Conformal and Disposable Antenna-Based Sensor for Non-Invasive Sweat Monitoring
Abstract
:1. Introduction
2. Materials and Methods
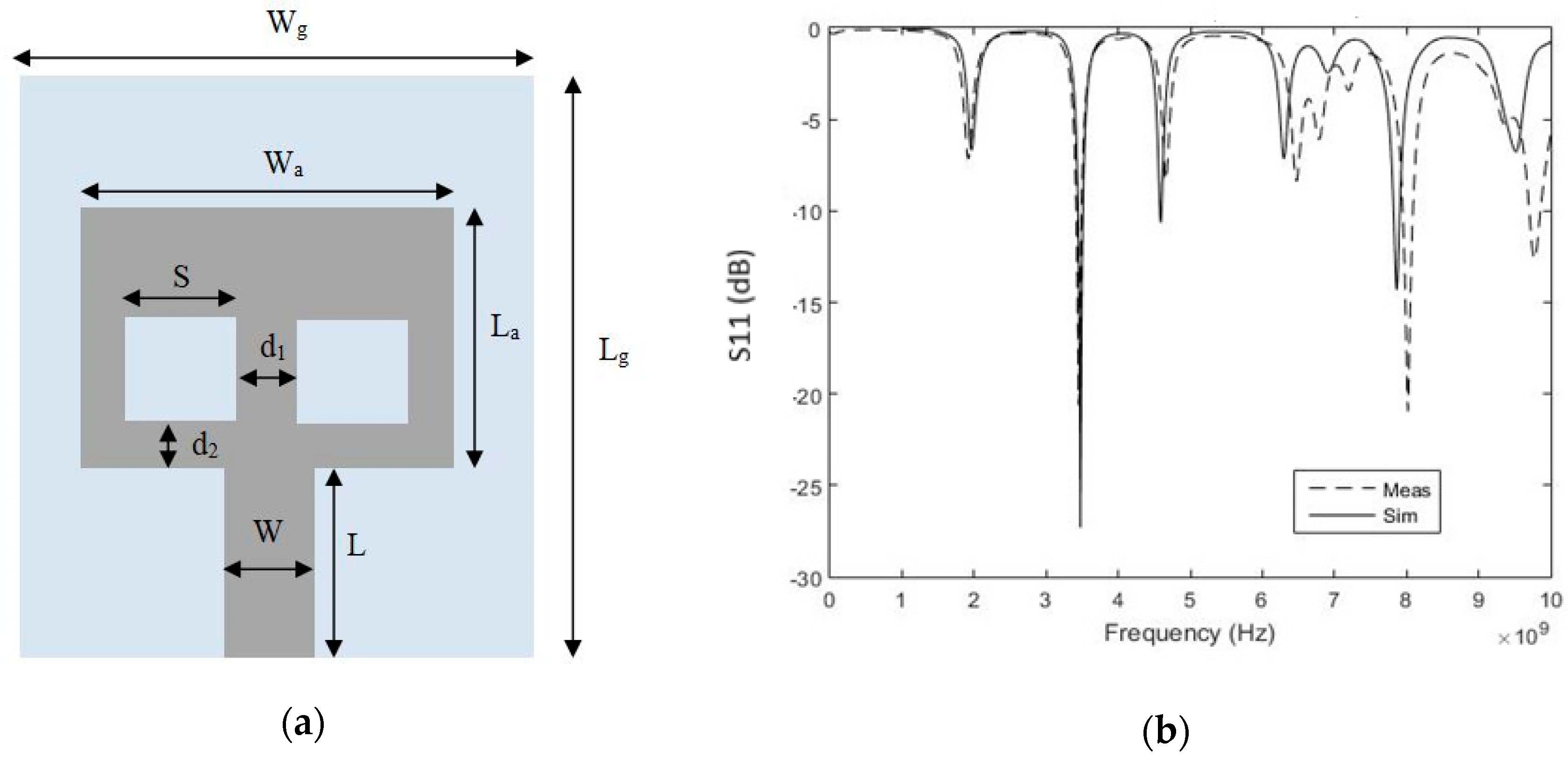
2.1. Sensor Design
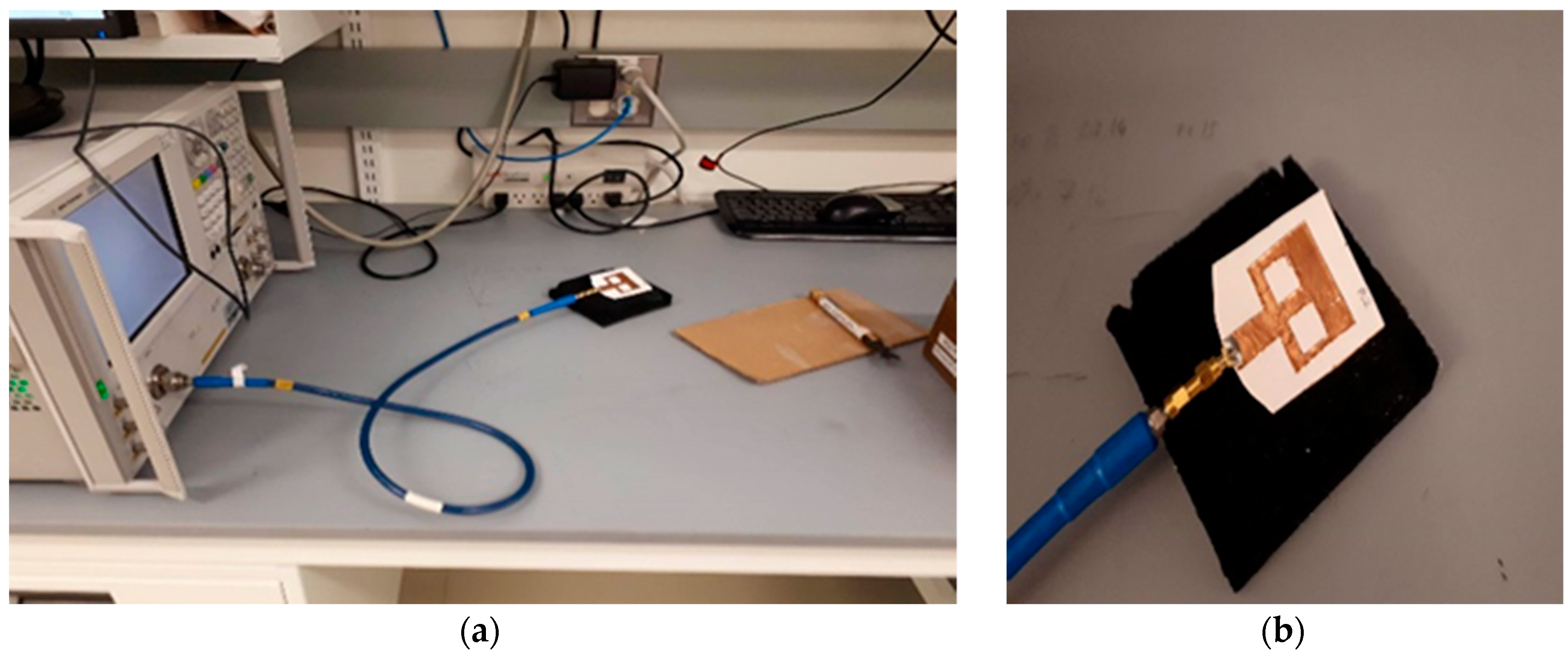
2.2. Sensing Methodology and Test Solutions
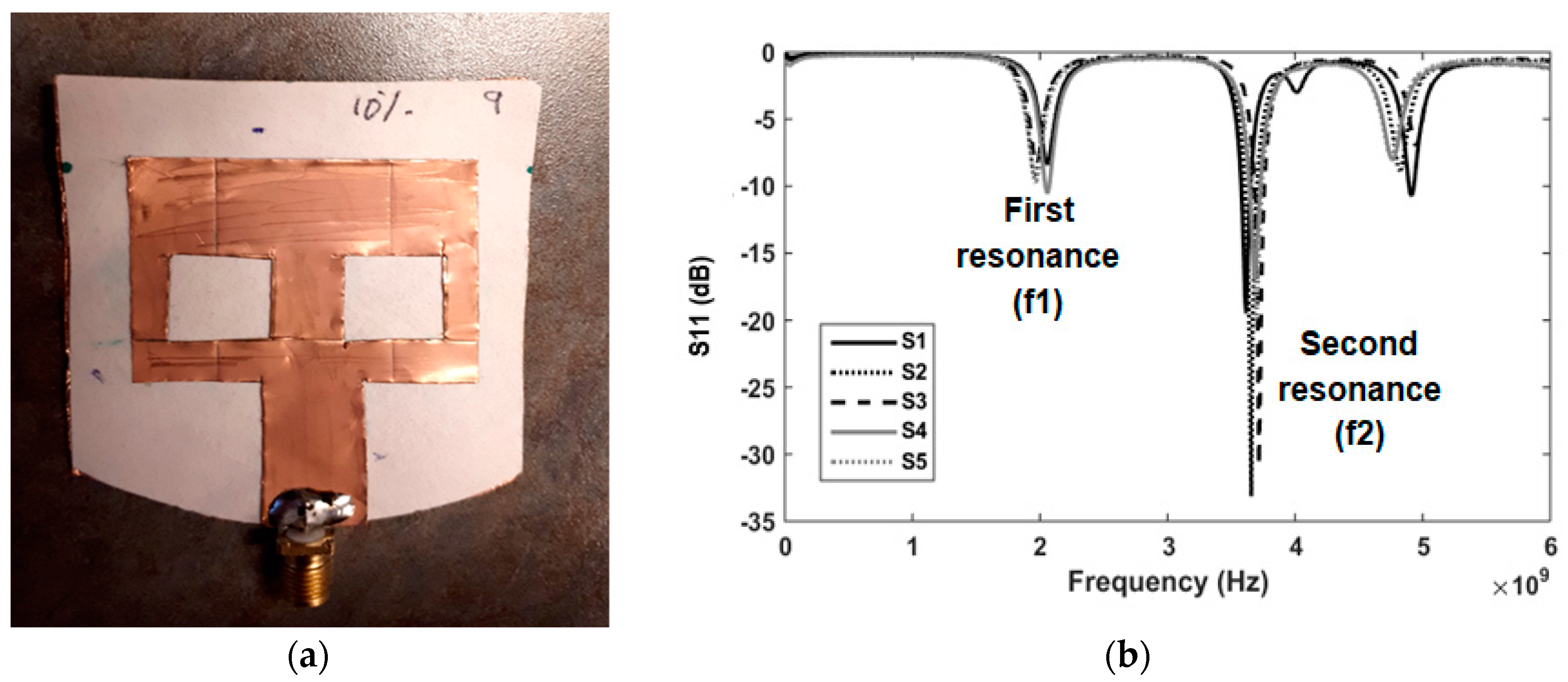
3. Results
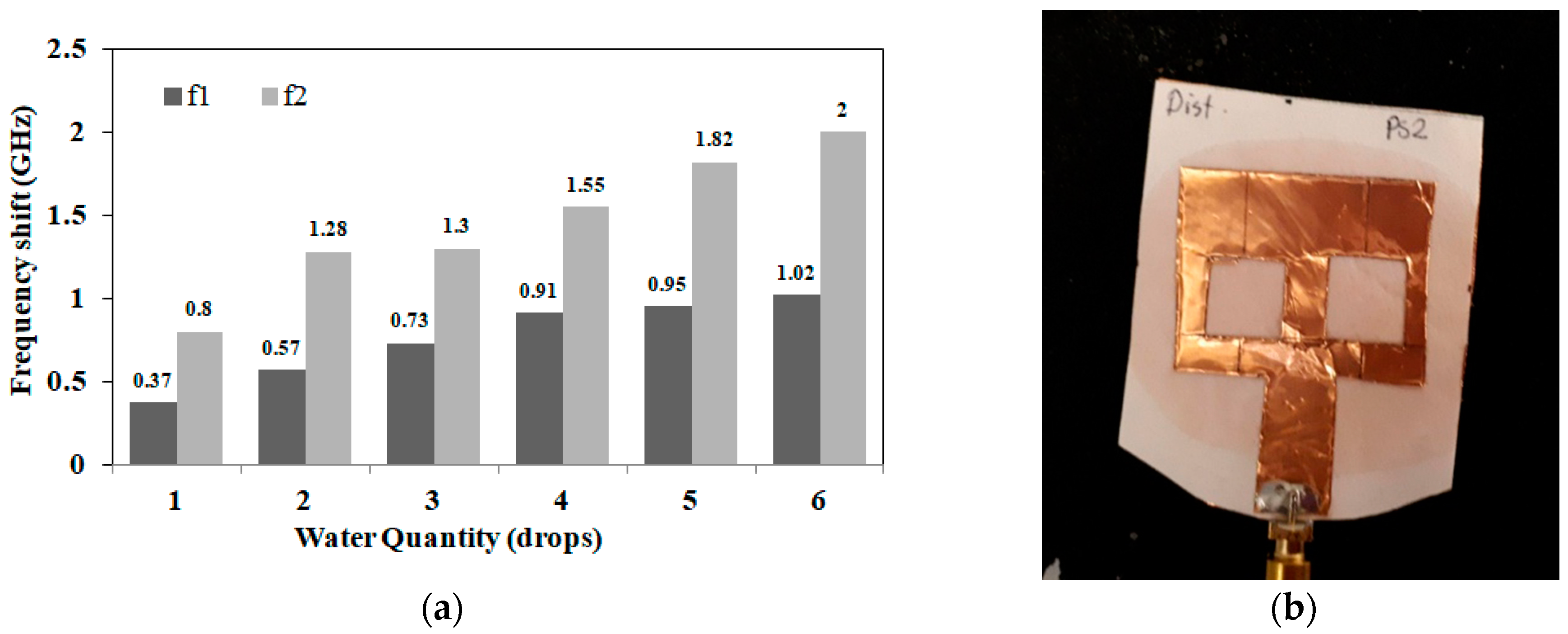
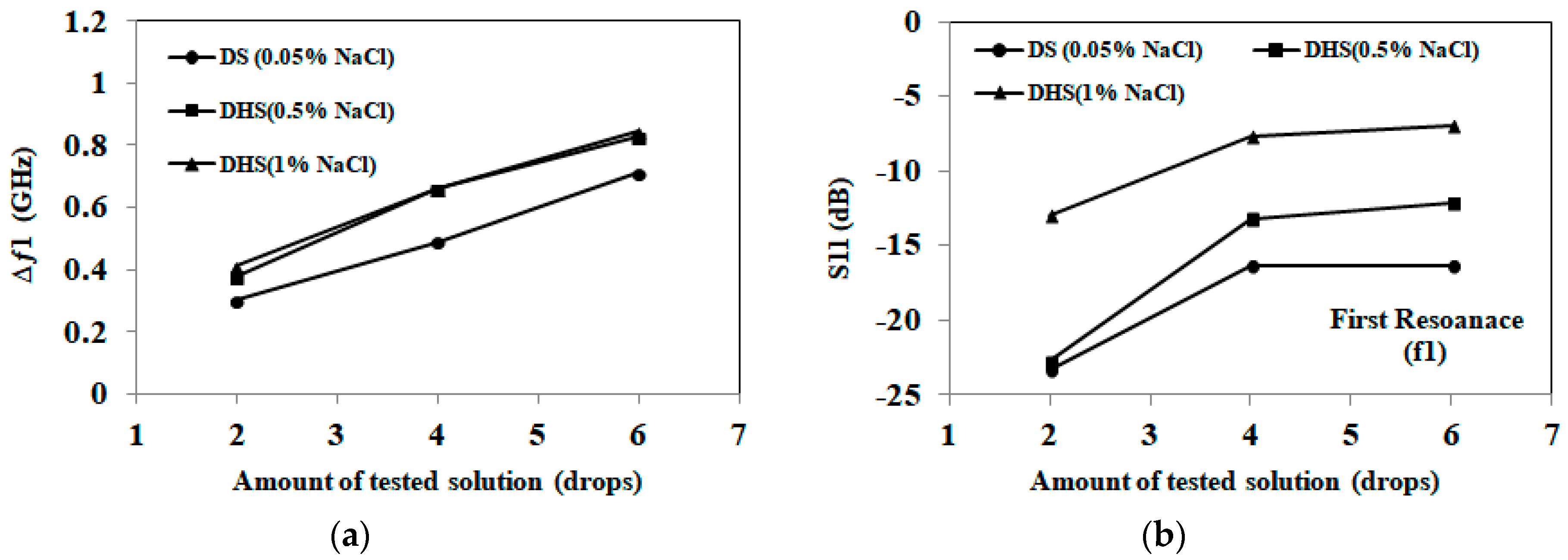
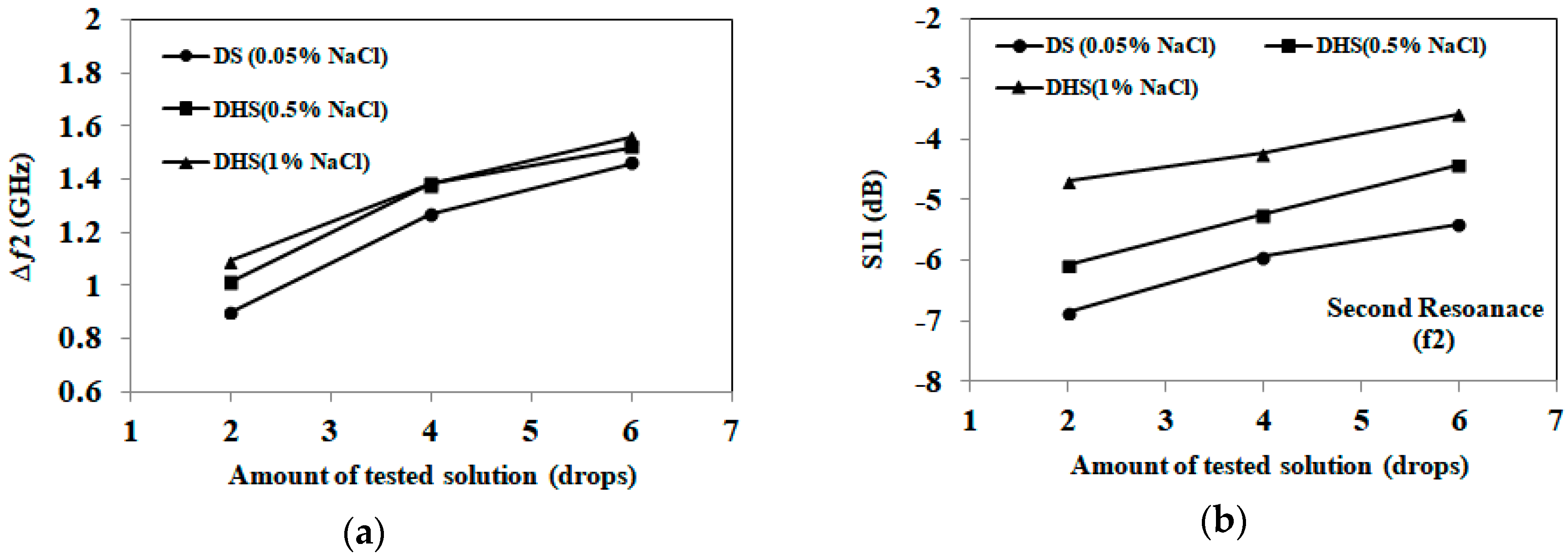
3.1. Detection of Applied Solution Quantity
3.2. Detection of Salt Concentrations
3.3. Case Studies: NaCl Solutions
3.4. Artificial Sweat Trials
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable Flexible Sensors: A Review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef] [Green Version]
- Mosenia, A.; Sur-Kolay, S.; Raghunathan, A.; Jha, N.K. Wearable Medical Sensor-Based System Design: A Survey. IEEE Trans. Multi-Scale Comput. Syst. 2017, 3, 124–138. [Google Scholar] [CrossRef]
- Wearable Sweat Sensor Could Monitor Dehydration, Fatigue. Available online: https://www.medicalnewstoday.com/articles/305751.php (accessed on 8 March 2018).
- Kurdak, S.S.; Shirreffs, S.M.; Maughan, R.J.; Ozgünen, K.T.; Zeren, C.; Korkmaz, S.; Yazici, Z.; Ersöz, G.; Binnet, M.S.; Dvorak, J. Hydration and sweating responses to hot-weather football competition. Scand. J. Med. Sci. Sports 2010, 20, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, J.L.; Bacon, W.E.; Harris, T.; McBean, A.M.; Foley, D.; Phillips, C. The burden and outcomes associated with dehydration among US elderly, 1991. Am. J. Public Health 1994, 84, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Benelam, B.; Wyness, L. Hydration and health: A review. Nutr. Bull. 2010, 35, 3–25. [Google Scholar] [CrossRef]
- Garrett, D.C.; Rae, N.; Fletcher, J.R.; Zarnke, S.; Thorson, S.; Hogan, D.B.; Fear, E.C. Engineering Approaches to Assessing Hydration Status. IEEE Rev. Biomed. Eng. 2018, 11, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.Z.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E.; Council, D. Understanding clinical dehydration and its treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Brendtke, R.; Wiehl, M.; Groeber, F.; Schwarz, T.; Walles, H.; Hansmann, J. Feasibility Study on a Microwave-Based Sensor for Measuring Hydration Level Using Human Skin Models. PLoS ONE 2016, 11, e0153145. [Google Scholar] [CrossRef] [PubMed]
- Daggett, P.; Deanfield, J.; Moss, F.; Reynolds, D. Severe hypernatraemia in adults. Br. Med. J. 1979, 1, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Ruiz, J.; Mas, R.; de Haro, C.; Cabruja, E.; Camero, R.; Alonso-Lomillo, M.A.; Muñoz, F.J. Early determination of cystic fibrosis by electrochemical chloride quantification in sweat. Biosens. Bioelectron. 2009, 24, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H.; Silver, S.M.; Hix, J.K. Treatment of Hyponatremia. In Hyponatremia; Springer: New York, NY, USA, 2013; pp. 221–250. ISBN 978-1-4614-6644-4. [Google Scholar]
- De Guzman, K.; Morrin, A. Screen-printed Tattoo Sensor towards the Non-invasive Assessment of the Skin Barrier. Electroanalysis 2017, 29, 188–196. [Google Scholar] [CrossRef]
- Walsh, N.P.; Laing, S.J.; Oliver, S.J.; Montague, J.C.; Walters, R.; Bilzon, J.L. Saliva parameters as potential indices of hydration status during acute dehydration. Med. Sci. Sports Exerc. 2004, 36, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta Physiol. 2004, 182, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.; Lau, K.-T.; Moyna, N.; O’Gorman, D.; Diamond, D.; Di Francesco, F.; Costanzo, D.; Salvo, P.; Trivella, M.G.; De Rossi, D.E. BIOTEX—Biosensing textiles for personalised healthcare management. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 364–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schazmann, B.; Morris, D.; Slater, C.; Beirne, S.; Fay, C.; Reuveny, R.; Moyna, N.; Diamond, D. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods 2010, 2, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, S.H.; Spilsbury, K.L.; Harvey, J.; Jackson, A.; Huang, J.; Platt, M.; Tok, A.; Nimmo, M.A. The detection and measurement of interleukin-6 in venous and capillary blood samples, and in sweat collected at rest and during exercise. Eur. J. Appl. Physiol. 2014, 114, 1207–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Ho, C.; Slappey, N.; Zhou, Z.; Snelgrove, S.E.; Brown, M.; Grabinski, A.; Guo, X.; Chen, Y.; Miller, K. A wearable conductivity sensor for wireless real-time sweat monitoring. Sens. Actuators B Chem. 2016, 227, 35–42. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.-Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.K.; Kenney, W.L. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. J. Appl. Physiol. 1987, 63, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Kenney, W.L.; Anderson, R.K. Responses of older and younger women to exercise in dry and humid heat without fluid replacement. Med. Sci. Sports Exerc. 1988, 20, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tankersley, C.G.; Smolander, J.; Kenney, W.L.; Fortney, S.M. Sweating and skin blood flow during exercise: Effects of age and maximal oxygen uptake. J. Appl. Physiol. 1991, 71, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Nakao, M.; Okudaira, S.; Ueda, H.; Araki, T. Seasonal variation in sweating responses of older and younger men. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Inbar, O.; Morris, N.; Epstein, Y.; Gass, G. Comparison of thermoregulatory responses to exercise in dry heat among prepubertal boys, young adults and older males. Exp. Physiol. 2004, 89, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, N.A.; Machado-Moreira, C.A. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem. Physiol. Med. 2013, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Brueck, A.; Iftekhar, T.; Stannard, A.B.; Yelamarthi, K.; Kaya, T. A Real-Time Wireless Sweat Rate Measurement System for Physical Activity Monitoring. Sensors 2018, 18, 533. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Hattori, K.; Ota, Y.; Kanai, T.; Shimizu, M.; Kobayashi, H.; Tokuda, Y. The measurement of axillary moisture for the assessment of dehydration among older patients: A pilot study. Exp. Gerontol. 2013, 48, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, A.; Tsiami, A.; Greene, C. Methods of Assessment of Hydration Status and their Usefulness in Detecting Dehydration in the Elderly. Curr. Res. Nutr. Food Sci. J. 2017, 5, 43–54. [Google Scholar] [CrossRef]
- Huang, J.; Harvey, J.; Fam, W.H.D.; Nimmo, M.A.; Tok, I.Y.A. Novel Biosensor for InterLeukin-6 Detection. Procedia Eng. 2013, 60, 195–200. [Google Scholar] [CrossRef]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.S.; Heled, Y.; Margaliot, M.; Shani, Y.; Laor, A.; Margaliot, S.; Bickels, E.E.; Shapiro, Y. Hydration status measurement by radio frequency absorptiometry in young athletes—A new method and preliminary results. Physiol. Meas. 2004, 25, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, I.; Serallés, J.; Mendoza, C.S.; Giancardo, L.; Daniel, L. A wearable physiological hydration monitoring wristband through multi-path non-contact dielectric spectroscopy in the microwave range. In Proceedings of the 2015 IEEE MTT-S 2015 International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-BIO), Taipei, Taiwan, 21–23 September 2015; pp. 60–61. [Google Scholar]
- Trenz, F.; Weigel, R.; Kissinger, D. Evaluation of a reflection based dehydration sensing method for wristwatch integration. In Proceedings of the 2016 21st International Conference on Microwave, Radar and Wireless Communications (MIKON), Krakow, Poland, 9–11 May 2016; pp. 1–3. [Google Scholar]
- Khaleel, H.R.; Al-Rizzo, H.M.; Abbosh, A.I. Design, Fabrication, and Testing of Flexible Antennas; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Raad, H.K.; Al-Rizzo, H.M.; Abbosh, A.I.; Hammoodi, A.I. A compact dual band polyimide based antenna for wearable and flexible telemedicine devices. Progress Electromagn. Res. C 2016, 63, 153–161. [Google Scholar] [CrossRef]
- Hassan, A.; Ali, S.; Hassan, G.; Bae, J.; Lee, C.H. Inkjet-printed antenna on thin PET substrate for dual band Wi-Fi communications. Microsyst. Technol. 2017, 23, 3701–3709. [Google Scholar] [CrossRef]
- Nguyen, H.-D.; Coupez, J.P.; Castel, V.; Person, C.; Delattre, A.; Crowther-Alwyn, L.; Borel, P. RF characterization of flexible substrates for new conformable antenna systems. In Proceedings of the 2016 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–5. [Google Scholar]
- Anagnostou, D.E. Organic Paper-Based Antennas; WIT Press: Billerica, MA, USA, 2014. [Google Scholar]
- Killard, A.J. Disposable sensors. Curr. Opin. Electrochem. 2017. [Google Scholar] [CrossRef]
- Moscato, S.; Moro, R.; Pasian, M.; Bozzi, M.; Perregrini, L. Innovative manufacturing approach for paper-based substrate integrated waveguide components and antennas. IET Microw. Antennas Propag. 2016, 10, 256–263. [Google Scholar] [CrossRef]
- Palazzi, V.; Mezzanotte, P.; Roselli, L. Design of a novel antenna system intended for harmonic RFID tags in paper substrate. In Proceedings of the 2015 IEEE Wireless Power Transfer Conference (WPTC), Boulder, CO, USA, 13–15 May 2015; pp. 1–4. [Google Scholar]
- Alimenti, F.; Mezzanotte, P.; Dionigi, M.; Virili, M.; Roselli, L. Microwave circuits in paper substrates exploiting conductive adhesive tapes. IEEE Microw. Wirel. Compon. Lett. 2012, 22, 660–662. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callewaert, C.; Buysschaert, B.; Vossen, E.; Fievez, V.; Van de Wiele, T.; Boon, N. Artificial sweat composition to grow and sustain a mixed human axillary microbiome. J. Microbiol. Methods 2014, 103, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Midander, K.; Julander, A.; Kettelarij, J.; Lidén, C. Testing in artificial sweat—Is less more? Comparison of metal release in two different artificial sweat solutions. Regul. Toxicol. Pharmacol. 2016, 81, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Alomari, M.; Sahin, B.; Snelgrove, S.E.; Edwards, J.; Mellinger, A.; Kaya, T. Real-time sweat analysis via alternating current conductivity of artificial and human sweat. Appl. Phys. Lett. 2015, 106, 133702. [Google Scholar] [CrossRef]
- Hoekstra, R.; Blondeau, P.; Andrade, F.J. IonSens: A Wearable Potentiometric Sensor Patch for Monitoring Total Ion Content in Sweat. Electroanalysis 2018, 30, 1536–1544. [Google Scholar] [CrossRef]
- Buchner, R.; Hefter, G.T.; May, P.M. Dielectric Relaxation of Aqueous NaCl Solutions. J. Phys. Chem. A 1999, 103, 1–9. [Google Scholar] [CrossRef]
- Chen, T.; Hefter, G.; Buchner, R. Dielectric Spectroscopy of Aqueous Solutions of KCl and CsCl. J. Phys. Chem. A 2003, 107, 4025–4031. [Google Scholar] [CrossRef]
- Gulich, R.; Köhler, M.; Lunkenheimer, P.; Loidl, A. Dielectric spectroscopy on aqueous electrolytic solutions. Radiat. Environ. Biophys. 2009, 48, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.N. Dielectric properties of human sweat fluid in the microwave range. Biophysics 2010, 55, 473–476. [Google Scholar] [CrossRef]
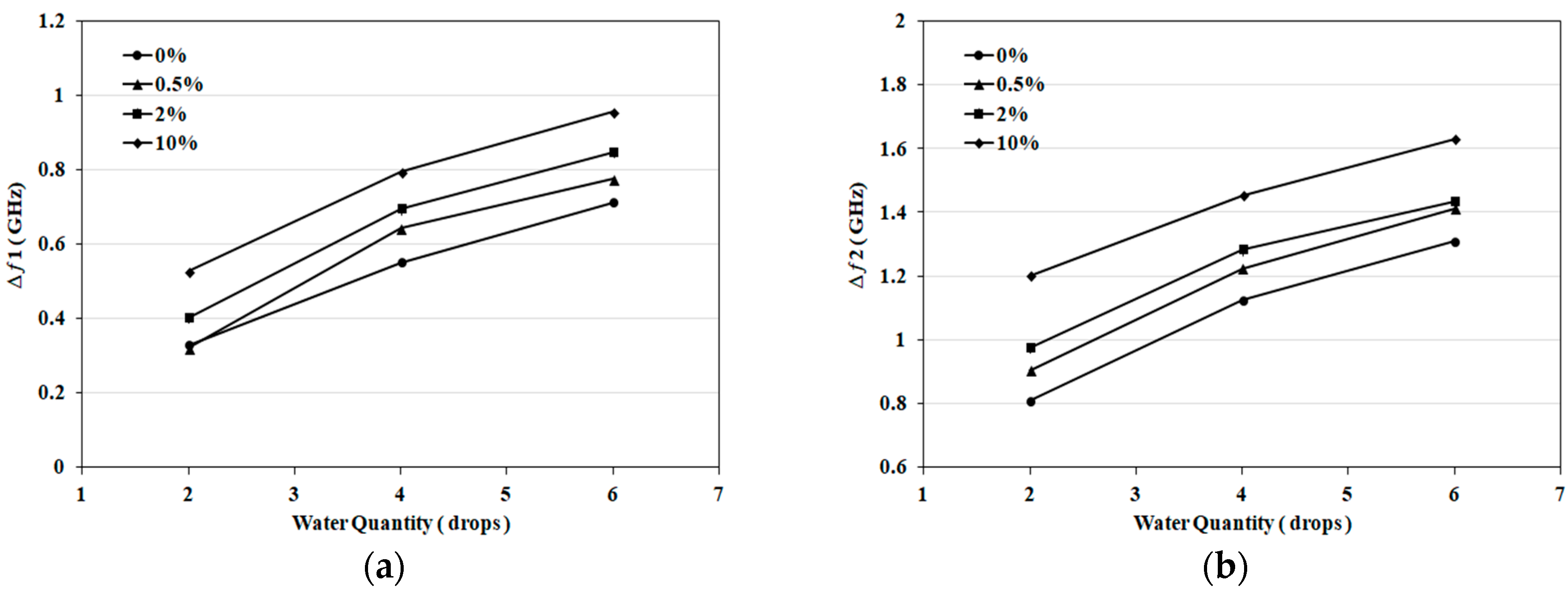
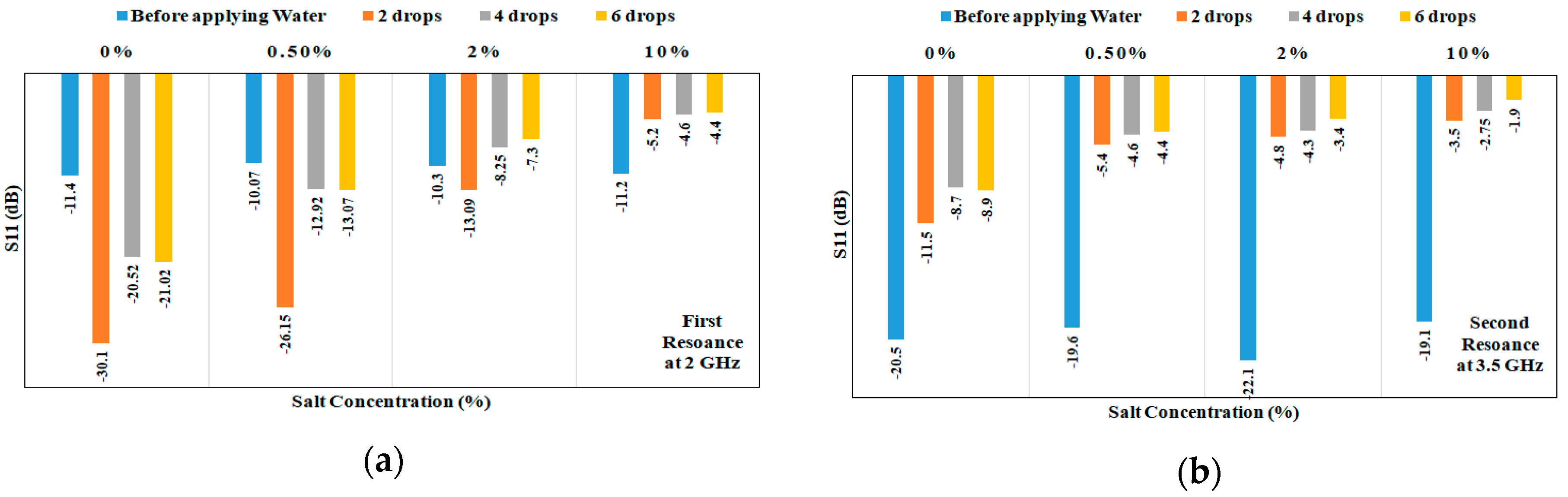
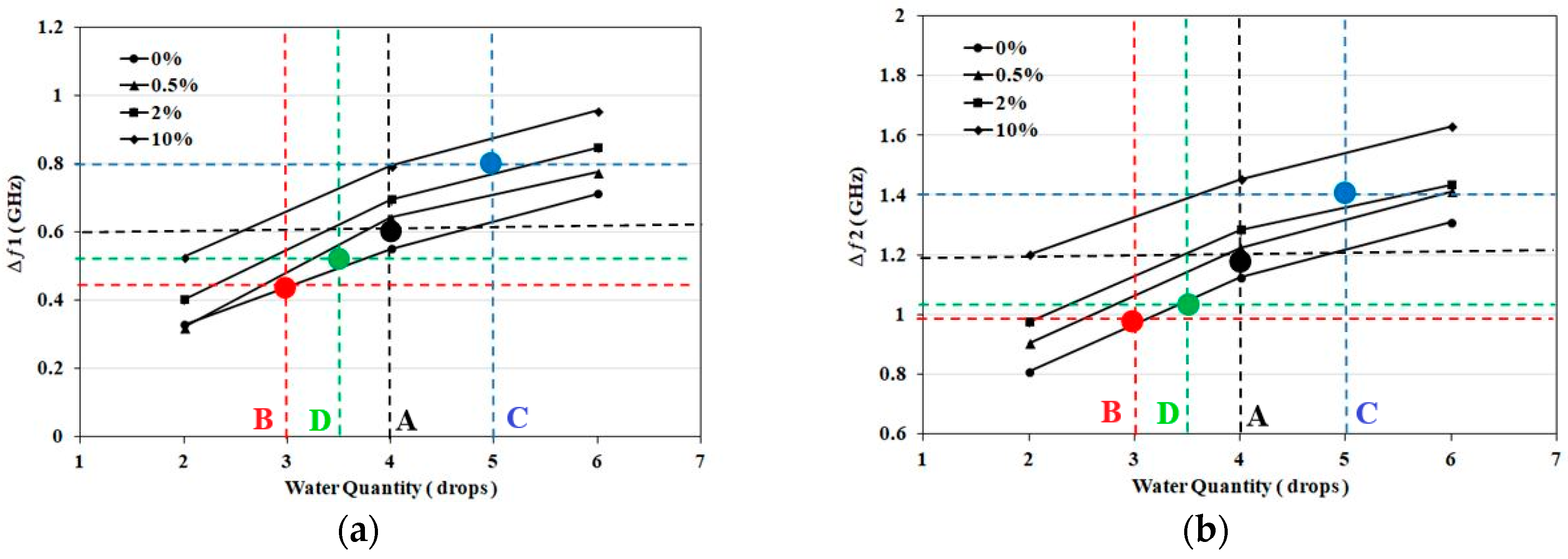

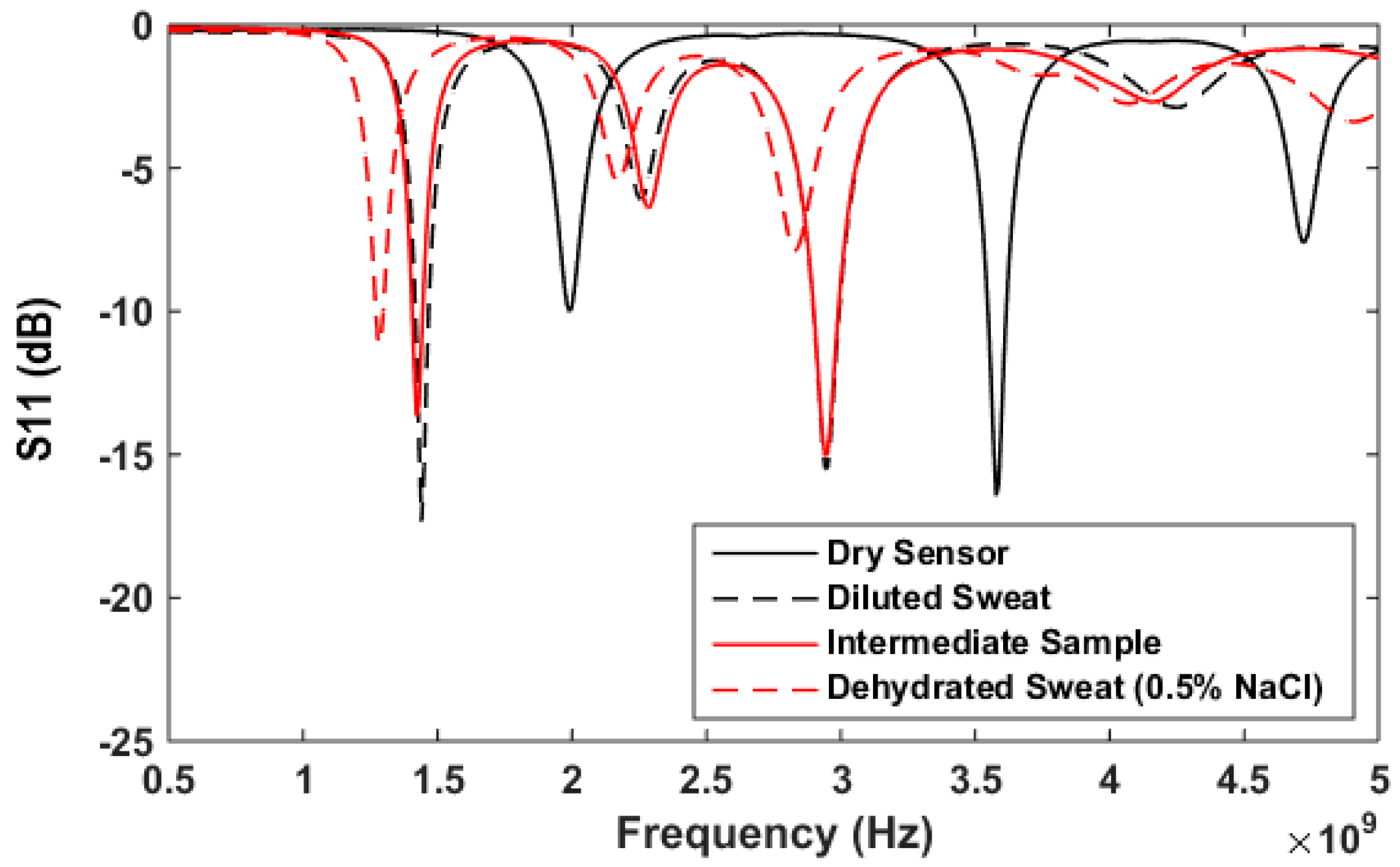
| Samples | First Band (GHZ) | Second Band (GHZ) | Detection Decision |
|---|---|---|---|
| Sample. A (4 drops, 0.5%) | Δƒ1=0.58 | Δƒ2=1.14 | Navg=4 (0–0.5%) |
| Sample. B (2 drops, 0%) | Δƒ1=0.44 | Δƒ2=0.97 | Navg=3 (0–0.5%) |
| Sample. C (4 drops, 10%) | Δƒ1=0.8 | Δƒ2=1.4 | Navg=5 (2–10%) |
| Sample. D (3 drops, 0%) | Δƒ1=0.51 | Δƒ2=1.03 | Navg=3.5 (0–0.5%) |
| Sample. E (6 drops, 2%) | Δƒ1=0.92 | Δƒ2=1.43 | Navg=7 (2–10%) |
| Sample. F (5 drops, 2%) | Δƒ1=0.8 | Δƒ2=1.39 | Navg= 5 (2–10%) |
| Sample. G (6 drops, 0%) | Δƒ1=0.72 | Δƒ2=1.33 | Navg=6 (0–0.5%) |
| Sample. H (3 drops, 0.5%) | Δƒ1=0.47 | Δƒ2=1.14 | Navg=3.5 (0–0.5%) |
| Sample. I (5 drops, 10%) | Δƒ1=0.9 | Δƒ2=1.51 | Navg=6 (2–10%) |
| Sample. J (4 drops, 2%) | Δƒ1=0.62 | Δƒ2=1.2 | Navg=4 (0.5–2%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldamak, A.R.; Fear, E.C. Conformal and Disposable Antenna-Based Sensor for Non-Invasive Sweat Monitoring. Sensors 2018, 18, 4088. https://doi.org/10.3390/s18124088
Eldamak AR, Fear EC. Conformal and Disposable Antenna-Based Sensor for Non-Invasive Sweat Monitoring. Sensors. 2018; 18(12):4088. https://doi.org/10.3390/s18124088
Chicago/Turabian StyleEldamak, Angie R., and Elise C. Fear. 2018. "Conformal and Disposable Antenna-Based Sensor for Non-Invasive Sweat Monitoring" Sensors 18, no. 12: 4088. https://doi.org/10.3390/s18124088
APA StyleEldamak, A. R., & Fear, E. C. (2018). Conformal and Disposable Antenna-Based Sensor for Non-Invasive Sweat Monitoring. Sensors, 18(12), 4088. https://doi.org/10.3390/s18124088






