Insight into the Mechanism of CO Oxidation on WO3(001) Surfaces for Gas Sensing: A DFT Study
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
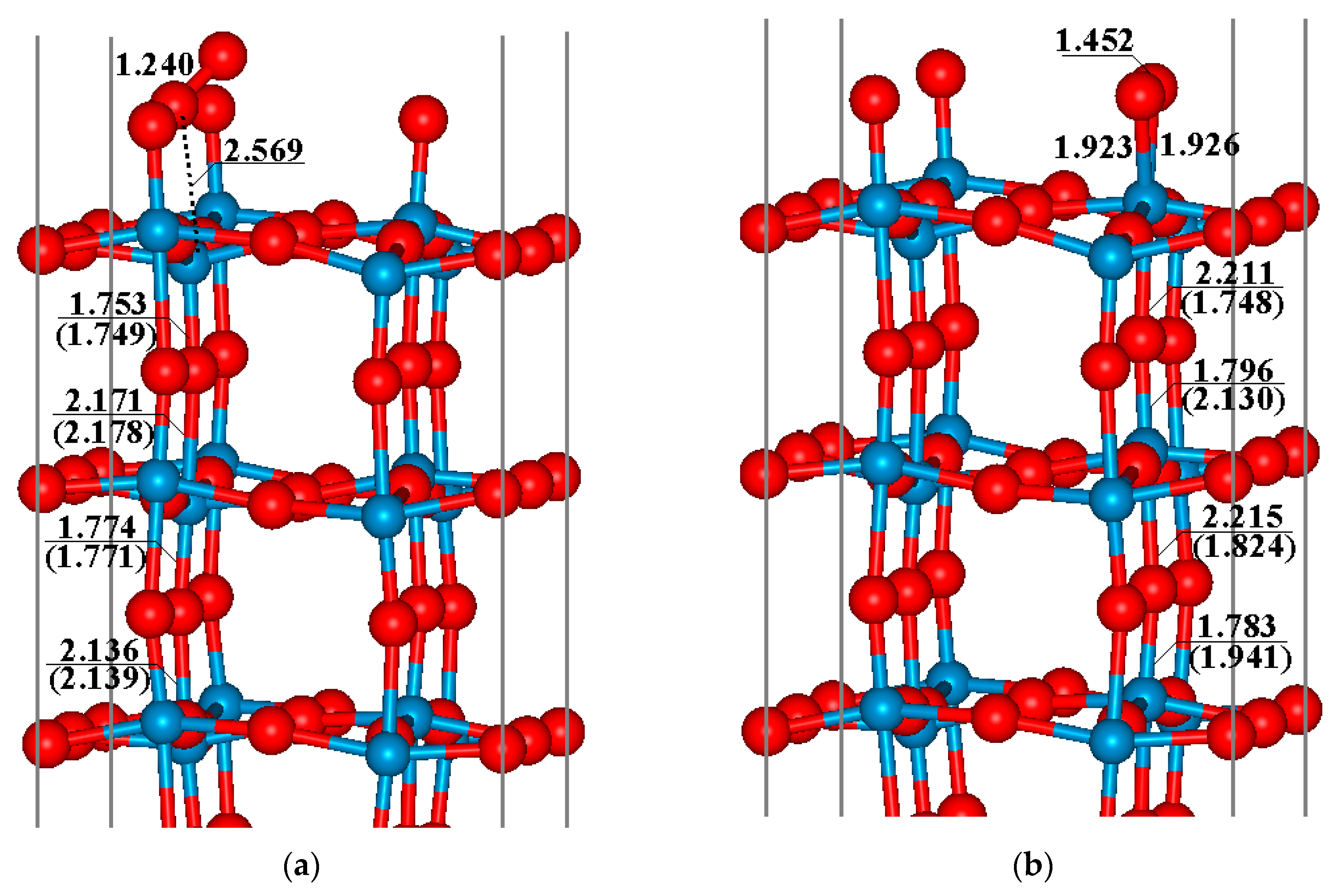
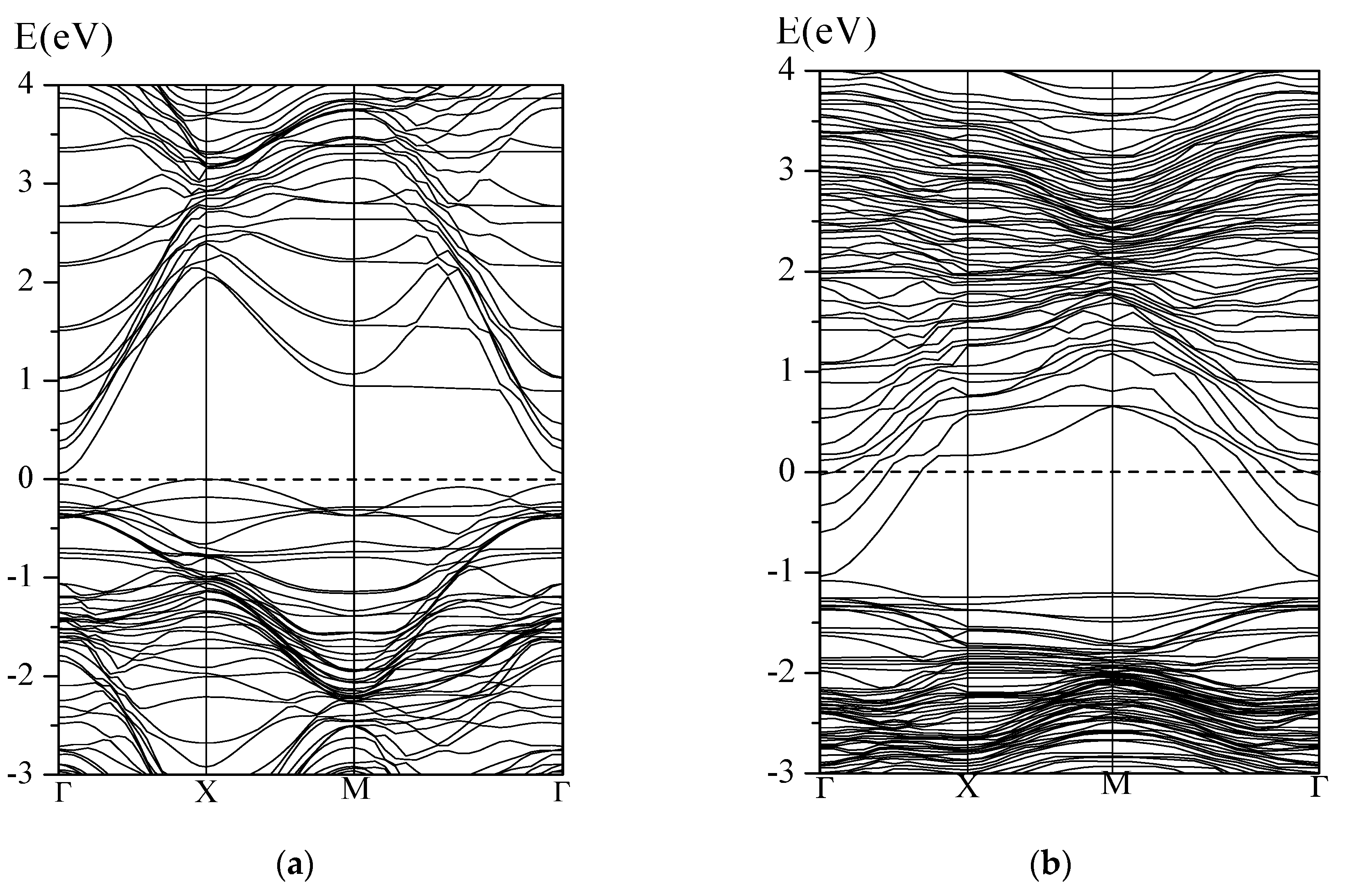
3.1. Surface Oxygen Species on WO3(001) Surface
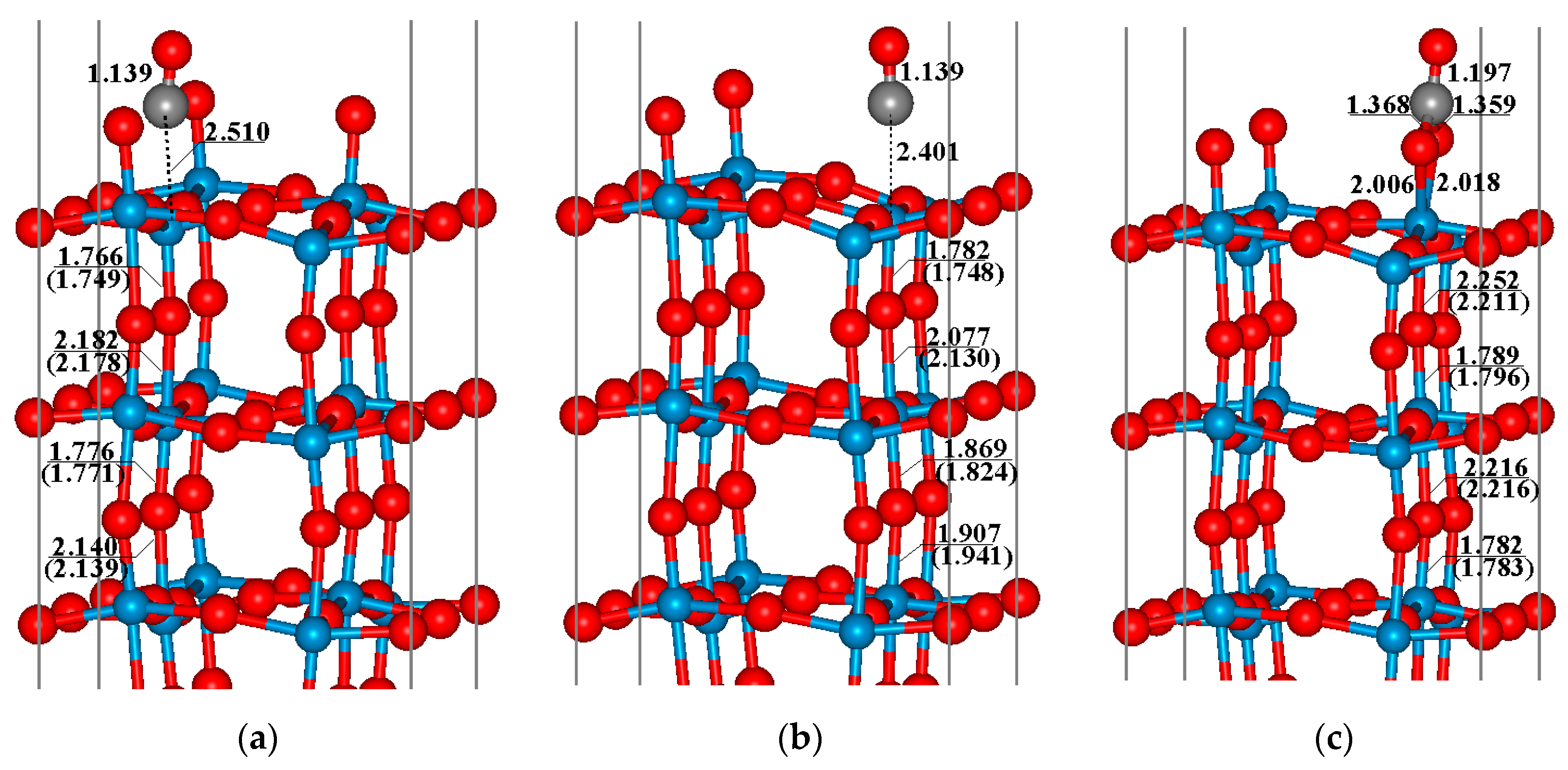
3.2. Adsorption of CO on WO3(001) Surfaces
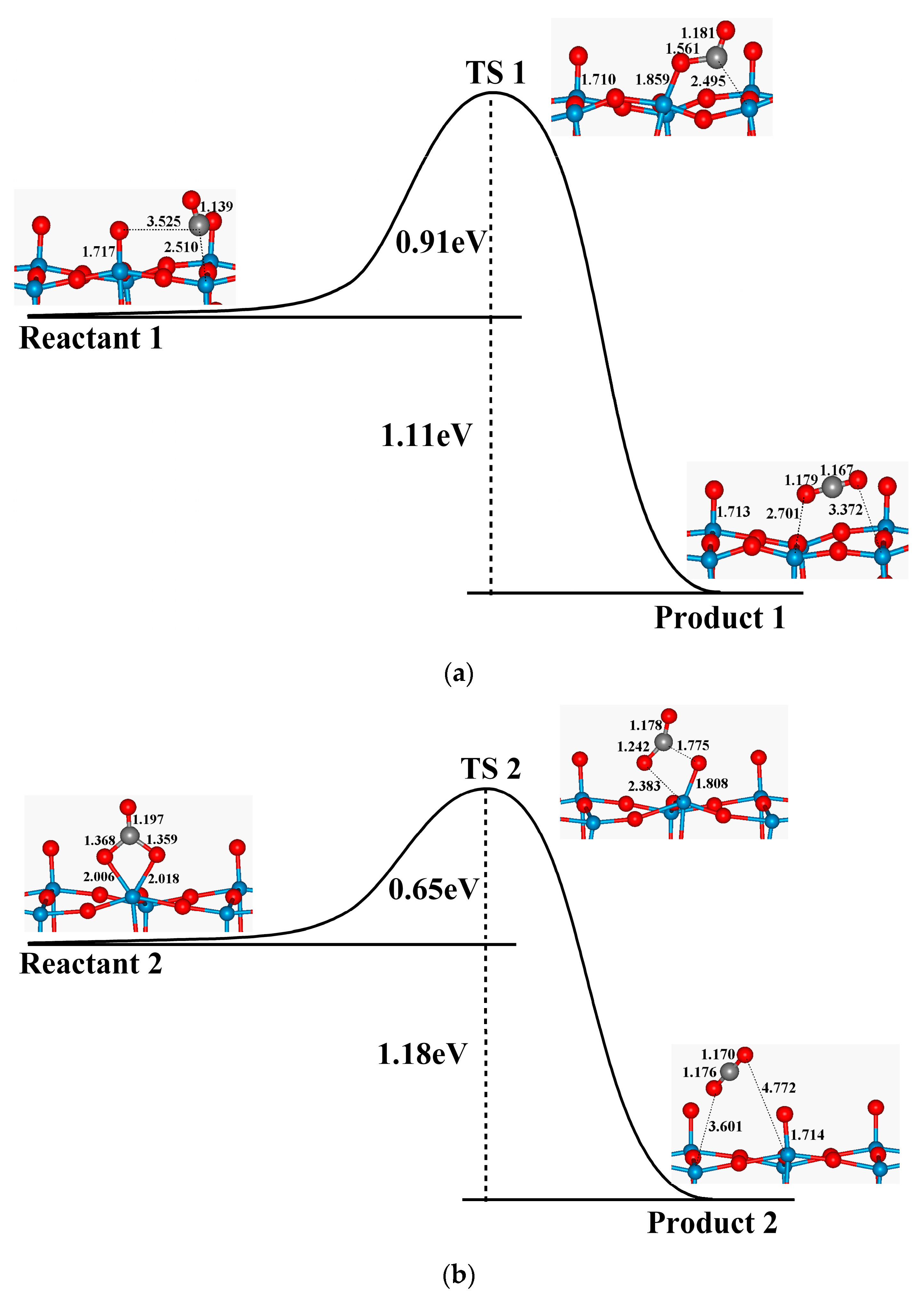
3.3. Oxidation of CO on WO3(001) Surfaces
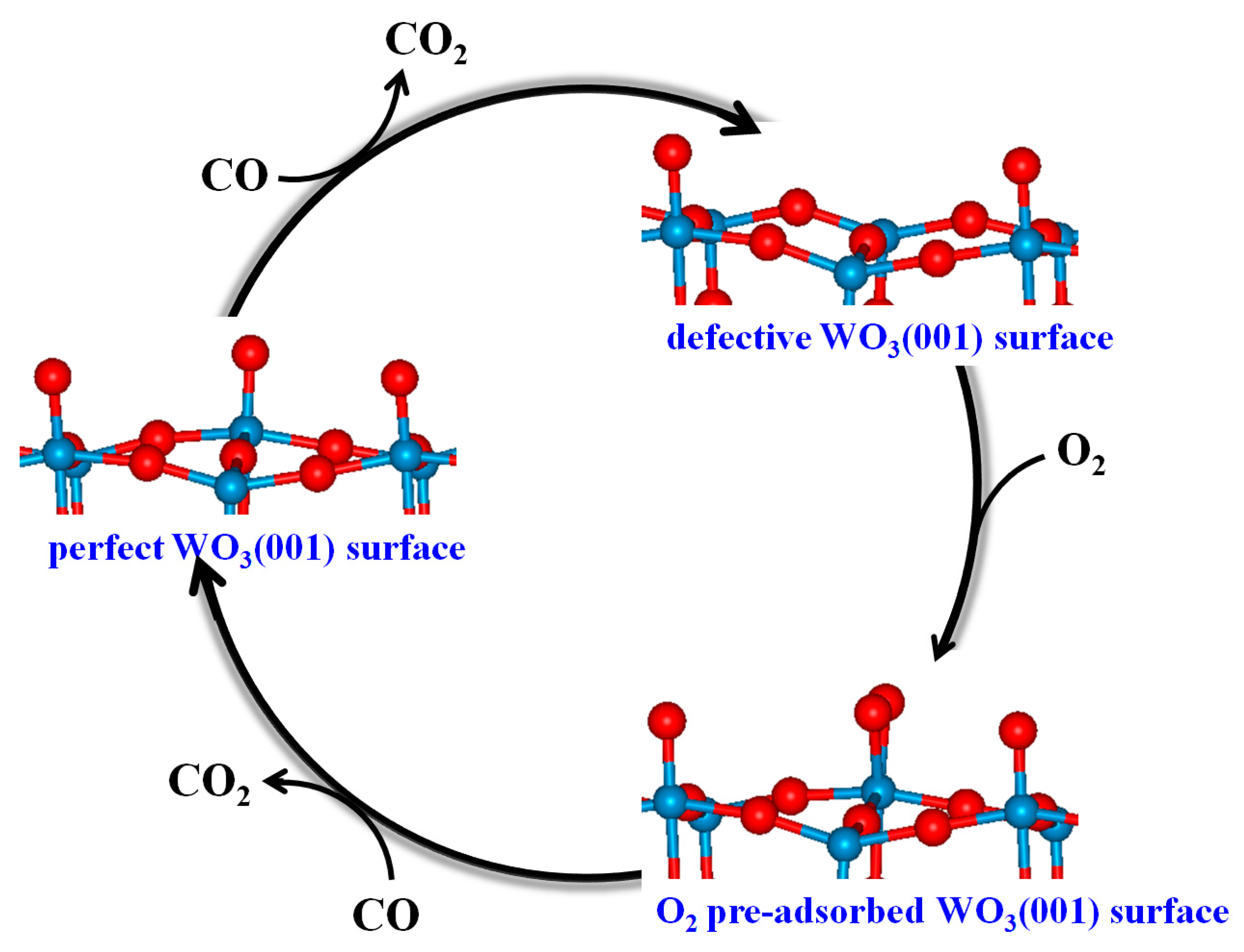
3.4. Mechanism of CO Oxidation on WO3(001) Surfaces
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Qin, Y.X.; Liu, M.; Ye, Z.H. A DFT study on WO3 nanowires with different orientations for NO2 sensing application. J. Mol. Struct. 2014, 1076, 546–553. [Google Scholar] [CrossRef]
- Qin, Y.X.; Hua, D.Y.; Liu, M. First-principles study on NO2-adsorbed tungsten oxide nanowires for sensing application. J. Alloys Compd. 2014, 587, 227–233. [Google Scholar] [CrossRef]
- Saadi, L.; Mauriat, C.L.; Oison, V.; Ouali, H.; Hayn, R. Mechanism of NOx sensing on WO3 surface: First principle calculations. Appl. Surf. Sci. 2014, 293, 76–79. [Google Scholar] [CrossRef]
- Maity, A.; Majumder, S.B. NO2 sensing and selectivity characteristics of tungsten oxide thin films. Sens. Actuators B 2015, 206, 423–429. [Google Scholar] [CrossRef]
- Qin, Y.X.; Ye, Z.H. DFT study on interaction of NO2 with the vacancy-defected WO3 nanowires for gas-sensing. Sens. Actuators B 2016, 222, 499–507. [Google Scholar] [CrossRef]
- Daté, M.; Okumura, M.; Tsubota, S.; Haruta, M. Vital role of moisture in the catalytic activity of supported gold nanoparticles. Angew. Chem. 2004, 43, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Kimble, M.L.; Castleman, A.W.; Mitrić, R.; Bürgel, C.; Koutecký, V.B. Reactivity of atomic gold anions toward oxygen and the oxidation of CO: Experiment and theory. J. Am. Chem. Soc. 2004, 126, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.Q.; Li, D.; Dai, Y.; Hu, Y.H.; Zhang, Y.H.; Liu, L.J.; Zhao, B.; Liu, B.; Sun, K.Q.; Dong, L.; et al. Effect of CO pretreatment on the performance of CuO/CeO2/γ-Al2O3 catalysts in CO + O2 reactions. Appl. Catal. 2009, 360, 26–32. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Pala, R.G.S.; Shapovalov, V.; Metiu, H. CO Oxidation by rutile TiO2(110) doped with V, W, Cr, Mo and Mn. J. Phys. Chem. C 2008, 112, 12398–12408. [Google Scholar] [CrossRef]
- Wang, H.F.; Gong, X.Q.; Guo, Y.L.; Guo, Y.; Lu, G.Z.; Hu, P. Structure and catalytic activity of gold in low-temperature CO oxidation. J. Phys. Chem. C 2009, 113, 6124–6131. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.J.; Xu, X.H.; Ding, Y. Theoretical study of the CO oxidation mediated by Au3+, Au3, and Au3−: Mechanism and charge state effect of gold on its catalytic activity. J. Phys. Chem. C 2009, 113, 18032–18039. [Google Scholar] [CrossRef]
- Farkas, A.; Mellau, G.C.; Over, H. Novel insight in the CO oxidation on RuO2(110) by in situ reflection-absorption infrared spectroscopy. J. Phys. Chem. C 2009, 113, 14341–14355. [Google Scholar] [CrossRef]
- Long, H.W.; Zeng, W.; Zhang, H. Synthesis of WO3 and its gas sensing: A review. J. Mater. Sci. Mater. Electron. 2015, 26, 4698–4707. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. Mater. Electron. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Azad, A.M.; Hammoud, M. Fine-tuning of ceramic-based chemical sensors via novel microstructural modification I: Low level CO sensing by tungsten oxide, WO3. Sens. Actuators B 2006, 119, 384–391. [Google Scholar] [CrossRef]
- Wu, R.J.; Chang, W.C.; Tsai, K.M.; Wu, J.G. The novel CO sensing material CoOOH–WO3 with Au and SWCNT performance enhancement. Sens. Actuators B Chem. 2009, 138, 35–41. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Haensch, A.; Barsan, N.; Weimar, U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B Chem. 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Ahsan, M.; Tefamichael, T.; Ionescu, M.; Bell, J.; Motta, N. Low temperature CO sensitive nanostructured WO3 thin films doped with Fe. Sens. Actuators B Chem. 2012, 162, 14–21. [Google Scholar] [CrossRef]
- Zappa, D.; Bertuna, A.; Comini, E.; Molinari, M.; Poli, N.; Sberveglier, G. Tungsten oxide nanowires for chemical detection. Anal. Methods 2015, 7, 2203–2209. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. DFT investigation on CO sensing characteristics of hexagonal and orthorhombic WO3 nanostructures. Superlattices Microstruct. 2015, 78, 22–39. [Google Scholar] [CrossRef]
- Oison, V.; Saadi, L.; Mauriat, C.L.; Hayn, R. Mechanism of CO and O3 sensing on WO3 surfaces: First principle study. Sens. Actuators B Chem. 2011, 160, 505–510. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, F.H.; Wang, X.; Zhao, W.; Fu, A.; Shen, Y.; Chen, S.; Yu, S. Mechanism of CO adsorption on hexagonal WO3(001) surface for gas sensing: A DFT study. Comput. Mater. Sci. 2013, 79, 691–697. [Google Scholar] [CrossRef]
- Tian, F.H.; Zhao, L.H.; Xue, X.Y.; Shen, Y.Y.; Jia, X.F.; Chen, S.G.; Wang, Z.H. DFT study of CO sensing mechanism on hexagonal WO3(001) surface: The role of oxygen vacancy. Appl. Surf. Sci. 2014, 311, 362–368. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamic simulation of the liquid-metal-amorphous semiconductor transition germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R. Atoms, molecules, solids and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, J.; Chen, W.J.; Fang, Z.X.; Li, Y.; Zhang, Y.F.; Huang, X.; Ding, K.N.; Ning, L.X.; Chen, W.K. Enhanced oxidation reactivity of WO3(001) surface through the formation of oxygen radical centers. J. Phys. Chem. C 2012, 116, 5067–5075. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Zhou, H.; Zhang, Y. Insight into the Mechanism of CO Oxidation on WO3(001) Surfaces for Gas Sensing: A DFT Study. Sensors 2017, 17, 1898. https://doi.org/10.3390/s17081898
Jin H, Zhou H, Zhang Y. Insight into the Mechanism of CO Oxidation on WO3(001) Surfaces for Gas Sensing: A DFT Study. Sensors. 2017; 17(8):1898. https://doi.org/10.3390/s17081898
Chicago/Turabian StyleJin, Hua, Hegen Zhou, and Yongfan Zhang. 2017. "Insight into the Mechanism of CO Oxidation on WO3(001) Surfaces for Gas Sensing: A DFT Study" Sensors 17, no. 8: 1898. https://doi.org/10.3390/s17081898
APA StyleJin, H., Zhou, H., & Zhang, Y. (2017). Insight into the Mechanism of CO Oxidation on WO3(001) Surfaces for Gas Sensing: A DFT Study. Sensors, 17(8), 1898. https://doi.org/10.3390/s17081898



