Author Contributions
O.I.G.P. wrote the code for the EPS and performed experiments to evaluate the electronics on the EPS and for the electrochemical experiments, A.I.M.-M.; established the requirements and characteristics that the potentiostat must have, performed experiments for the electrochemical experiments, proposed the experimental methodology and setup conditions, analyzed the results, evaluated all functionality and performance of the potentiostat, wrote the article, corresponding author O.I.G.P.; proposed the methodology for evaluating the errors and precision on the EPS, J.C.-F.; developed the architecture circuit design on the potentiostat, J.M.R.-D.; reviewed the code written for the EPS, A.Á.-O.; reviewed the overall functionality of the EPS from the point of view of the embedded electronics, funding acquisition, G.D.-A.
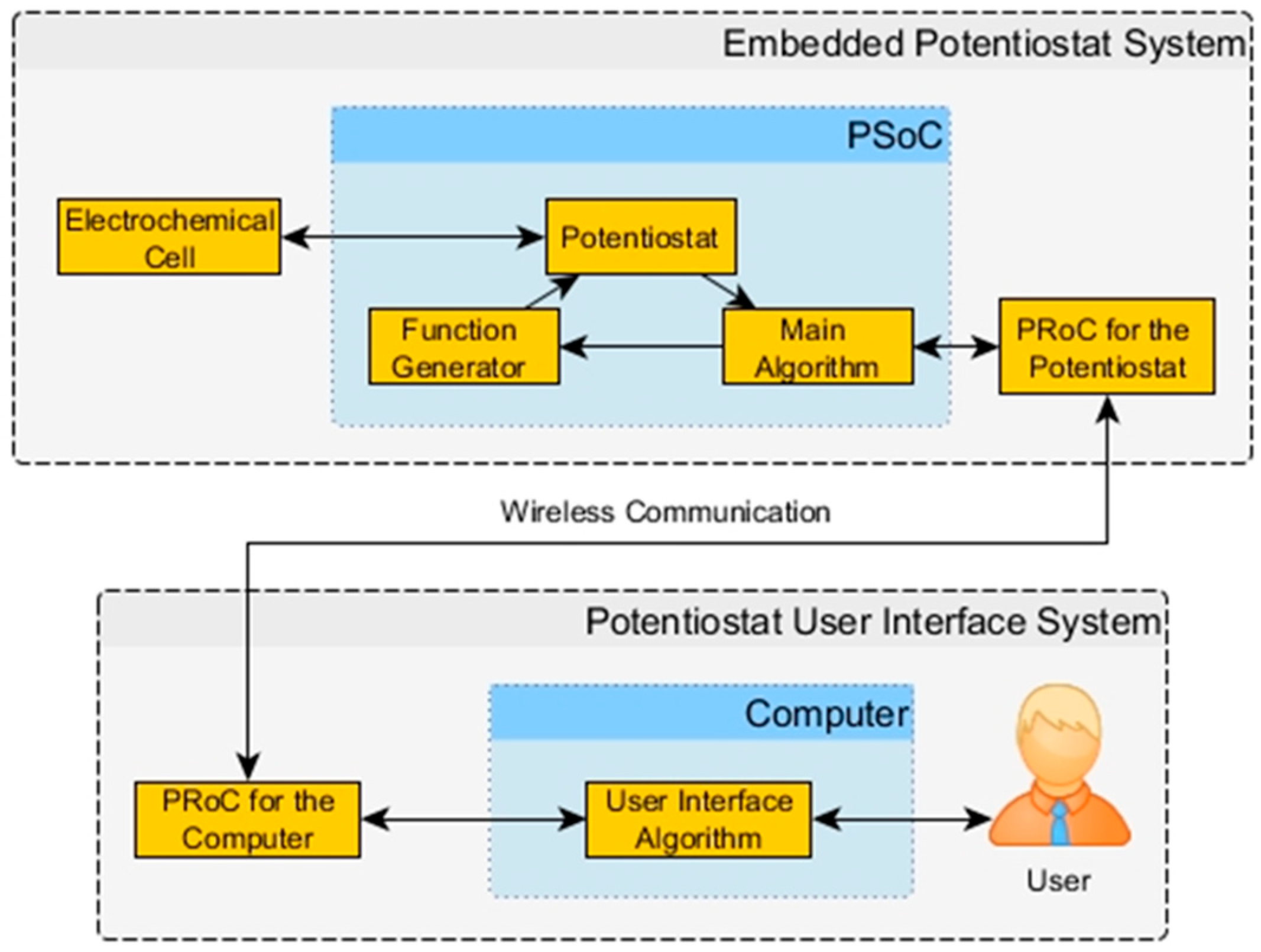
Figure 1.
Block Diagram of the Embedded Potentiostat System (EPS) with its User Interface.
Figure 1.
Block Diagram of the Embedded Potentiostat System (EPS) with its User Interface.
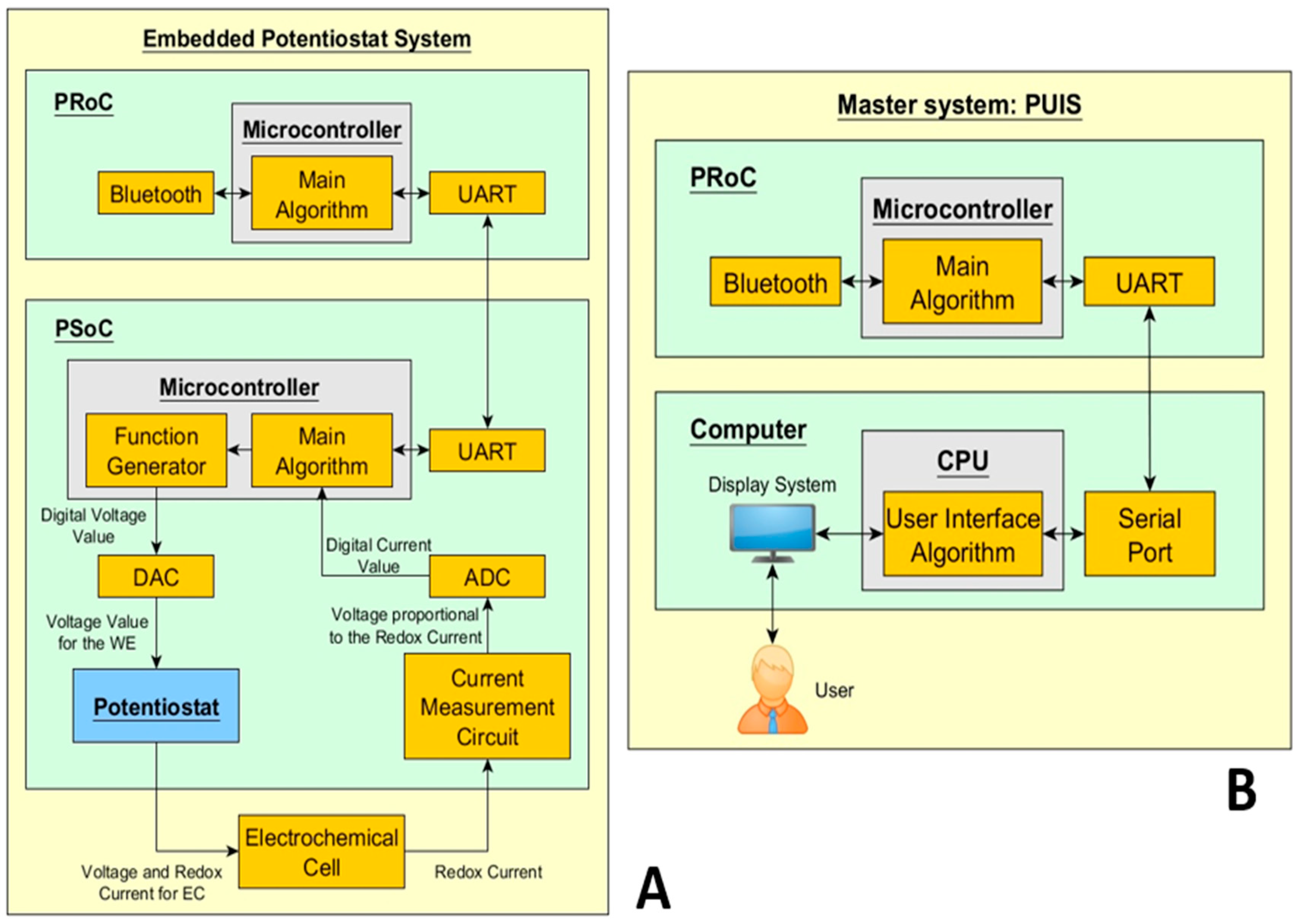
Figure 2.
Slave-master scheme: Embedded and User Interface systems (EPS and PUIS) driving the electrochemical cell.
Figure 2.
Slave-master scheme: Embedded and User Interface systems (EPS and PUIS) driving the electrochemical cell.
Figure 3.
Modular Potentiostat: (A) Slave system of the EPS. (B) Master system with the structure PUIS.
Figure 3.
Modular Potentiostat: (A) Slave system of the EPS. (B) Master system with the structure PUIS.
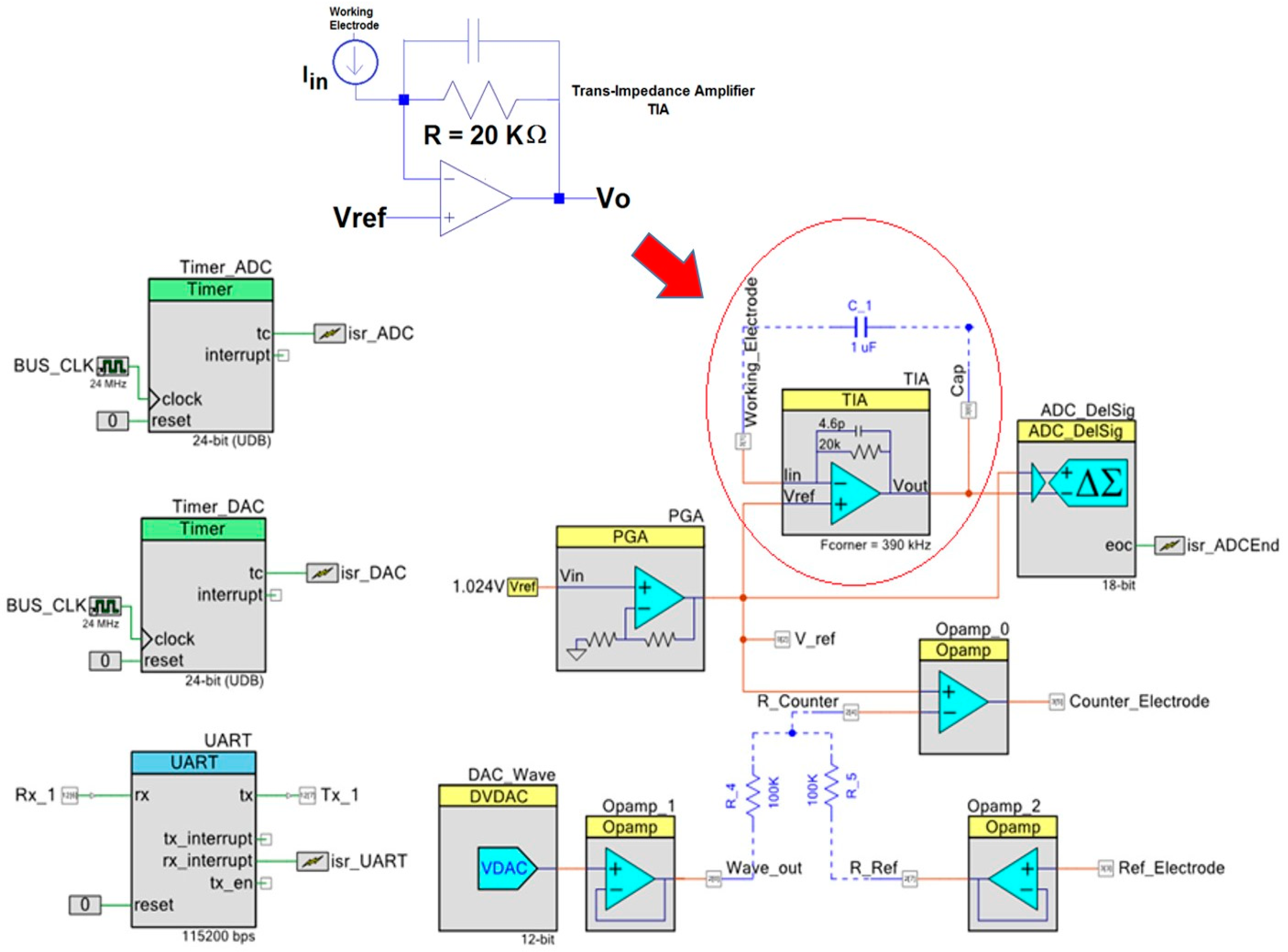
Figure 4.
Digital peripherals and analog front end devices of the PSoC. Digital to Analog Converter (DAC), Analog to Digital converter (ADC), Input-Output Port (UART), Trans-Impedance Amplifier (TIA).
Figure 4.
Digital peripherals and analog front end devices of the PSoC. Digital to Analog Converter (DAC), Analog to Digital converter (ADC), Input-Output Port (UART), Trans-Impedance Amplifier (TIA).
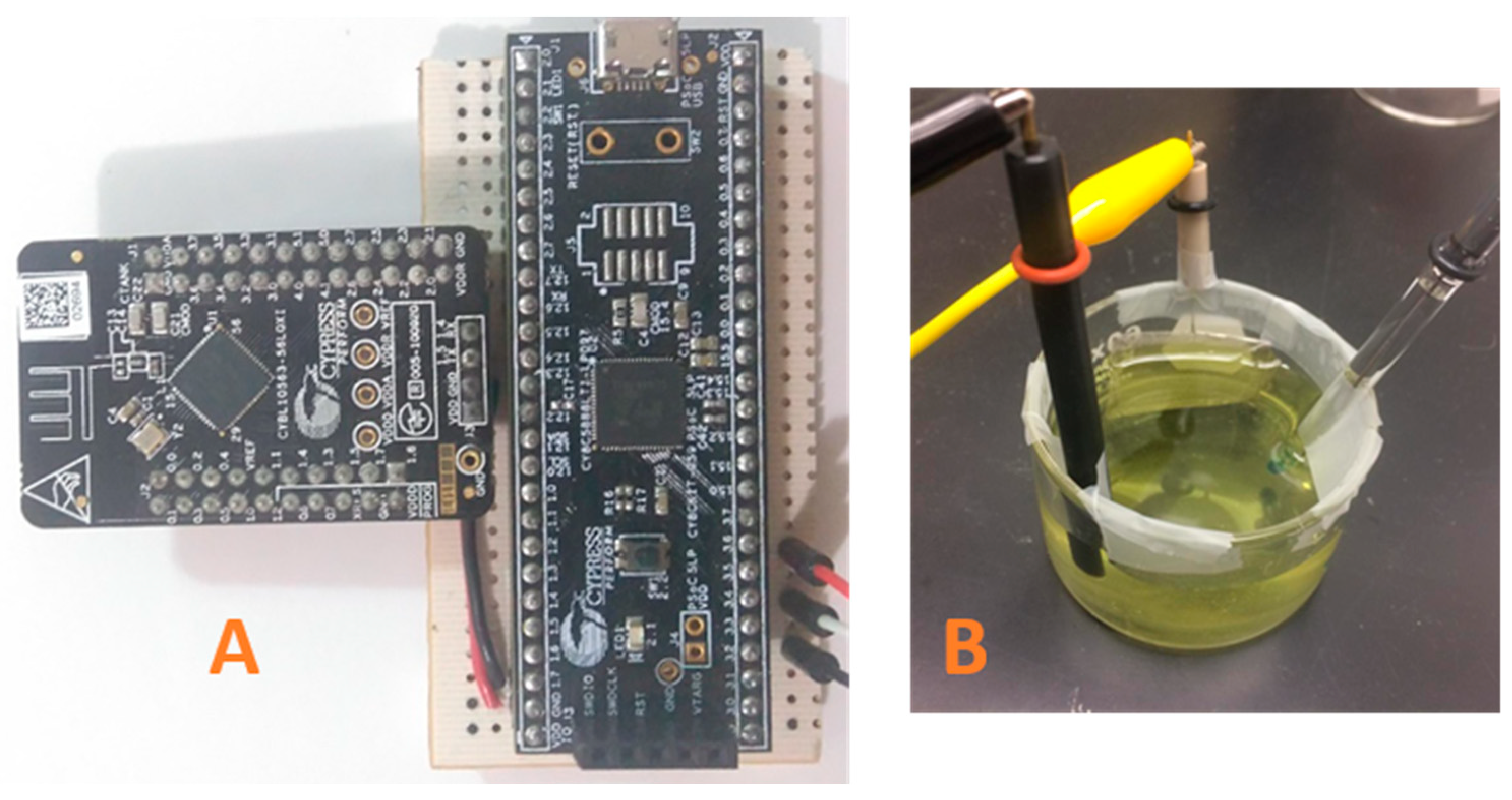
Figure 5.
(A) Prototype of the EPS. (B) Electrochemical cell (height = 35 mm, diameter = 60 mm), solution volume of 50 mL of K3[Fe(CN)6] and KCl.
Figure 5.
(A) Prototype of the EPS. (B) Electrochemical cell (height = 35 mm, diameter = 60 mm), solution volume of 50 mL of K3[Fe(CN)6] and KCl.
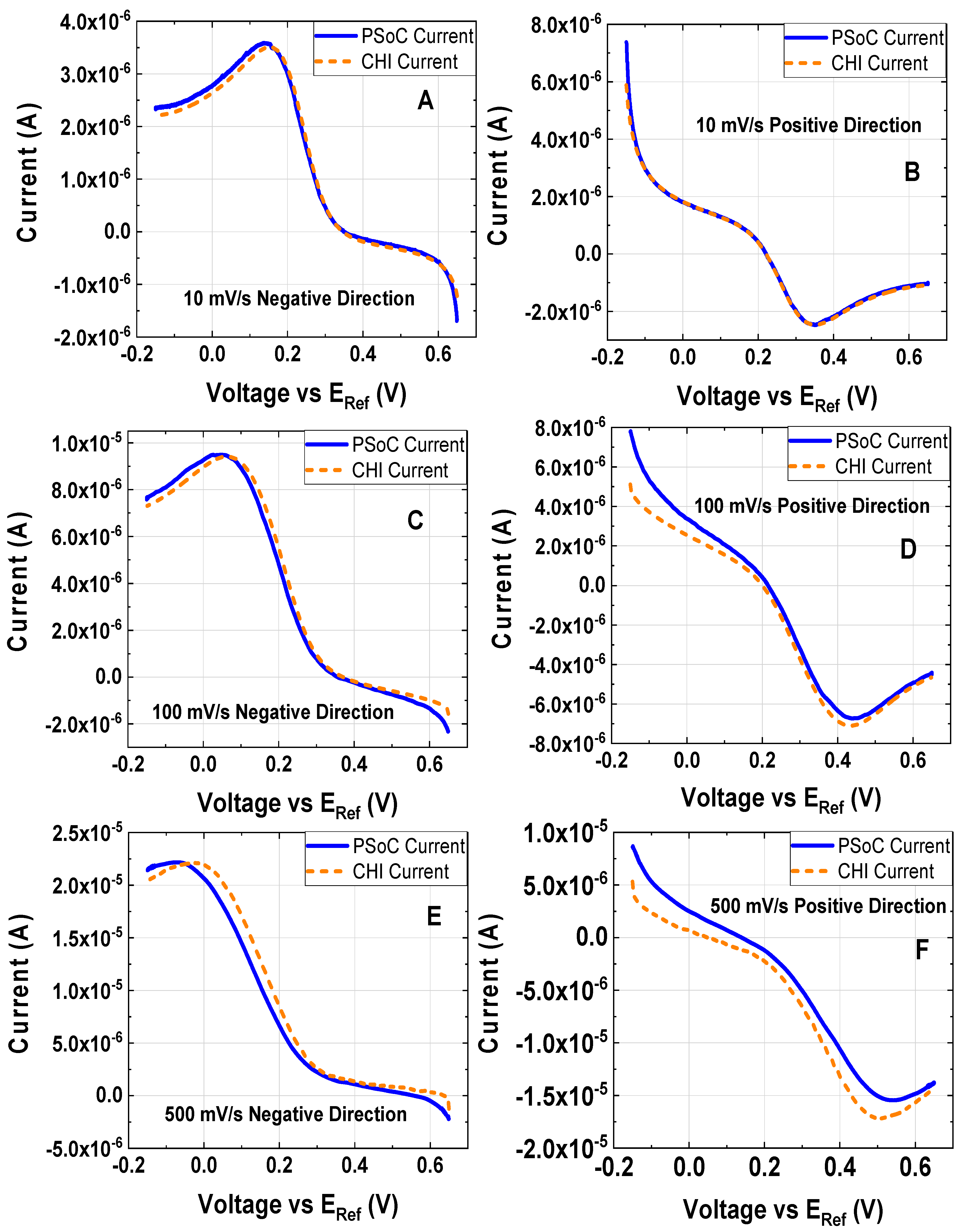
Figure 6.
Cyclic voltammetry at different scan rates of 1 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively: (A) 10 mV/s (experiment under the conditions 1). (B) 100 mV/s (experiment under the conditions 2). (C) 250 mV/s (experiment under the conditions 3). (D) 500 mV/s (experiment under the conditions 4).
Figure 6.
Cyclic voltammetry at different scan rates of 1 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively: (A) 10 mV/s (experiment under the conditions 1). (B) 100 mV/s (experiment under the conditions 2). (C) 250 mV/s (experiment under the conditions 3). (D) 500 mV/s (experiment under the conditions 4).
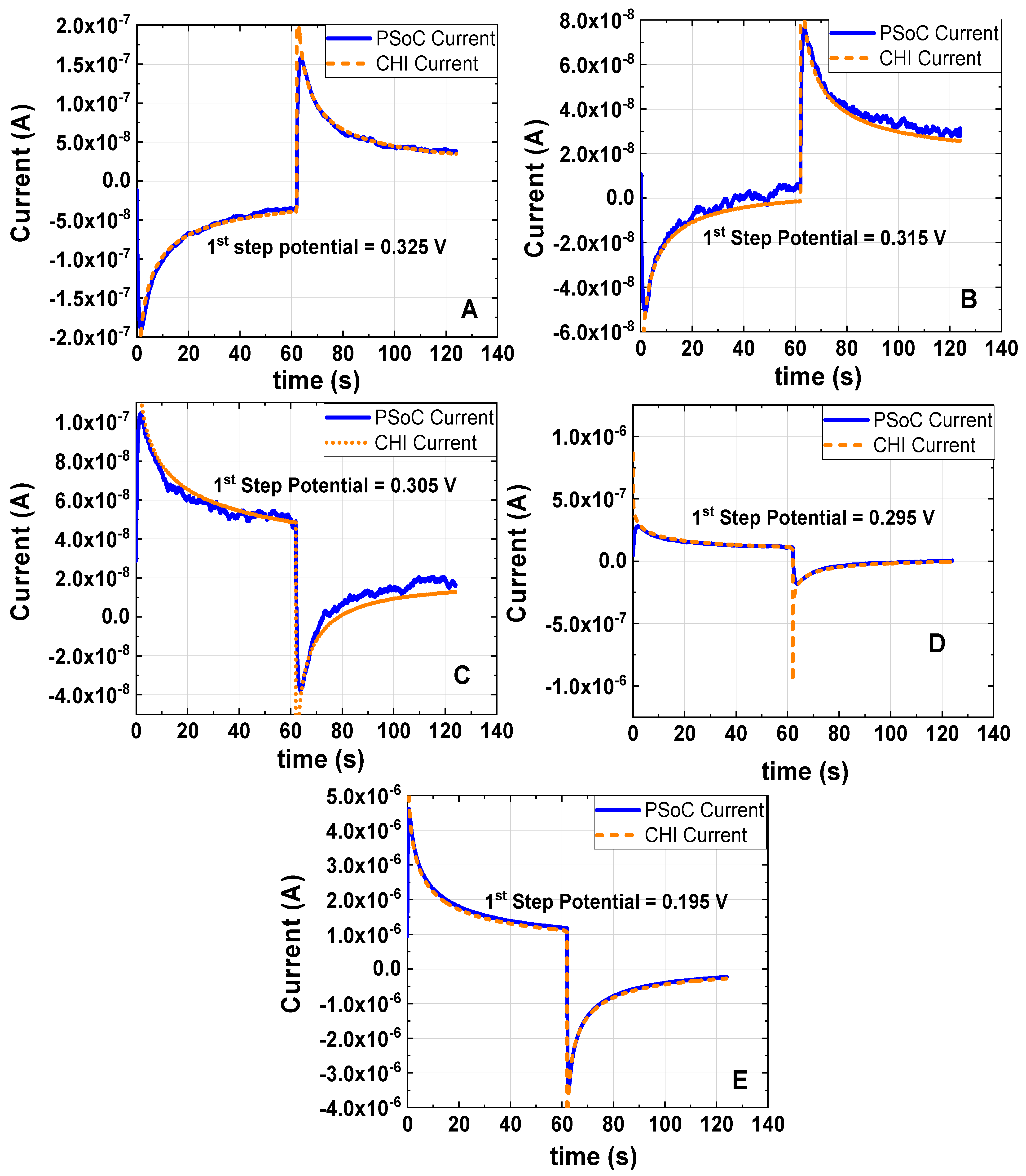
Figure 7.
Double Step Chronoamperometry at different first step potentials of 1 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively. All DSC were done with a pulse width of 62 s and the last step was setup at 0.310 V vs. Ag/AgCl: (A) first step potential 0.325 V vs. Ag/AgCl (experiment under the conditions 5). (B) first step potential 0.315 V vs. Ag/AgCl (experiment under the conditions 6). (C) first step potential 0.305 V vs. Ag/AgCl (experiment under the conditions 7). (D) first step potential 0.295 V vs. Ag/AgCl (experiment under the conditions 8). (E) first step potential 0.195 V vs. Ag/AgCl (experiment under the conditions 9).
Figure 7.
Double Step Chronoamperometry at different first step potentials of 1 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively. All DSC were done with a pulse width of 62 s and the last step was setup at 0.310 V vs. Ag/AgCl: (A) first step potential 0.325 V vs. Ag/AgCl (experiment under the conditions 5). (B) first step potential 0.315 V vs. Ag/AgCl (experiment under the conditions 6). (C) first step potential 0.305 V vs. Ag/AgCl (experiment under the conditions 7). (D) first step potential 0.295 V vs. Ag/AgCl (experiment under the conditions 8). (E) first step potential 0.195 V vs. Ag/AgCl (experiment under the conditions 9).
Figure 8.
Linear sweep voltammetry at different scan rates at different first step potentials of 1 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively. All voltages are reported vs. Ag/AgCl. (A) Scan rate 10 mV/s, initial and final voltage are 0.65 V and −0.15 V, respectively (experiment under the conditions 10). (B) Scan rate 10 mV/s, initial and final voltage are −0.15 V and 0.65 V, respectively (experiment under the conditions 11). (C) Scan rate 100 mV/s, initial and final voltage are 0.65 V and −0.15 V, respectively (experiment under the condition 12). (D) Scan rate 100 mV/s, initial and final voltage are −0.15 V and 0.65 V, respectively (experiment under the conditions 13). (E) Scan rate 500 mV/s, initial and final voltage are 0.65 V and −0.15 V, respectively (experiment under the conditions 14). (F) Scan rate 500 mV/s, initial and final voltage are −0.15 V and 0.65 V, respectively (experiment under the conditions 15).
Figure 8.
Linear sweep voltammetry at different scan rates at different first step potentials of 1 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively. All voltages are reported vs. Ag/AgCl. (A) Scan rate 10 mV/s, initial and final voltage are 0.65 V and −0.15 V, respectively (experiment under the conditions 10). (B) Scan rate 10 mV/s, initial and final voltage are −0.15 V and 0.65 V, respectively (experiment under the conditions 11). (C) Scan rate 100 mV/s, initial and final voltage are 0.65 V and −0.15 V, respectively (experiment under the condition 12). (D) Scan rate 100 mV/s, initial and final voltage are −0.15 V and 0.65 V, respectively (experiment under the conditions 13). (E) Scan rate 500 mV/s, initial and final voltage are 0.65 V and −0.15 V, respectively (experiment under the conditions 14). (F) Scan rate 500 mV/s, initial and final voltage are −0.15 V and 0.65 V, respectively (experiment under the conditions 15).
![Sensors 18 04490 g008 Sensors 18 04490 g008]()
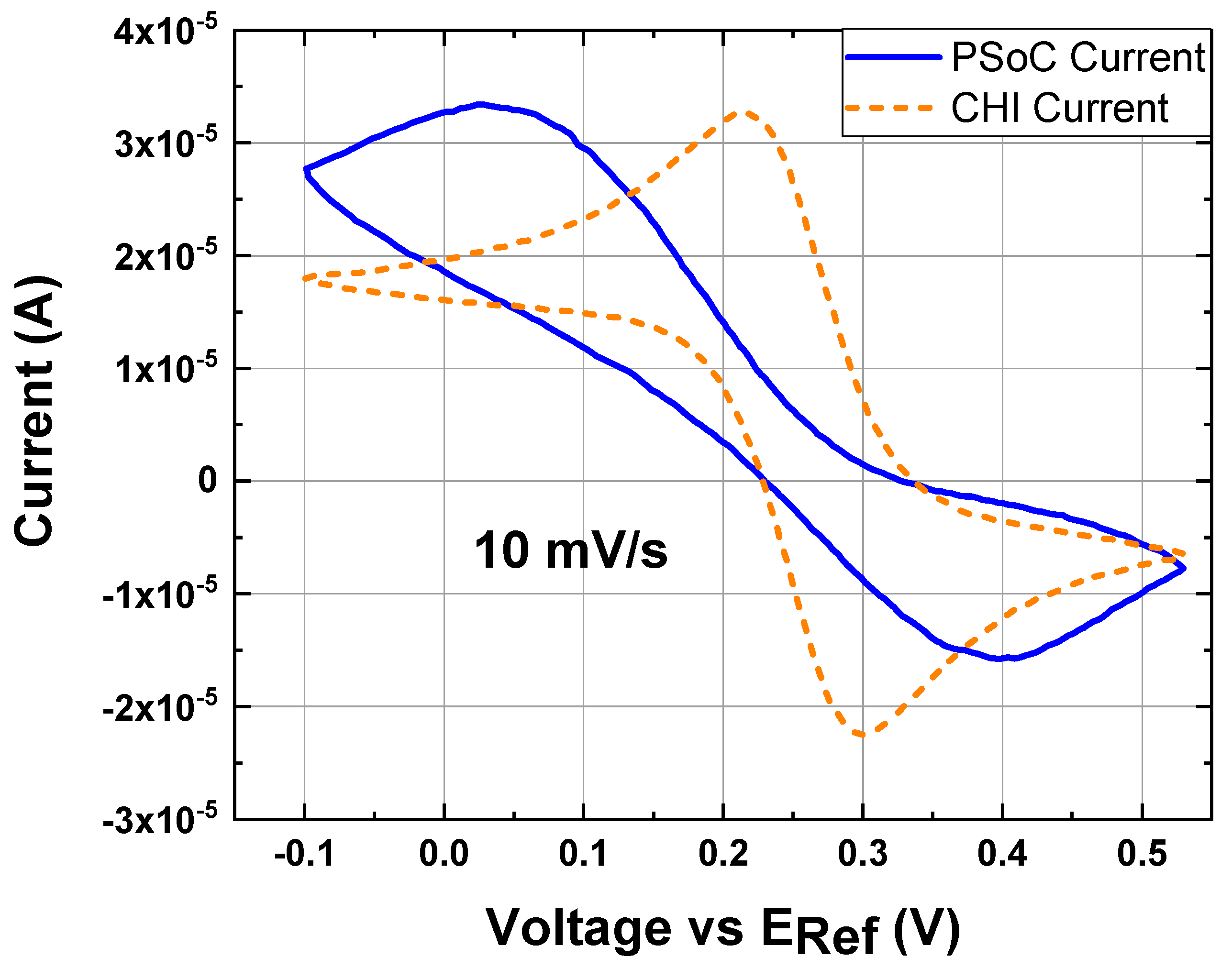
Figure 9.
Cyclic voltammetry at 10 mV/s, 10 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively; experiment under the conditions 16: (Blue line) CV response by the EPS. (Orange line) CV response by the commercial potentiostat.
Figure 9.
Cyclic voltammetry at 10 mV/s, 10 mM K3[Fe(CN)6] and 0.5 M KCl as the electrolyte support; the working electrode, the counter electrode and the reference electrode were a disk glassy carbon electrode (diameter ϕ = 3 mm), a platinum wire and Ag/AgCl electrode, respectively; experiment under the conditions 16: (Blue line) CV response by the EPS. (Orange line) CV response by the commercial potentiostat.
Table 1.
Sequences for the surface activation on the WE.
Table 1.
Sequences for the surface activation on the WE.
| | Sequence 1 | Sequence 2 | Sequence 3 | Sequence 4 |
|---|
| Scan Rate | 500 mV/s | 250 mV/s | 100 mV/s | 50 mV/s |
| Cycles | 50 | 25 | 10 | 5 |
| Method | Cyclic Voltammetry |
| Initial Voltage | 0.25 V |
| Maximum Voltage | 0.65 V |
| Minimum Voltage | −0.15 V |
| Initial Scan Direction | anodic direction |
Table 2.
Randles-Sevcik parameters and expected maximum current from CV and LSV experiments.
Table 2.
Randles-Sevcik parameters and expected maximum current from CV and LSV experiments.
| | Solution 1 | Solution 2 |
|---|
| K3[Fe(CN)6], analyte concentration | 1 mM | 10 mM |
| KCl, Electrolyte support concentration | 0.5 M |
| # of e− per mole oxidized or reduced | 1 |
| Analyte Diffusion Coefficient | 7.23 µcm2/s [36] |
| Scan Rate | 10 mV/s |
| Room Temperature | 25 °C |
| Surface Area of WE | 0.071 cm2 |
| Randles-Sevcik Current Peak | 5.11 µA | 51.1 µA |
Table 3.
CV conditions for each experiment.
Table 3.
CV conditions for each experiment.
| | Condition 1 | Condition 2 | Condition 3 | Condition 4 |
|---|
| Scan Rate | 10 mV/s | 100 mV/s | 250 mV/s | 500 mV/s |
| Initial Voltage | 0.25 V |
| Minimum Voltage | −0.15 V |
| Maximum Voltage | 0.65 V |
| Recorded cycle for comparison | fifth |
| Initial Scan Direction | anodic direction |
| Analyte concentration | 1 mM K3[Fe(CN)6] |
| Electrolyte support concentration | 0.5 M KCl |
Table 4.
DSC conditions for each experiment.
Table 4.
DSC conditions for each experiment.
| | Cond. 5 | Cond. 6 | Cond. 7 | Cond. 8 | Cond. 9 |
|---|
| First Step | 0.325 V | 0.315 V | 0.305 V | 0.295 V | 0.195 V |
| Last Step | 0.310 V |
| Pulse Width | 62 s |
| Quite time | 62 s |
| Analyte | 1 mM K3[Fe(CN)6] |
| Electrolyte | 0.5 M KCl |
Table 5.
LSV conditions for the experiments.
Table 5.
LSV conditions for the experiments.
| | Cond. 10 | Cond. 11 | Cond. 12 | Cond. 13 | Cond. 14 | Cond. 15 |
|---|
| Initial Voltage | 0.65 V | −0.15 V | 0.65 V | −0.15 V | 0.65 V | −0.15 V |
| Final Voltage | −0.15 V | 0.65 V | −0.15 V | 0.65 V | −0.15 V | 0.65 V |
| Scan Rate | 10 mV/s | 10 mV/s | 100 mV/s | 100 mV/s | 500 mV/s | 500 mV/s |
| Analyte | 1 mM K3[Fe(CN)6] |
| Electrolyte | 0.5 M KCl |
| Experiment under the: | MEP, % | HEP, % | Mean Error, A | Highest Error, A | Full Scale, A | Method |
|---|
| Conditions 1 | 2.887 | 5.226 | 1.98 × 10 −7 | 3.58 × 10 −7 | 6.852 × 10 −6 | CV |
| Conditions 2 | 1.691 | 5.721 | 2.88 × 10 −7 | 9.75 × 10 −7 | 1.704 × 10 −5 | CV |
| Conditions 3 | 2.226 | 7.653 | 5.62 × 10 −7 | 1.93 × 10 −6 | 2.527 × 10 −5 | CV |
| Conditions 4 | 3.397 | 11.178 | 1.18 × 10 −6 | 3.89 × 10 −6 | 3.481 × 10 −5 | CV |
| Conditions 5 | 0.319 | 5.185 | 5.35 × 10 −9 | 8.69 × 10 −8 | 1.676 × 10 −6 | DSC |
| Conditions 6 | 0.587 | 5.386 | 3.6 × 10 −9 | 3.30 × 10 −8 | 6.134 × 10 −7 | DSC |
| Conditions 7 | 0.780 | 5.480 | 5.04 × 10 −9 | 3.54 × 10 −8 | 6.461 × 10 −7 | DSC |
| Conditions 8 | 0.562 | 6.169 | 1.03 × 10 −8 | 1.13 × 10 −7 | 1.827 × 10 −6 | DSC |
| Conditions 9 | 0.414 | 4.758 | 7.73 × 10 −8 | 8.87 × 10 −7 | 1.865 × 10 −5 | DSC |
| Conditions 10 | 2.019 | 5.994 | 9.55 × 10 −8 | 2.83 × 10 −7 | 4.728 × 10 −6 | LSV |
| Conditions 11 | 0.585 | 5.464 | 4.88 × 10 −8 | 4.56 × 10 −7 | 8.341 × 10 −6 | LSV |
| Conditions 12 | 2.837 | 8.429 | 3.12 × 10 −7 | 9.26 × 10 −7 | 1.098 × 10 −5 | LSV |
| Conditions 13 | 5.081 | 17.825 | 6.2 × 10 −7 | 2.18 × 10 −6 | 1.220 × 10 −5 | LSV |
| Conditions 14 | 4.243 | 14.483 | 9.94 × 10 −7 | 3.39 × 10 −6 | 2.342 × 10 −5 | LSV |
| Conditions 15 | 7.655 | 19.173 | 1.72 × 10 −6 | 4.32 × 10 −6 | 2.252 × 10 −5 | LSV |
Table 7.
Lower Limit of Detection analysis. *** Data did not evaluate in the study.
Table 7.
Lower Limit of Detection analysis. *** Data did not evaluate in the study.
| | Mean Error, A | LLD from 5% of the Mean Error, A |
|---|
| Conditions 6 | 3.6 × 10 −9 | *** |
| Conditions 7 | 5.04 × 10−9 | *** |
| Conditions Average | 4.32 × 10−9 | 86.44 × 10−9 |
Table 8.
Condition to explore the highest limit of detection.
Table 8.
Condition to explore the highest limit of detection.
| | Conditions 16 |
|---|
| Scan Rate | 10 mV/s |
| Initial Voltage | 0.40 V |
| Minimum Voltage | −0.10 V |
| Maximum Voltage | 0.53 V |
| Cycle | Fifth |
| Initial Scan Direction | Positive |
| Analyte | 10 mM K3[Fe(CN)6] |
| Electrolyte Support | 0.5 M KCl |
Table 9.
Electrochemical conditions evaluated. *** Data did not evaluate in the study.
Table 9.
Electrochemical conditions evaluated. *** Data did not evaluate in the study.
| | CV | LSV | DSC |
|---|
| Analyte Concentration | 1 mM K3[Fe(CN)6] | 1 mM K3[Fe(CN)6] | 1 mM K3[Fe(CN)6] |
| Voltage Range | −0.15 to 0.65 V | −0.15 to 0.65 V | 0.195 to 0.325 V |
| Peak Current Range | −3.0 to 4.0 µA | −2.5 to 3.5 µA | *** |
| Maximum Step | *** | *** | 0.310 to 0.195 V |
| Minimum Step | *** | *** | 0.310 to 0.315 V |
| Maximum Current at 62 s | *** | *** | 1.2 µA |
| Minimum Current at 62 s | *** | *** | 43.22 nA |
| Scan Rate Range | 10 to 500 mV/s | 10 to 500 mV/s | *** |
| SPS Range | 50 to 2000 SPS | 50 to 2000 SPS | 50 to 2000 SPS |
Table 10.
Principal electric features of the EPS.
Table 10.
Principal electric features of the EPS.
| Voltage Range: ±2 V | Samples per Second: 50 to 2000 |
| Current Range: ±3 µA | Scan Rate: 10 to 500 mV/s |
Table 11.
Additional features of the EPS with a source voltage of 5 volts.
Table 11.
Additional features of the EPS with a source voltage of 5 volts.
| ADC Offset Voltage: −61.056 µV | EPS consumption at Stand By: 137.5 mW |
| TIA Offset Voltage: −3.36034 mV | EPS consumption at 2000 SPS: 207.5 mW |
Table 12.
TIA resistor calculated and bias current at the inverting input.
Table 12.
TIA resistor calculated and bias current at the inverting input.
| TIA Resistors | Value Calculated | Bias Current at the Inverting Input |
|---|
| R1 | 18,971.46 Ω | 3.40 × 10−8 A |
| R2 | 28,636.77 Ω | 2.06 × 10−8 A |
| R3 | 38,272.14 Ω | 1.67 × 10−8 A |
| R4 | 77,433.47 Ω | 6.77 × 10−9 A |
| R5 | 116,828.09 Ω | 5.35 × 10−9 A |
| R6 | 244,583.12 Ω | 2.59 × 10−9 A |
| R7 | 490,370.41 Ω | 1.27 × 10−9 A |
| R8 | 981,623.90 Ω | 6.89 × 10−10 A |
Table 13.
Electrochemical Instruments Comparison. *** Data not available.
Table 13.
Electrochemical Instruments Comparison. *** Data not available.
| Electrochemical Instrument | Jafari et al. [44] | Dorta-Quinones et al. [45] | Bozorgzadeh et al. [46] | Dryden et al. [10] | EmStat 3+
Embedded/OEM [47] | WaveNow AFTP1 [48] | Sun et al. [11] | Giordano et al. [49] | Muñoz et al. (This Article) |
|---|
| Highest Current Detection Limit | 350 nA | 430 nA | 950 nA | 22 mA | 100 mA | 100 mA | 200 µA | 50 µA | 3 µA |
| Lowest Current Detection Limit | 8.6 pA | *** | *** | 600 fA | 1 pA | 80 nA | 1 nA | 100 nA | 86 nA |
| Electronic Chip Area | 3 mm × 3 mm | 1.5 mm × 1.0 mm | 3.16 mm × 3.16 mm | *** | *** | *** | *** | *** | *** |
| PCB Area | *** | 4.7 cm × 1.9 cm | *** | 8 cm × 8 cm | 5.5 cm × 4.1 cm | 16.5 cm × 10 cm | 3.9 cm × 1.62 cm | 9.7 cm × 5.7 cm | ~(8 cm × 6 cm) |
| Maximum Samples Per Second | 2 ksps | 10 ksps | 10 ksps | 30 ksps | Less than 1 Ksps | 1 Ksps | 200 ksps | *** | 2 Ksps |
| ADC Effective Number of Bits | 9 bits | 10.95 bits | *** | 21.3 bits at 1.45 ksps | *** | *** | *** | *** | 18 |
| Wireless Connectivity | Ultra-Wideband Transmitter | Ultra-Wideband Transmitter | FSK Transmission with Manchester Encoding | No | Bluetooth or Wifi | No | No | Bluetooth | Bluetootth |
| Number of Techniques Implemented | 1 | 1 | 2 | More than 3 | 9 | 33 | 3 | 4 | At least 3 |
| Channels | 54 | 1 | 1 | 1 | up to 16 | 1 | 2 | 1 | 1 |
| SoC Present | Yes | Yes | Yes | No | *** | *** | No | No | Yes |
| Capabilities additional to a potentiostat | No | No | No | Yes | Yes | Yes | Yes | No | Yes, flexibility to reconfigure the analog front end |
| Maximum Power Consumption | 1543.3 µW for each channel | 30 µW | Approximately 0.4 mW | Less than 1 W | 2.5 W | 10 W | 111 mW | *** | 207 mW at 5 V |