A Fluorescence Sensing Determination of 2,4,6-Trinitrophenol Based on Cationic Water-Soluble Pillar[6]arene Graphene Nanocomposite
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Apparatus and Instruments
2.3. Synthesis of the PCP6-rGO Hybrid Nanomaterial
2.4. Fluorescent Experiments
3. Results and Discussion
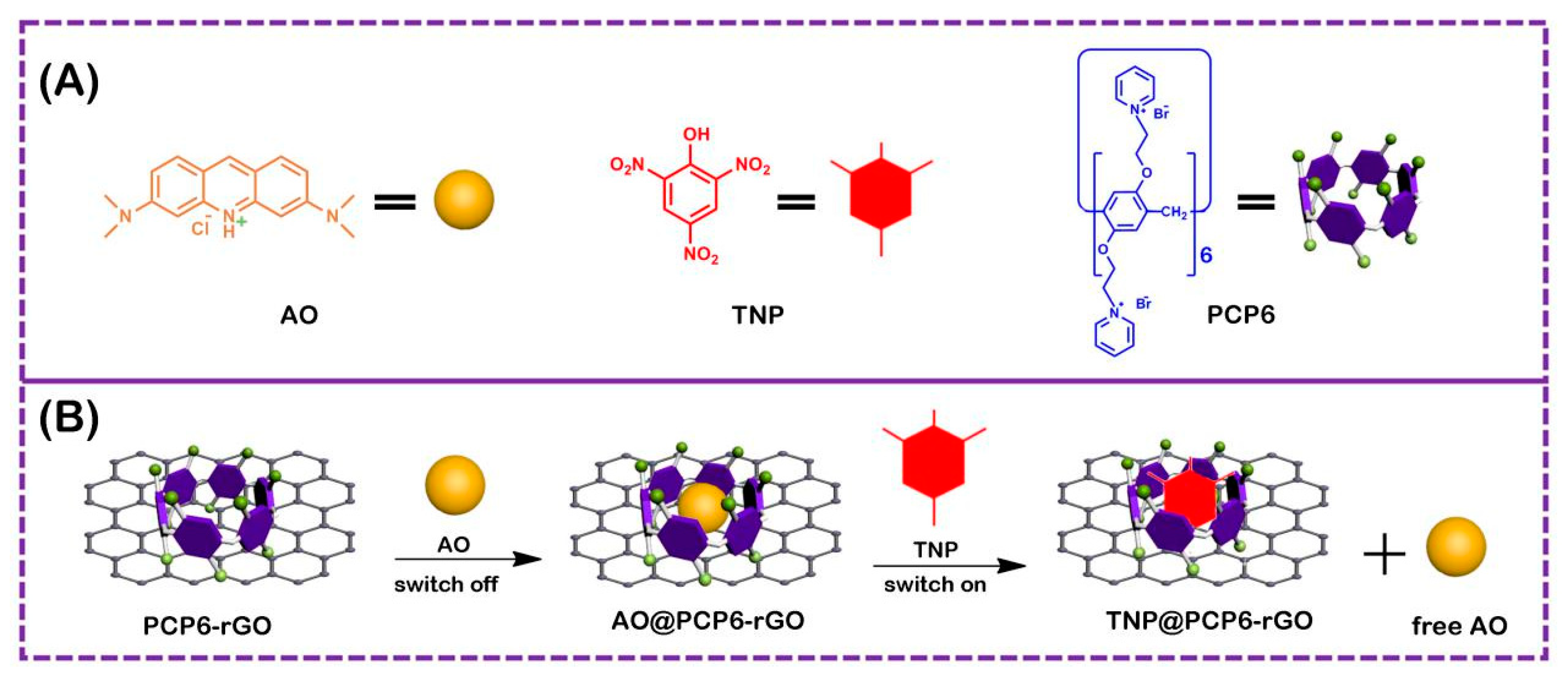
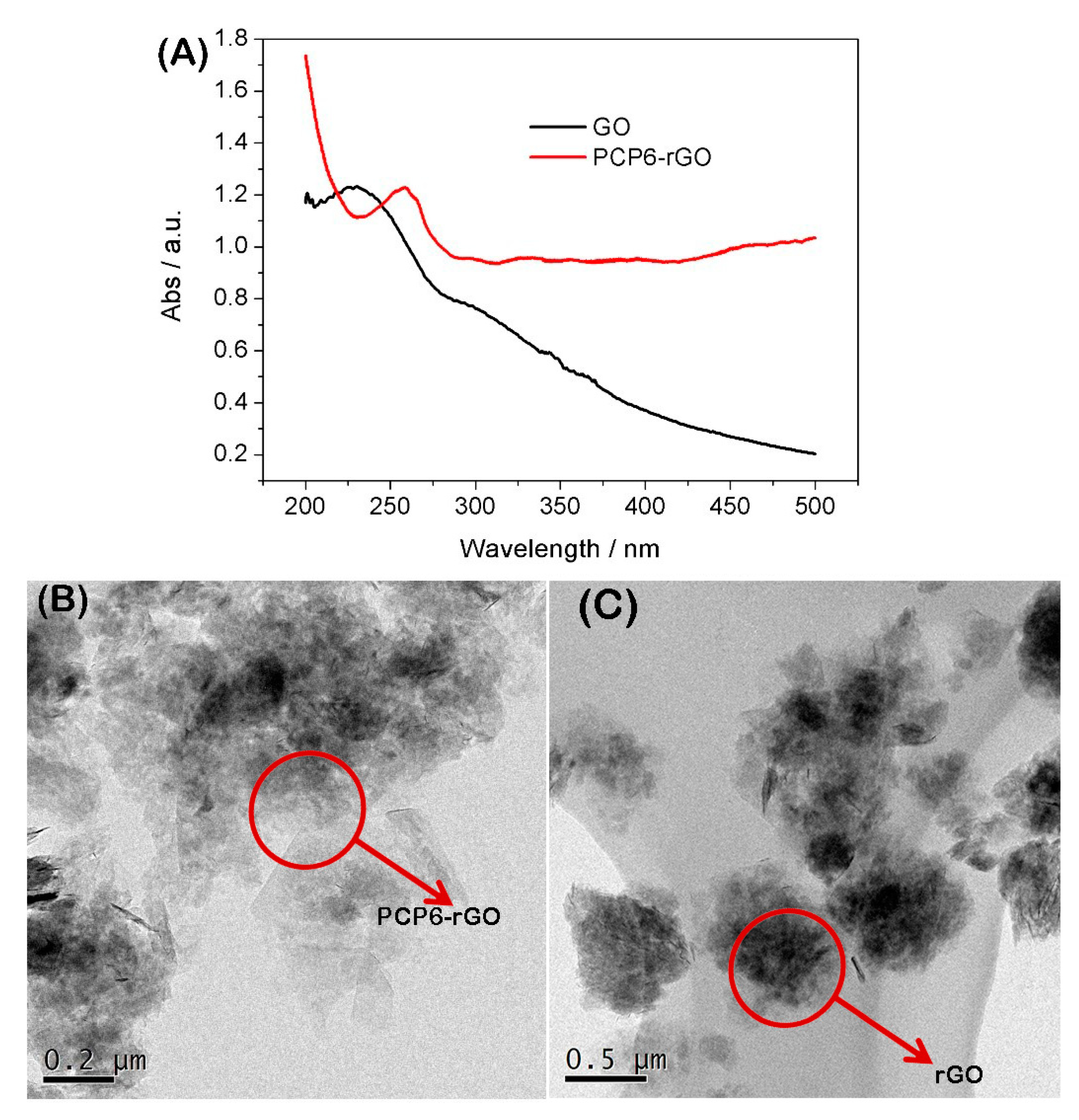
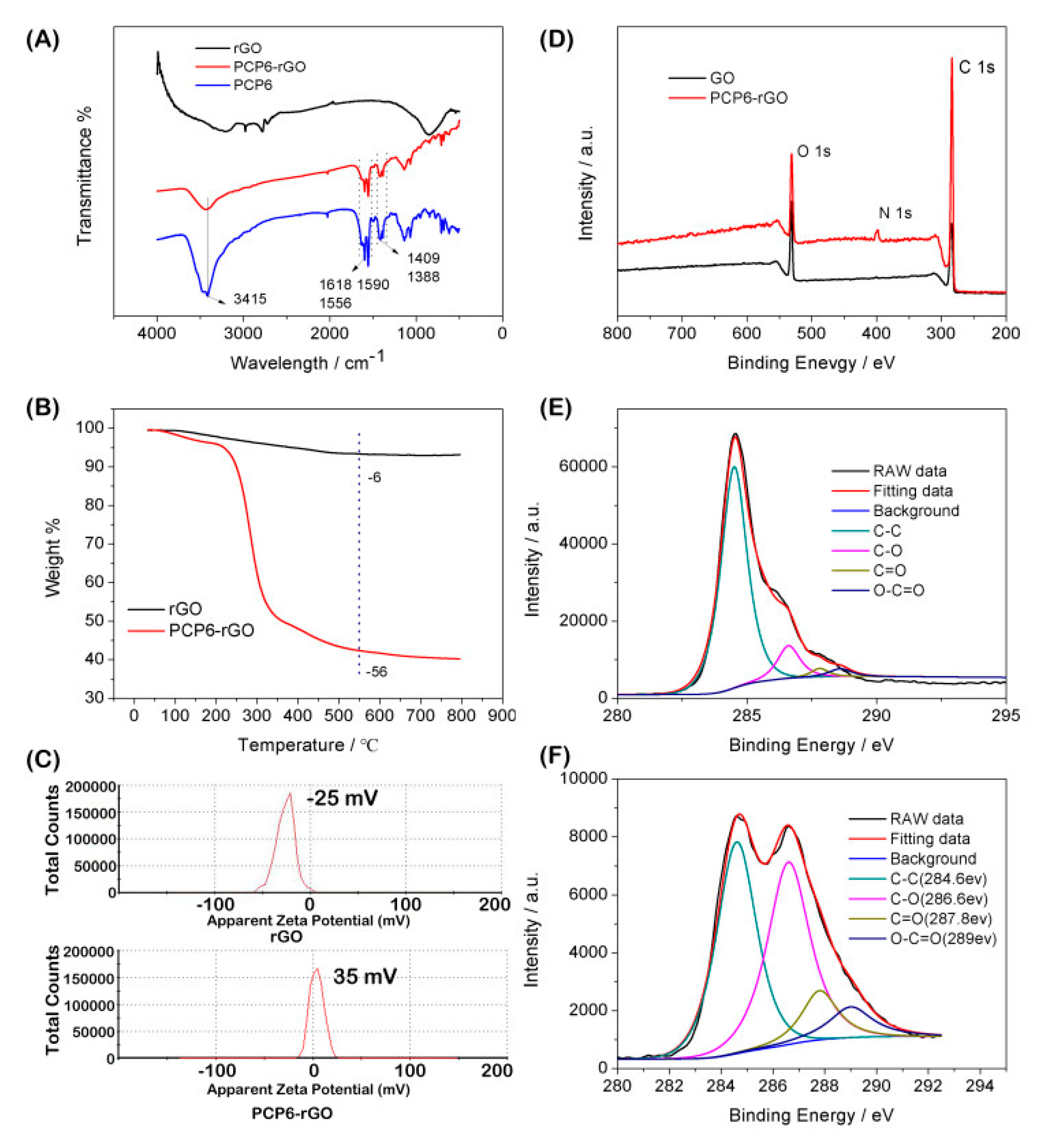
3.1. Characterization of the PCP6-rGO Hybrid Nanomaterial
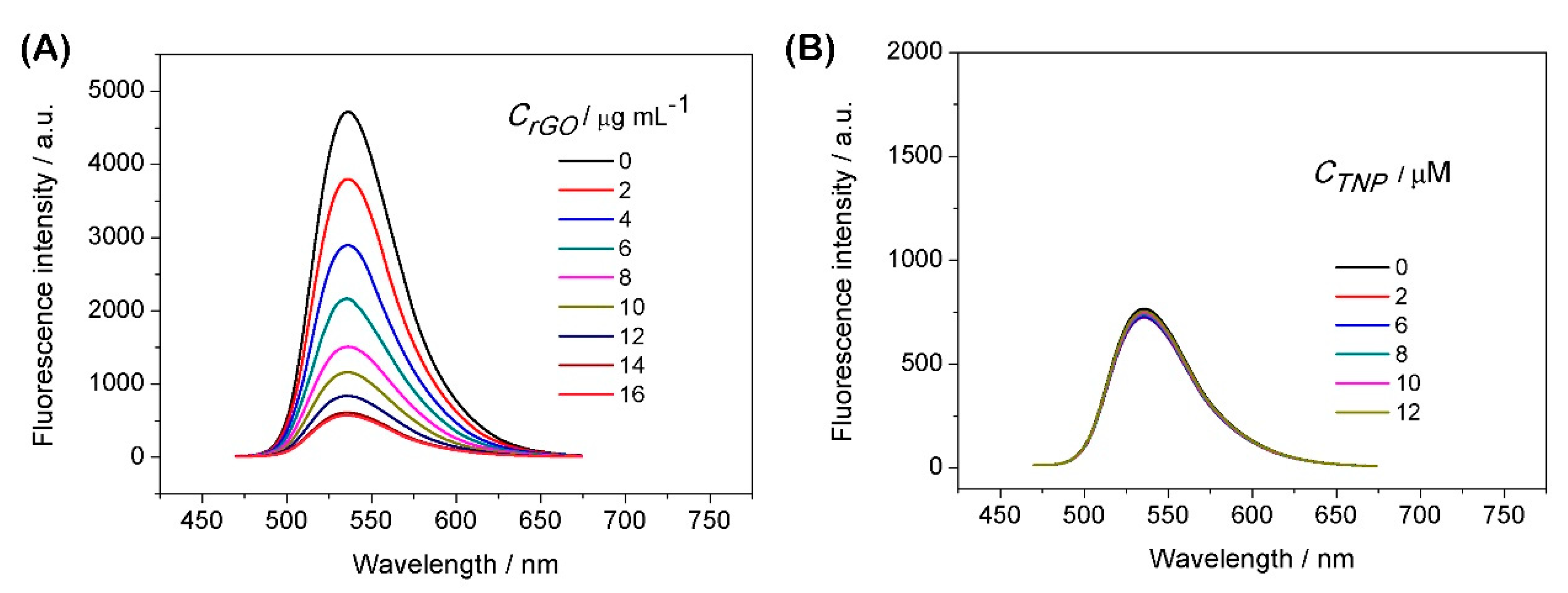
3.2. Fluorescence Spectra Analysis
3.3. The Analysis of Host–Guest Recognition
3.4. Selectivity and Practical Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salinas, Y.; Martinez-Manez, R.; Marcos, M.D.; Sancenon, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar]
- Ma, Y.; Huang, S.; Deng, M.; Wang, L. White upconversion luminescence nanocrystals for the simultaneous and selective detection of 2,4,6-trinitrotoluene and 2,4,6-trinitrophenol. ACS Appl. Mater. Interfaces 2014, 6, 7790–7796. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Feng, J.; Liu, J.; Ma, S.; Chen, X. One-pot synthesis of fluorescent silicon nanoparticles for sensitive and selective determination of 2,4,6-trinitrophenol in aqueous solution. Anal. Chem. 2017, 89, 3001–3008. [Google Scholar] [CrossRef]
- Guerra-Diaz, P.; Gura, S.; Almirall, J.R. Dynamic planar solid phase microextraction–ion mobility spectrometry for rapid field air sampling and analysis of illicit drugs and explosives. Anal. Chem. 2010, 82, 2826–2835. [Google Scholar] [CrossRef]
- Uzer, A.; Ercag, E.; Apak, R. Selective spectrophotometric determination of trinitrotoluene, trinitrophenol, dinitrophenol and mononitrophenol. Anal. Chim. Acta 2004, 505, 83–93. [Google Scholar] [CrossRef]
- Luggar, R.; Farquharson, M.; Horrocks, J.; Lacey, R. Multivariate analysis of statistically poor EDXRD spectra for the detection of concealed explosives. X-ray Spectrom. 1998, 27, 87–94. [Google Scholar] [CrossRef]
- Tesarova, E.; Sykora, D.; Voznakova, Z. GC and HPLC determination of nitrophenol related pesticides. Fresenius Environ. Bull. 1995, 4, 609–616. [Google Scholar]
- Zhang, H.Y.; Wang, M.; Zhao, J.Y.; Shi, Z.H. Sandwich-type spontaneous injection of nitrophenols for capillary electrophoresis analysis. Anal. Methods 2012, 4, 2177–2182. [Google Scholar]
- Yamauchi, Y.; Ido, M.; Ohta, M.; Maeda, H. High performance liquid chromatography with an electrochemical detector in the cathodic mode as a tool for the determination of p-nitrophenol and assay of acid phosphatase in urine samples. Chem. Pharm. Bull. 2004, 52, 552–555. [Google Scholar]
- Yan, F.; He, Y.Y.; Ding, L.H.; Su, B. Highly ordered binary assembly of silica mesochannels and surfactant micelles for extraction and electrochemical analysis of trace nitroaromatic explosives and pesticides. Anal. Chem. 2015, 87, 4436–4441. [Google Scholar] [CrossRef]
- Senol, A.M.A.; Onganer, Y.; Meral, K. An unusual “off-on” fluorescence sensor for iron(III) detection basedon fluorescein–reduced graphene oxide functionalized withpolyethyleneimine. Sens. Actuators B 2017, 239, 343–351. [Google Scholar]
- Xu, G.X.; Zeng, S.W.; Zhang, B.T.; Swihart, M.T.; Yong, K.T.; Prasad, P.N. New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine. Chem. Rev. 2016, 115, 12234–12327. [Google Scholar]
- Rong, M.C.; Lin, L.P.; Song, X.H.; Zhao, T.T.; Zhong, Y.X.; Yan, J.W.; Wang, Y.R.; Chen, X. A label-free fluorescence sensing approach for selective and sensitive detection of 2,4,6-trinitrophenol (TNP) in aqueous solution using graphitic carbon nitride nanosheets. Anal. Chem. 2015, 87, 1288–1296. [Google Scholar]
- Ma, H.W.; Li, F.; Yao, L.; Feng, Y.T.; Zhang, Z.X.; Zhang, M. Dual-emissive electropolymerization films for the ratiometric fluorescence detection of TNT and TNP with high sensitivity and selectivity. Sens. Actuators B 2018, 259, 380–386. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Li, F.F.; Cui, W.R.; Liang, R.P.; Qiu, J.D. Facile and green approach to the synthesis of boron nitride quantum dots for 2,4,6-trinitrophenol sensing. ACS Appl. Mater. Interfaces 2018, 10, 7315–7323. [Google Scholar] [CrossRef]
- Cao, L.H.; Shi, F.; Zhang, W.M.; Zang, S.Q.; Mak, T.C.W. Selective sensing of Fe3+ and Al3+ ions and detection of 2,4,6-trinitrophenol by a water-stable terbium-based metal-organic framework. Chem. Eur. J. 2015, 21, 15705–15712. [Google Scholar] [CrossRef]
- Haldar, D.; Dinda, D.; Saha, S.K. High selectivity in water soluble MoS2 quantum dots for sensing nitro explosives. J. Mater. Chem. C 2016, 4, 6321–6326. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.N. Molybdenum disulfide quantum dots as a photoluminescence sensing platform for 2,4,6-trinitrophenol detection. Anal. Chem. 2014, 86, 7463–7470. [Google Scholar]
- Sang, N.N.; Zhan, C.X.; Cao, D.P. Highly sensitive and selective detection of 2,4,6-trinitrophenol using covalent-organic polymer luminescent probes. J. Mater. Chem. A 2015, 3, 92–96. [Google Scholar]
- Dong, S.J.; Hu, J.S.; Wu, K.; Zheng, M.D. A Mg(II)-MOF as recyclable luminescent sensor for detecting TNP with high selectivity and sensitivity. Inorg. Chem. Commun. 2018, 95, 111–116. [Google Scholar]
- Jigyasa, J.K.R. “ON-OFF” novel fluorescent chemosensors based on nanoaggregates of triaryl imidazoles for superselective detection of nitro-explosive trinitrophenol in multiple solvent systems. Sens. Actuators B Chem. 2018, 259, 990–1005. [Google Scholar]
- Ma, X.; Zhao, Y. Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar]
- Cragg, P.J.; Sharma, K. Pillar[5]arenes: Fascinating cyclophanes with a bright future. Chem. Soc. Rev. 2012, 41, 597–607. [Google Scholar]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.; Nakamoto, Y. para-bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar]
- Ogoshi, T.; Yamagishi, T.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef]
- Shao, L.; Sun, J.F.; Hua, B.; Huang, F.H. An AIEE fluorescent supramolecular cross-linked polymer network based on pillar[5]arene host–guest recognition: Construction and application in explosive detection. Chem. Commun. 2018, 54, 4866–4869. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: Synthesis and host–guest inclusion for enhanced electrochemical performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Mao, X.W.; Liu, T.; Bi, J.H.; Luo, L.; Tian, D.M.; Li, H.B. The synthesis of pillar[5]arene functionalized graphene as a fluorescent probe for paraquat in living cells and mice. Chem. Commun. 2016, 52, 4385–4388. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar]
- Yu, G.C.; Zhou, J.; Shen, J.; Tang, G.P.; Huang, F.H. Cationic pillar[6]arene/ATP host–guest recognition: Selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem. Sci. 2016, 7, 4073–4078. [Google Scholar]
- Wu, J.R.; Mu, A.; Li, B.; Wang, C.Y.; Fang, L.; Yang, Y.W. Desymmetrized Leaning Pillar[6]arene. Angew. Chem. 2018, 130, 1–7. [Google Scholar]
- Zhao, G.F.; Yang, L.; Wu, S.L.; Zhao, H.; Tang, E.; Li, C.P. The synthesis of amphiphilic pillar[5]arene functionalized reduced graphene oxide and its application as novel fluorescence sensing platform for the determination of acetaminophen. Biosens. Bioelectron. 2017, 91, 863–869. [Google Scholar] [CrossRef]
- Mao, X.W.; Tian, D.M.; Li, H.B. p-Sulfonated calix[6]arene modified graphene as a ‘turn on’ fluorescent probe for L-carnitine in living cells. Chem. Commun. 2012, 48, 4851–4853. [Google Scholar]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.Y.; Wang, H.L.; Casalongue, H.S.; Vinh, D.; Dai, H.J. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar]
- Yang, L.; Zhao, H.; Li, Y.C.; Ran, X.; Deng, G.G.; Zhang, Y.Q.; Ye, H.Z.; Zhao, G.F.; Li, C.P. Indicator displacement assay for cholesterol electrochemical sensing using a calix[6]arene functionalized graphene-modified electrode. Analyst 2016, 141, 270–278. [Google Scholar]
- Fu, G.; Tao, L.; Zhang, M.; Chen, Y.; Tang, Y.; Lin, J.; Lu, T. One-pot, water-based and high-yield synthesis of tetrahedral palladium nanocrystal decorated grapheme. Nanoscale 2013, 5, 8007–8014. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, L.; Li, Y.C.; Ran, X.; Ye, H.Z.; Zhao, G.F.; Zhang, Y.Q.; Li, F.; Li, C.P. A comparison study of macrocyclic hosts functionalized reduced graphene oxide for electrochemical recognition of tadalafil. Biosens. Bioelectron. 2017, 89, 361–369. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, Z.H.; Sun, J.F.; Yang, J. Pillar[6]arene/acridine orange host–guest complexes as colorimetric and fluorescence sensors for choline compounds and further application in monitoring enzymatic reactions. Sens. Actuators B 2018, 255, 1430–1435. [Google Scholar] [CrossRef]

| Sample | Added (μM) | Found (µM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Tap water | 0 | 0 | - | - |
| 2 | 1.95 ± 0.06 | 3.1 | 97.5 | |
| 5 | 5.07 ± 0.15 | 2.9 | 101.4 | |
| 10 | 9.89 ± 0.32 | 3.2 | 98.9 | |
| Lake water | 0 | 0 | - | - |
| 2 | 1.97 ± 0.11 | 5.5 | 98.5 | |
| 5 | 4.97 ± 0.09 | 1.8 | 99.4 | |
| 10 | 10.09 ± 0.21 | 2.1 | 100.9 | |
| Soil | 0 | 0 | - | - |
| 2 | 2.06 ± 0.12 | 5.8 | 103 | |
| 5 | 4.97 ± 0.19 | 3.8 | 99.4 | |
| 10 | 9.95 ± 0.17 | 1.7 | 99.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Zhang, T.; Zeng, W.; He, S.; Liu, X.; Tian, H.; Shi, J.; Cao, T. A Fluorescence Sensing Determination of 2,4,6-Trinitrophenol Based on Cationic Water-Soluble Pillar[6]arene Graphene Nanocomposite. Sensors 2019, 19, 91. https://doi.org/10.3390/s19010091
Tan X, Zhang T, Zeng W, He S, Liu X, Tian H, Shi J, Cao T. A Fluorescence Sensing Determination of 2,4,6-Trinitrophenol Based on Cationic Water-Soluble Pillar[6]arene Graphene Nanocomposite. Sensors. 2019; 19(1):91. https://doi.org/10.3390/s19010091
Chicago/Turabian StyleTan, Xiaoping, Tingying Zhang, Wenjie Zeng, Shuhua He, Xi Liu, Hexiang Tian, Jianwei Shi, and Tuanwu Cao. 2019. "A Fluorescence Sensing Determination of 2,4,6-Trinitrophenol Based on Cationic Water-Soluble Pillar[6]arene Graphene Nanocomposite" Sensors 19, no. 1: 91. https://doi.org/10.3390/s19010091
APA StyleTan, X., Zhang, T., Zeng, W., He, S., Liu, X., Tian, H., Shi, J., & Cao, T. (2019). A Fluorescence Sensing Determination of 2,4,6-Trinitrophenol Based on Cationic Water-Soluble Pillar[6]arene Graphene Nanocomposite. Sensors, 19(1), 91. https://doi.org/10.3390/s19010091





