Determination of HPLC-UV Fingerprints of Spanish Paprika (Capsicum annuum L.) for Its Classification by Linear Discriminant Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standard Solutions
2.2. Instrumentation
2.3. Samples and Sample Treatment
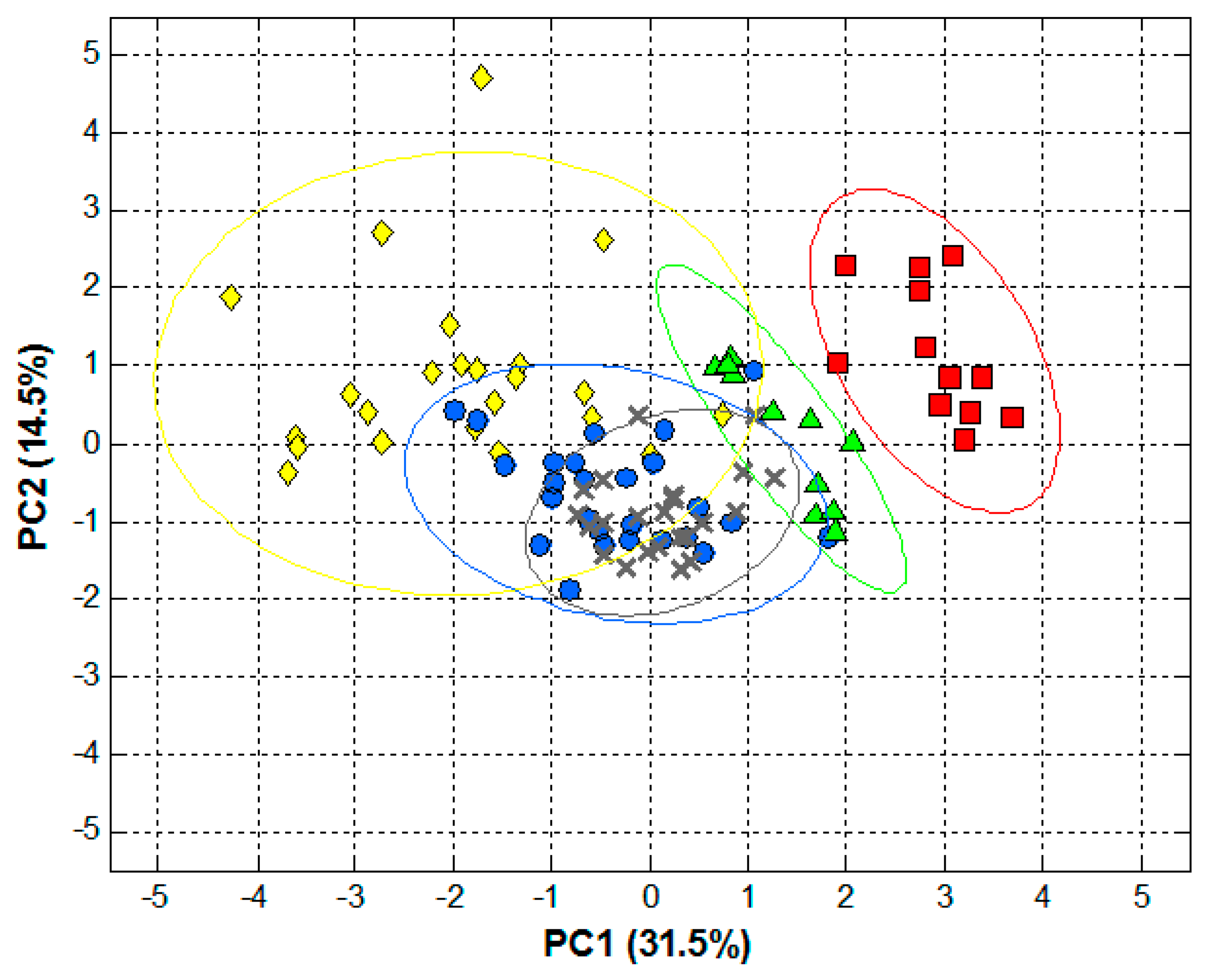
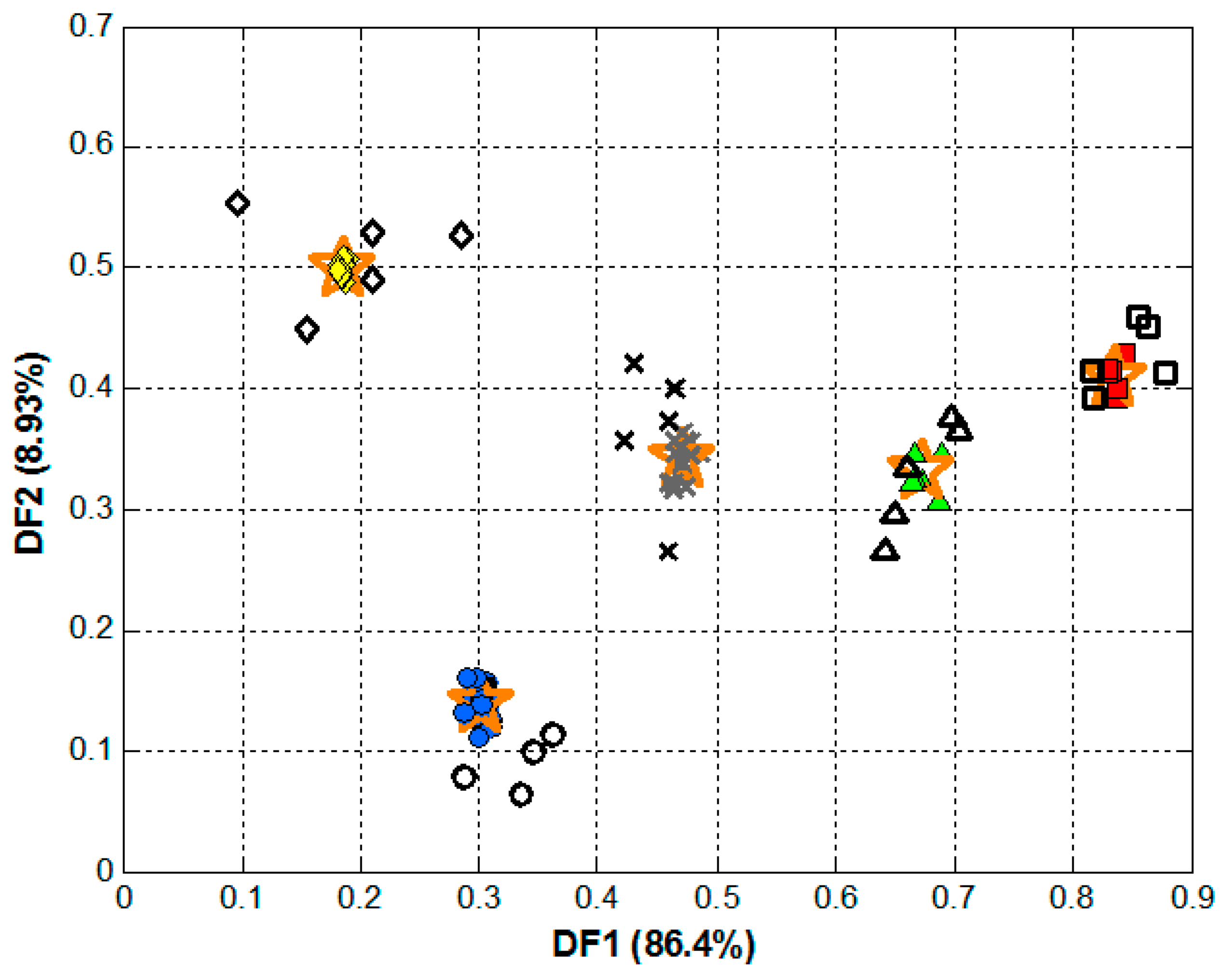
2.4. Data Analysis
3. Results and Discussion
3.1. HPLC-UV Method
3.2. Sample Extraction Optimization
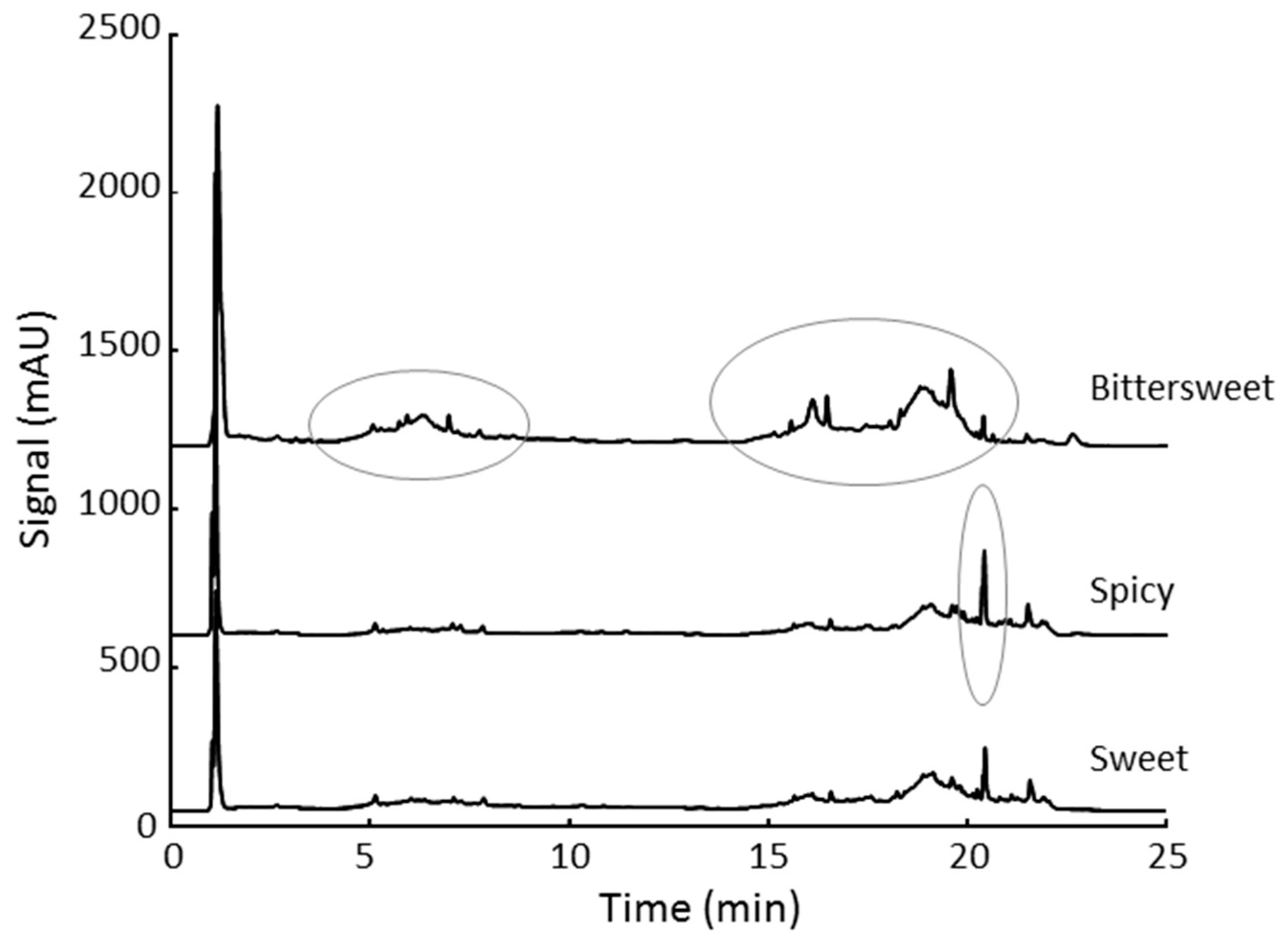
3.3. Qualitative Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Govindarajan, V.S.; Sathyanarayana, N.M. Capsicum—Production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–474. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Lee, S.S. Capsaicin in hot chili pepper: Carcinogen, co-carcinogen or anticarcinogen? Food Chem. Toxicol. 1996, 34, 313–316. [Google Scholar] [CrossRef]
- Márkus, F.; Daood, H.G.; Kapitány, J.; Biacs, P.A. Change in the carotenoid and antioxidant content of spice red pepper (Paprika) as a function of ripening and some technological factors. J. Agric. Food Chem. 1999, 47, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D.; Gómez-Ladrón de Guevara, R.; Mínguez-Mosquera, M.I. Carotenoid Biosynthesis Changes in Five Red Pepper (Capsicum annuum L.) Cultivars during Ripening. Cultivar Selection for Breeding. J. Agric. Food Chem. 2000, 48, 3857–3864. [Google Scholar] [CrossRef] [PubMed]
- Pol, L.C.; Siddiq, M.; Shahzad, T. Chili, Peppers, and Paprika. In Handbook of Vegetables and Vegtable Processing; Siddiq, M., Uebersax, M.A., Eds.; John Wiley & Sons: Hoboken NJ, USA, 2018; pp. 633–660. [Google Scholar]
- Anuario de Estadistica 2016. Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente, Gobierno de España, Madrid 2017. Available online: http://www.mapama.gob.es/estadistica/pags/anuario/2016/AE16.pdf (accessed on 17 February 2017).
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols—Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Serrano, M.; Núñez, O.; Saurina, J. Chemometrics in the polyphenolic characterization of natural products by liquid chromatography and capillary electrophoresis. In Chemometrics. Methods, Applications and New Research; Luna, A.S., Ed.; Nova Science: New York, NY, USA, 2017. [Google Scholar]
- Saurina, J.; Sentellas, S. Determination of Phenolic Compounds in Food Matrices: Applications to Characterization and Authentication. In Fast Liquid Chromatography-Mass Spectrometry Methods in Food and Environmental Analysis; Núñez, O., Gallart-Ayala, H., Eds.; Imperial College Press: London, UK, 2015. [Google Scholar]
- Nagy, Z.; Daood, H.; Ambrózy, Z.; Helyes, L. Determination of Polyphenols, Capsaicinoids, and Vitamin C in New Hybrids of Chili Peppers. J. Anal. Methods Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Haesen, N.; Câmara, J.S. A new and improved strategy combining a dispersive-solid phase extraction-based multiclass method with ultra high pressure liquid chromatography for analysis of low molecular weight polyphenols in vegetables. J. Chromatogr. A 2012, 1260, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Characterization of fruit products by capillary zone electrophoresis and liquid chromatography using the compositional profiles of polyphenols: Application to authentication of natural extracts. J. Agric. Food Chem. 2014, 62, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Mudric, S.Z.; Gašic, U.M.; Dramicanin, A.M.; Ciric, I.Z.; Milojkovic-Opsenica, D.M.; Popovic-Dordevic, J.B.; Momirovic, N.M.; Tešic, Z.L. The polyphenolics and carbohydrates as indicators of botanical and geographical origin of Serbian autochthonous clones of red spice paprika. Food Chem. 2017, 217, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Valcárcel, M.; Herrero-Martínez, J.M.; Cebolla-Cornejo, J.; Roselló, S. Fast simultaneous determination of prominent polyphenols in vegetables and fruits by reversed phase liquid chromatography using a fused-core column. Food Chem. 2015, 169, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Puigventós, L.; Navarro, M.; Alechaga, É.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Determination of polyphenolic profiles by liquid chromatography-electrospray-tandem mass spectrometry for the authentication of fruit extracts. Anal. Bioanal. Chem. 2015, 407, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Jurado, J.M.; Alcázar, Á.; De Pablos, F. Geographical characterization of Spanish PDO paprika by multivariate analysis of multielemental content. Talanta 2014, 128, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Martín, A.; Aranda, E.; Bartolomé, T.; Córdoba, M.G. Detection of Smoked Paprika “Pimentón de La Vera” adulteration by Free Zone Capillary Electrophoresis (FZCE). J. Agric. Food Chem. 2006, 54, 4141–4147. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Martín, A.; Aranda, E.; Bartolomé, T.; de Córdoba, M.G. Application of temperature-induced phase partition of proteins for the detection of smoked paprika adulteration by free zone capillary electrophoresis (FZCE). Food Chem. 2007, 105, 1219–1227. [Google Scholar] [CrossRef]
- Hernández, A.; Aranda, E.; Martín, A.; Benito, M.J.; Bartolomé, T.; Córdoba, M.D.G. Efficiency of DNA typing methods for detection of smoked paprika “pimenton de la Vera” adulteration used in the elaboration of dry-cured iberian pork sausages. J. Agric. Food Chem. 2010, 58, 11688–11694. [Google Scholar] [CrossRef] [PubMed]
- Cetó, X.; Céspedes, F.; del Valle, M. Comparison of methods for the processing of voltammetric electronic tongues data. Microchim. Acta 2013, 180, 319–330. [Google Scholar] [CrossRef]
- Ciulu-Costinescu, F.; Chifiriuc, M.C.; Popa, M.; Bleotu, C.; Neamtu, J.; Averis, L.M.E.; Bubulica, M.V.; Simionescu, A.; Aldea, I.M.; Belu, I. Screening of Polyphenol Content and In vitro Studies of Antioxidant, Antibacterial and Cytotoxic Activities of Capsicum Annuum Extracts. Rev. Chim. 2015, 66, 1261–1266. [Google Scholar]
- Pardo-Mates, N.; Vera, A.; Barbosa, S.; Hidalgo-Serrano, M.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Characterization, classification and authentication of fruit-based extracts by means of HPLC-UV chromatographic fingerprints, polyphenolic profiles and chemometric methods. Food Chem. 2017, 221, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Larrauri, A.; Núñez, O.; Hernández-Cassou, S.; Saurina, J. Determination of polyphenols in white wines by liquid chromatography: Application to the characterization of alella (Catalonia, Spain) wines using chemometric methods. J. AOAC Int. 2017, 100, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Farrés-Cebrián, M.; Seró, R.; Saurina, J.; Núñez, O. HPLC-UV Polyphenolic Profiles in the Classification of Olive Oils and Other Vegetable Oils via Principal Component Analysis. Separations 2016, 3, 33. [Google Scholar] [CrossRef]
- Wagner, M.S.; Castner, D.G. Characterization of adsorbed protein films by time-of-flight secondary ion mass spectrometry with principal component analysis. Langmuir 2001, 17, 4649–4660. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wichein, D.W. Applied Multivariate Statistical Analysis; Pearson International Edition: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Cetó, X.; Voelcker, N.H.; Prieto-Simón, B. Bioelectronic tongues: New trends and applications in water and food analysis. Biosens. Bioelectron. 2016, 79, 608–626. [Google Scholar] [CrossRef] [PubMed]
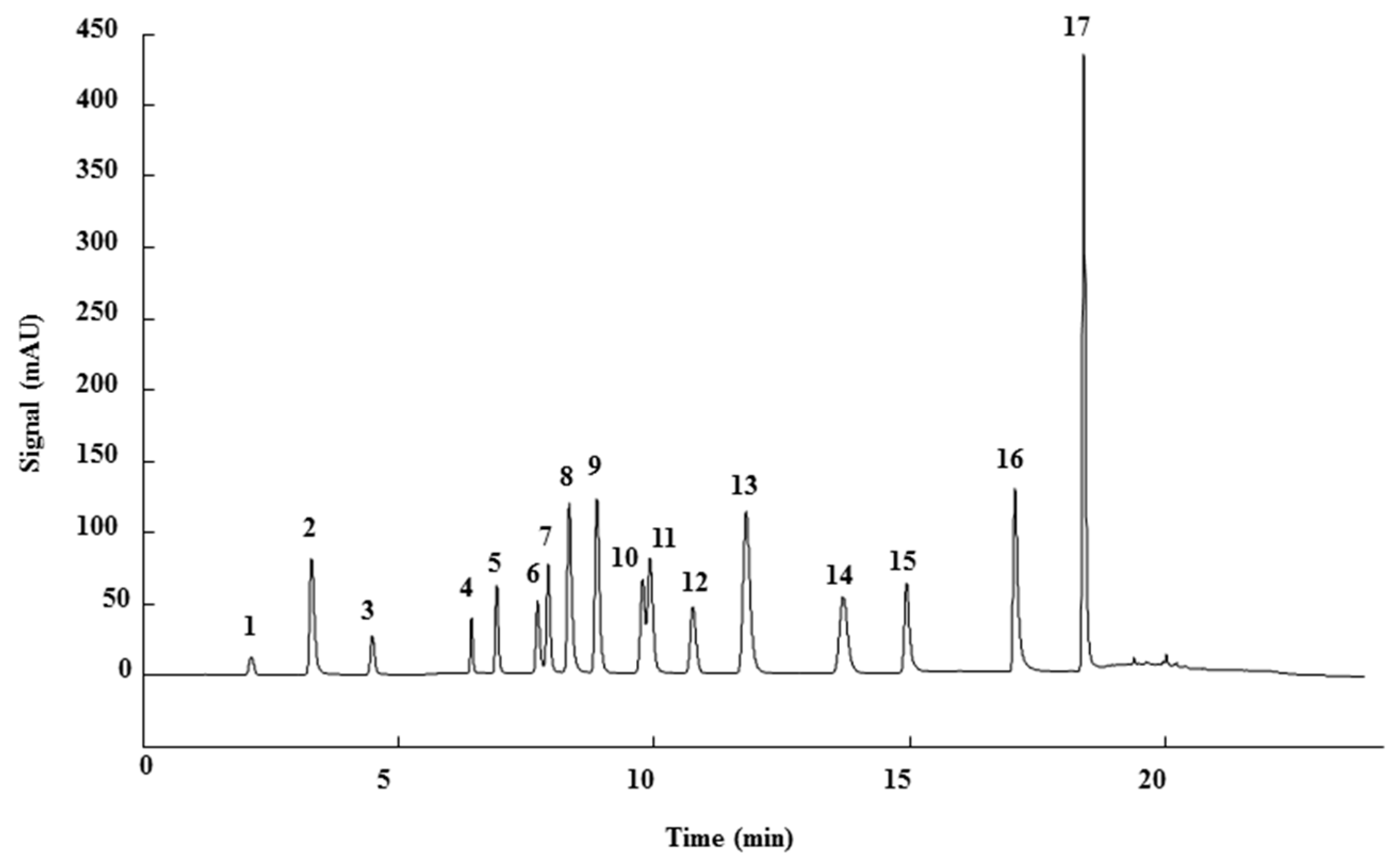
| Peak | Phenolic compound | Family | Structure | CAS Number |
|---|---|---|---|---|
| 1 | Arbutine | Phenolic glucoside |  | 497-76-7 |
| 2 | Gallic acid | Phenolic acid |  | 149-91-7 |
| 3 | Homogentisic acid | Phenolic acid |  | 451-13-8 |
| 4 | Tyrosol | Other phenolics |  | 501-94-0 |
| 5 | 4-Hydroxybenzoic acid | Phenolic acid |  | 99-96-7 |
| 6 | Chlorogenic acid | Phenolic acid |  | 327-97-9 |
| 7 | Vanillic acid | Phenolic acid |  | 121-34-6 |
| 8 | Caffeic acid | Phenolic acid |  | 331-39-5 |
| 9 | Syringic acid | Phenolic acid |  | 530-57-4 |
| 10 | Syringaldehyde | Phenolic aldehyde |  | 134-96-3 |
| 11 | Ethyl gallate | Phenolic acid |  | 831-61-8 |
| 12 | Umbelliferon | Coumarin |  | 93-35-6 |
| 13 | p-Coumaric acid | Phenolic acid |  | 501-98-4 |
| 14 | Ferulic acid | Phenolic acid |  | 537-98-4 |
| 15 | Polydatin | Stilben |  | 65914-17-2 |
| 16 | Resveratrol | Stilben |  | 501-36-0 |
| 17 | trans-Cinnamic acid | Cinnamic acid |  | 140-10-3 |
| Peak | Polyphenol | ILOD (µg/L) | ILOQ (µg/L) | Linearity (r2) | Sensitivity | run-to-run precision (%RSD, n = 5) | day-to-day precision (%RSD, n = 3 × 5) | Trueness (% Error) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 1 | Level 2 | Level 3 | Level 4 | Level 1 | Level 2 | Level 3 | Level 4 | |||||||||
| 1 | Arbutine | 38.0 | 126.7 | 0.9996 | 6.29 | 0.1 | 0.1 | 0.1 | 0.4 | 0.4 | 3.9 | 1.4 | 19.3 | 0.5 | 0.7 | 3.1 | 7.9 | |||
| 2 | Gallic acid | 33.6 | 112.0 | 0.9997 | 36.70 | 0.1 | 0.05 | 0.4 | 0.3 | 0.4 | 2.7 | 2.1 | 10.7 | 1.0 | 0.7 | 1.6 | 2.5 | |||
| 3 | Homogentisic acid | 31.7 | 105.7 | 0.9996 | 11.50 | 0.1 | 0.2 | 0.4 | 0.4 | 1.8 | 4.5 | 9.1 | 17.2 | 1.0 | 0.3 | 0.4 | 5.1 | |||
| 4 | Tyrosol | 31.3 | 104.3 | 0.9996 | 9.07 | 0.1 | 0.1 | 0.1 | 0.7 | 2.1 | 3.3 | 4.7 | 9.1 | 1.0 | 0.2 | 5.3 | 13.3 | |||
| 5 | 4-Hydroxybenzoic acid | 13.4 | 44.7 | 0.9996 | 18.05 | 0.04 | 0.1 | 0.1 | 0.2 | 1.8 | 3.3 | 3.7 | 10.0 | 1.0 | 1.3 | 4.7 | 4.3 | |||
| 6 | Chlorogenic acid | 33.3 | 111.0 | 0.9996 | 19.24 | 0.03 | 0.1 | 0.1 | 0.3 | 1.1 | 4.4 | 8.1 | 8.6 | 1.1 | 0.6 | 0.1 | 7.2 | |||
| 7 | Vanillic acid | 12.5 | 41.7 | 0.9999 | 26.39 | 0.1 | 0.1 | 0.2 | 0.3 | 0.8 | 3.2 | 3.3 | 13.5 | 0.04 | 1.0 | 4.6 | 1.0 | |||
| 8 | Caffeic acid | 13.2 | 44.0 | 0.9996 | 48.28 | 0.05 | 0.03 | 0.1 | 0.2 | 1.1 | 3.1 | 5.5 | 14.9 | 0.3 | 0.9 | 2.1 | 8.6 | |||
| 9 | Syringic acid | 12.8 | 42.7 | 0.9996 | 53.45 | 0.1 | 0.1 | 0.1 | 0.2 | 2.1 | 3.4 | 4.2 | 10.3 | 1.1 | 1.1 | 5.3 | 5.7 | |||
| 10 | Syringaldehyde | 31.0 | 103.3 | 0.9999 | 28.06 | 0.1 | 0.1 | 0.1 | 0.1 | 1.6 | 3.2 | 2.3 | 8.1 | 0.6 | 0.7 | 4.0 | 25.7 | |||
| 11 | Ethyl gallate | 13.0 | 43.3 | 0.9996 | 41.57 | 0.1 | 0.1 | 0.3 | 0.2 | 2.0 | 5.7 | 10.8 | 14.3 | 1.0 | 3.4 | 0.5 | 1.3 | |||
| 12 | Umbelliferon | 31.2 | 104.0 | 0.9996 | 25.50 | 0.1 | 0.03 | 0.1 | 0.5 | 1.1 | 3.8 | 5.9 | 17.7 | 0.3 | 0.2 | 2.4 | 8.9 | |||
| 13 | p-Coumaric acid | 14.3 | 47.7 | 0.9999 | 75.10 | 0.1 | 0.2 | 0.1 | 0.1 | 1.2 | 4.2 | 6.0 | 13.6 | 0.8 | 1.1 | 0.1 | 4.4 | |||
| 14 | Ferulic acid | 31.1 | 103.7 | 0.9999 | 46.79 | 0.1 | 0.04 | 0.1 | 0.2 | 1.4 | 3.9 | 6.3 | 14.9 | 0.9 | 0.9 | 1.6 | 5.2 | |||
| 15 | Polydatin | 33.0 | 110.7 | 0.9999 | 37.26 | 0.1 | 0.2 | 0.5 | 0.4 | 1.4 | 2.5 | 7.9 | 13.7 | 0.5 | 2.0 | 0.6 | 8.7 | |||
| 16 | Resveratrol | 31.1 | 103.7 | 0.9999 | 63.62 | 0.04 | 0.2 | 0.3 | 0.4 | 1.4 | 4.5 | 8.2 | 18.3 | 1.2 | 0.6 | 4.6 | 3.7 | |||
| 17 | trans-Cinnamic acid | 12.6 | 42.0 | 0.9999 | 138.66 | 0.1 | 0.1 | 0.4 | 2.7 | 3.3 | 2.7 | 5.6 | 8.1 | 1.4 | 0.5 | 8.3 | 29.2 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetó, X.; Serrano, N.; Aragó, M.; Gámez, A.; Esteban, M.; Díaz-Cruz, J.M.; Núñez, O. Determination of HPLC-UV Fingerprints of Spanish Paprika (Capsicum annuum L.) for Its Classification by Linear Discriminant Analysis. Sensors 2018, 18, 4479. https://doi.org/10.3390/s18124479
Cetó X, Serrano N, Aragó M, Gámez A, Esteban M, Díaz-Cruz JM, Núñez O. Determination of HPLC-UV Fingerprints of Spanish Paprika (Capsicum annuum L.) for Its Classification by Linear Discriminant Analysis. Sensors. 2018; 18(12):4479. https://doi.org/10.3390/s18124479
Chicago/Turabian StyleCetó, Xavier, Núria Serrano, Miriam Aragó, Alejandro Gámez, Miquel Esteban, José Manuel Díaz-Cruz, and Oscar Núñez. 2018. "Determination of HPLC-UV Fingerprints of Spanish Paprika (Capsicum annuum L.) for Its Classification by Linear Discriminant Analysis" Sensors 18, no. 12: 4479. https://doi.org/10.3390/s18124479
APA StyleCetó, X., Serrano, N., Aragó, M., Gámez, A., Esteban, M., Díaz-Cruz, J. M., & Núñez, O. (2018). Determination of HPLC-UV Fingerprints of Spanish Paprika (Capsicum annuum L.) for Its Classification by Linear Discriminant Analysis. Sensors, 18(12), 4479. https://doi.org/10.3390/s18124479









