Extracellular Electrophysiology in the Prostate Cancer Cell Model PC-3
Abstract
1. Introduction
2. Materials and Methods
2.1. General Cellular Culturing Method
2.2. Standard Cellular Viability Assays in Water
2.3. MEA Experimental Details
3. Results and Discussion
3.1. Sensor, Cell Adhesion and Viability
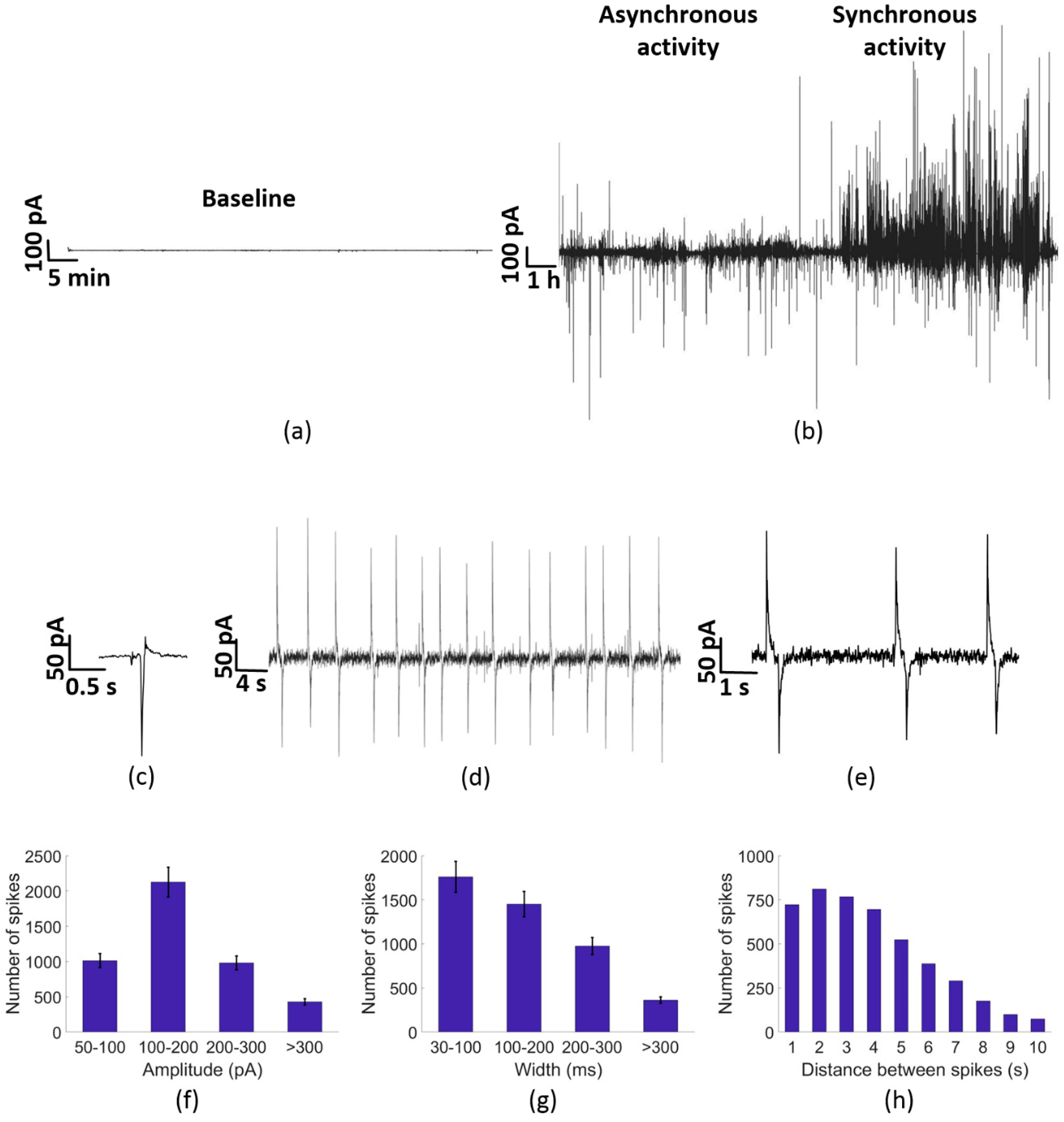
3.2. Electrical Activity of PC-3 Cells
3.3. Calcium Channel Inhibition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2015. Available online: https://seer.cancer.gov/csr/1975_2015/ (accessed on 18 April 2018).
- Weiner, A.B.; Matulewicz, R.S.; Eggener, S.E.; Schaeffer, E.M. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016, 19, 395. [Google Scholar] [CrossRef] [PubMed]
- Buzzoni, C.; Auvinen, A.; Roobol, M.J.; Carlsson, S.; Moss, S.M.; Puliti, D.; Lujan, M. Metastatic prostate cancer incidence and prostate-specific antigen testing: New insights from the European Randomized Study of Screening for Prostate Cancer. Eur. Urol. 2015, 68, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Office of National Statistic. Cancer Registration Statistics, England: 2018. 2016. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/final2016 (accessed on 18 April 2018).
- Dozmorov, M.G.; Hurst, R.E.; Culkin, D.J.; Kropp, B.P.; Frank, M.B.; Osban, J.; Lin, H.K. Unique patterns of molecular profiling between human prostate cancer LNCaP and PC-3 cells. Prostate 2009, 69, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Cell L. BC Cancer Agency. 2014 Nov 21; Cell L. 2001. Available online: http://capcelllines.ca (accessed on 18 April 2018).
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current mouse and cell models in prostate cancer research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Del Vecchio, V.; Palma, G.; Barbieri, A.; Falco, M.; Luciano, A.; de Biase, D.; Perdonà, S.; Facchini, G.; Arra, C. Mouse models in prostate cancer translational research: From xenograft to PDX. BioMed Res. Int. 2016, 2016, 9750795. [Google Scholar] [CrossRef]
- Pulukuri, S.M.; Gondi, C.S.; Lakka, S.S.; Jutla, A.; Estes, N.; Gujrati, M.; Rao, J.S. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J. Biol. Chem. 2005, 280, 36529–36540. [Google Scholar] [CrossRef]
- Harrison, I.P.; Selemidis, S. Understanding the biology of reactive oxygen species and their link to cancer: NADPH oxidases as novel pharmacological targets. Clin. Exp. Pharmacol. Physiol. 2014, 41, 533–542. [Google Scholar] [CrossRef]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef]
- Eimon, P.M.; Ghannad-Rezaie, M.; De Rienzo, G.; Allalou, A.; Wu, Y.; Gao, M.; Roy, A.; Skolnick, J.; Yanik, M.F. Brain activity patterns in high-throughput electrophysiology screen predict both drug efficacies and side effects. Nat. Commun. 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, N.; Maguy, A.; De Simone, S.A.; Miragoli, M.; Jousset, F.; Rohr, S. TGF-β1 (Transforming Growth Factor-β1) Plays a Pivotal Role in Cardiac Myofibroblast Arrhythmogenicity. Circulation 2017, 10, e004567. [Google Scholar] [PubMed]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Pardo, L.A. Ion channels: Functional expression and therapeutic potential in cancer: Colloquium on Ion Channels and Cancer. EMBO Rep. 2008, 9, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and tranporters in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Bidaux, G.; Flourakis, M.; Shuba, Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007, 14, 1295. [Google Scholar] [CrossRef]
- Arcangeli, A.; Becchetti, A. Novel perspectives in cancer therapy: Targeting ion channels. Drug Resist. Updates 2015, 21, 11–19. [Google Scholar] [CrossRef]
- Abdul, M.; Hoosein, N. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 2002, 186, 99–105. [Google Scholar] [CrossRef]
- Yildirim, S.; Altun, S.; Gumushan, H.; Patel, A.; Djamgoz, M.B. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 2012, 323, 58–61. [Google Scholar] [CrossRef]
- Zhang, L.; Barritt, G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004, 64, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Gkika, D.; Flourakis, M.; Lemonnier, L.; Prevarskaya, N. PSA reduces prostate cancer cell motility by stimulating TRPM8 activity and plasma membrane expression. Oncogene 2010, 29, 4611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yue, D.; Li, K.; Liu, Y.L.; Ren, C.S.; Wang, P. The role of TRPC6 in HGF-induced cell proliferation of human prostate cancer DU145 and PC-3 cells. Asian J. Androl. 2010, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, C.; Kilch, T.; Kappel, S.; Dörr, K.; Jung, V.; Stöckle, M.; Peinelt, C. Differential redox regulation of Ca2+ signaling and viability in normal and malignant prostate cells. Biophys. J. 2015, 109, 1410–1419. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Szatkowski, M.; Mycielska, M.; Knowles, R.; Kho, A.L.; Djamgoz, M.B.A. Electrophysiological recordings from the rat prostate gland in vitro: Identified single-cell and transepithelial (lumen) potentials. BJU Int. 2000, 86, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.R.; Schlett, P.; Schneider, L.; Dröge, M.; Mailänder, V.; Gomes, H.L.; Blom, P.W.M.; De Leeuw, D.M. Low frequency electric current noise in glioma cell populations. J. Mater. Chem. B 2015, 3, 5035–5039. [Google Scholar] [CrossRef]
- Rocha, P.R.; Schlett, P.; Kintzel, U.; Mailänder, V.; Vandamme, L.K.; Zeck, G.; Gomes, H.L.; Biscarini, F.; De Leeuw, D.M. Electrochemical noise and impedance of Au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci. Rep. 2016, 6, 34843. [Google Scholar] [CrossRef]
- Rocha, P.R.; Medeiros, M.C.; Kintzel, U.; Vogt, J.; Araújo, I.M.; Mestre, A.L.; Biscarini, F. Extracellular electrical recording of pH-triggered bursts in C6 glioma cell populations. Sci. Adv. 2016, 2, e1600516. [Google Scholar] [CrossRef]
- Thomas, C.A., Jr.; Springer, P.A.; Loeb, G.E.; Berwald-Netter, Y.; Okun, L.M. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Hoogerwerf, A.C.; Wise, K.D. A three-dimensional microelectrode array for chronic neural recording. IEEE Trans. Biomed. Eng. 1994, 41, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Nordhausen, C.T.; Maynard, E.M.; Normann, R.A. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 1996, 726, 129–140. [Google Scholar] [CrossRef]
- Berdondini, L.; Overstolz, T.; De Rooij, N.F.; Koudelka-Hep, M.; Wany, M.; Seitz, P. High-density microelectrode arrays for electrophysiological activity imaging of neuronal networks. In Proceedings of the ICECS 2001, 8th IEEE International Conference on Electronics, Circuits and Systems (Cat. No.01EX483), Malta, Malta, 2–5 September 2001; Volume 3, pp. 1239–1242. [Google Scholar]
- Wang, K.; Fishman, H.A.; Dai, H.; Harris, J.S. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett. 2006, 6, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Obien, M.E.J.; Deligkaris, K.; Bullmann, T.; Bakkum, D.J.; Frey, U. Revealing neuronal function through microelectrode array recordings. Front. Neurosci. 2015, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Eversmann, B.; Jenkner, M.; Hofmann, F.; Paulus, C.; Brederlow, R.; Holzapfl, B.; Gabl, R. A 128/spl times/128 CMOS biosensor array for extracellular recording of neural activity. IEEE J. Solid-State Circuits 2003, 38, 2306–2317. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Mestre, A.; Inácio, P.; Asgarif, S.; Araújo, I.M.; Hubbard, P.C.; Biscarini, F. An electrical method to measure low-frequency collective and synchronized cell activity using extracellular electrodes. Sens. Bio-Sens. Res. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Teulon, J.; Ronco, P.M.; Geniteau-Legendre, M.; Baudouin, B.; Estrade, S.; Cassingena, R.; Vandewalle, A. Transformation of renal tubule epithelial cells by simian virus-40 is associated with emergence of Ca2+-insensitive K+ channels and altered mitogenic sensitivity to K+ channel blockers. J. Cell. Physiol. 1992, 151, 113–125. [Google Scholar] [CrossRef]
- Sohma, Y.; Harris, A.; Wardle, C.J.C.; Gray, M.A.; Argent, B.E. Maxi K+ channels on human vas deferens epithelial cells. J. Membr. Biol. 1994, 141, 69–82. [Google Scholar] [CrossRef]
- Logsdon, N.J.; Kang, J.; Togo, J.A.; Christian, E.P.; Aiyar, J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997, 272, 32723–32726. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Van Coppenolle, F.; Le Bourhis, X.; Belhaj, A.; Prevarskaya, N. Potassium channels in rat prostate epithelial cells. FEBS Lett. 1999, 459, 15–21. [Google Scholar] [CrossRef]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca2+ waves: Mechanisms and function. Physiol. Rev. 2012, 92, 1359–1392. [Google Scholar] [CrossRef] [PubMed]
- Kuga, N.; Sasaki, T.; Takahara, Y.; Matsuki, N.; Ikegaya, Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 2011, 31, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Zell, R.A.; Erickson, G.R.; Grande, D.A.; Rubin, C.T.; McLeod, K.J.; Donahue, H.J. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. J. Orthop. Res. 1999, 17, 421–429. [Google Scholar] [CrossRef] [PubMed]


| Day 1 | Day 2 | Day 3 | Day 4 | Day 8 | |
|---|---|---|---|---|---|
| Average | 42.0000 | 93.7500 | 156.6250 | 285.0000 | 1193.6250 |
| S.E.M | 4.5591 | 9.4449 | 16.8395 | 43.4133 | 128.5749 |
| Normalized average | 0.0352 | 0.0785 | 0.1312 | 0.2089 | 1.0000 |
| Normalized S.E.M | 2.42 × 10−3 | 5.02 × 10−3 | 8.95 × 10−3 | 2.75 × 10−2 | 6.84 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello, M.; Ge, H.; Aracil, C.; Moschou, D.; Estrela, P.; Manuel Quero, J.; I. Pascu, S.; R. F. Rocha, P. Extracellular Electrophysiology in the Prostate Cancer Cell Model PC-3. Sensors 2019, 19, 139. https://doi.org/10.3390/s19010139
Cabello M, Ge H, Aracil C, Moschou D, Estrela P, Manuel Quero J, I. Pascu S, R. F. Rocha P. Extracellular Electrophysiology in the Prostate Cancer Cell Model PC-3. Sensors. 2019; 19(1):139. https://doi.org/10.3390/s19010139
Chicago/Turabian StyleCabello, Miguel, Haobo Ge, Carmen Aracil, Despina Moschou, Pedro Estrela, Jose Manuel Quero, Sofia I. Pascu, and Paulo R. F. Rocha. 2019. "Extracellular Electrophysiology in the Prostate Cancer Cell Model PC-3" Sensors 19, no. 1: 139. https://doi.org/10.3390/s19010139
APA StyleCabello, M., Ge, H., Aracil, C., Moschou, D., Estrela, P., Manuel Quero, J., I. Pascu, S., & R. F. Rocha, P. (2019). Extracellular Electrophysiology in the Prostate Cancer Cell Model PC-3. Sensors, 19(1), 139. https://doi.org/10.3390/s19010139








