In An introduction to Statistical Learning; James, G., Witten, D., Hastie, T., Tibshirani, R., Eds.; Springer: New York, NY, USA, 2013; pp. 175–201. [Google Scholar]
- Cristianini, N.; Shawe-Taylor, N. An Introduction to Support Vector Machines and Other Kernel-Based Learning Methods; Cambridge University Press: New York, NY, USA, 2000. [Google Scholar]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Ng, A.Y. L1 vs. L2 regularization, and rotational invariance. In Proceedings of the Twenty-First International Conference on Machine Learning (ICML ’04), Banff, AB, Canada, 4–8 July 2004; p. 78. [Google Scholar]
- Florkowski, C.M. Sensitivity, specificity, receiver-operating characteristic (roc) curves and likelihood ratios: Communicating the performance of diagnostic tests. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S83–S87. [Google Scholar]
| N Total = 172 | CP | CN | Δ | p Value |
|---|---|---|---|---|
| Male | 66% | 70% | ||
| Female | 34% | 30% | ||
| Age | 62 (56–67) | 71 (66–75) | 9 | 0.13 |
| UPDRS I | 6 (3–10.5) | 8 (5–13) | −2 | 0.02 |
| UPDRS II | 13 (10–18) | 7 (4–12) | 6 | 0.0001 |
| UPDRS III | 27 (19–37) | 25 (18–35) | 2 | 0.3 |
| UPDRS IV | 7 (4–9) | 1 (0–4) | 6 | 0.0001 |
| UPDRS Total | 54 (46–72) | 43 (32–59) | 11 | 0.001 |
| Median BKS | 20.8 (16.4–25.4) | 24 (21.7–27) | −3.2 | 0.0001 |
| PTB | 37.5 (19.1–55.9) | 47.7 (35–65.1) | 10.2% (1.6 h) | 0.0004 |
| Median DKS | 5.1 (2.4–13.5) | 2 (0.9–3.8) | 3.1 | 0.0001 |
| PTD | 23.9 (10.2–46.7) | 7.3 (2.9–13.3) | 16.6% (2.7 h) | 0.0001 |
| DBSS | 0.96 (0.82–0.99) | 0.16 (0.02–0.5) | 0.8 | 0.0001 |
| doses/day | 5 (5–6) | 4 (3–4) | 1 | 0.0001 |
| PTT | 0.8 (0.3–2.1) | 0.8 (0.4–2) | 0 | 0.6 |
| PTI | 4.1 (1.5–.8) | 4.8 (2.8–8.9) | 0.7 | 0.02 |
| PKG Variable | Joint Mutual Information | Clinical Information Represented by the PKG Data |
|---|---|---|
| Doses of l-dopa | 0.25 | Dose of levodopa/day |
| DKS 75 | 0.11 | Severity of dyskinesia |
| BKS 25 | 0.08 | Severity of bradykinesia |
| BKS 75 | 0.08 | Fluctuation in bradykinesia |
| PT in DK | 0.06 | Time in “troublesome” dyskinesia |
| PTI | 0.05 | Time asleep during the day |
| PTT | 0.04 | Time with tremor |
| PTO | 0.03 | Time “off” |
| Construction Set | Test Set | Full Data Set | ||||
|---|---|---|---|---|---|---|
| Linear | Non-Linear | Linear | Non-Linear | Linear | Non-Linear | |
| Area | 0.92 | 0.94 | 0.93 | 0.93 | 0.93 | 0.93 |
| Optimum score | 58.3 | 61.1 | 58.3 | 61.1 | 58.3 | 61.1 |
| Sensitivity | 85.5 | 91.7 | 80 | 89 | 80 | 89 |
| Specificity | 84.6 | 84.6 | 88.5 | 86.6 | 88.5 | 86.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodakarami, H.; Farzanehfar, P.; Horne, M. The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies. Sensors 2019, 19, 2241. https://doi.org/10.3390/s19102241
Khodakarami H, Farzanehfar P, Horne M. The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies. Sensors. 2019; 19(10):2241. https://doi.org/10.3390/s19102241
Chicago/Turabian StyleKhodakarami, Hamid, Parisa Farzanehfar, and Malcolm Horne. 2019. "The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies" Sensors 19, no. 10: 2241. https://doi.org/10.3390/s19102241
APA StyleKhodakarami, H., Farzanehfar, P., & Horne, M. (2019). The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies. Sensors, 19(10), 2241. https://doi.org/10.3390/s19102241




