Non-Destructive Analysis of the Internal Anatomical Structures of Mosquito Specimens Using Optical Coherence Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Species Identification
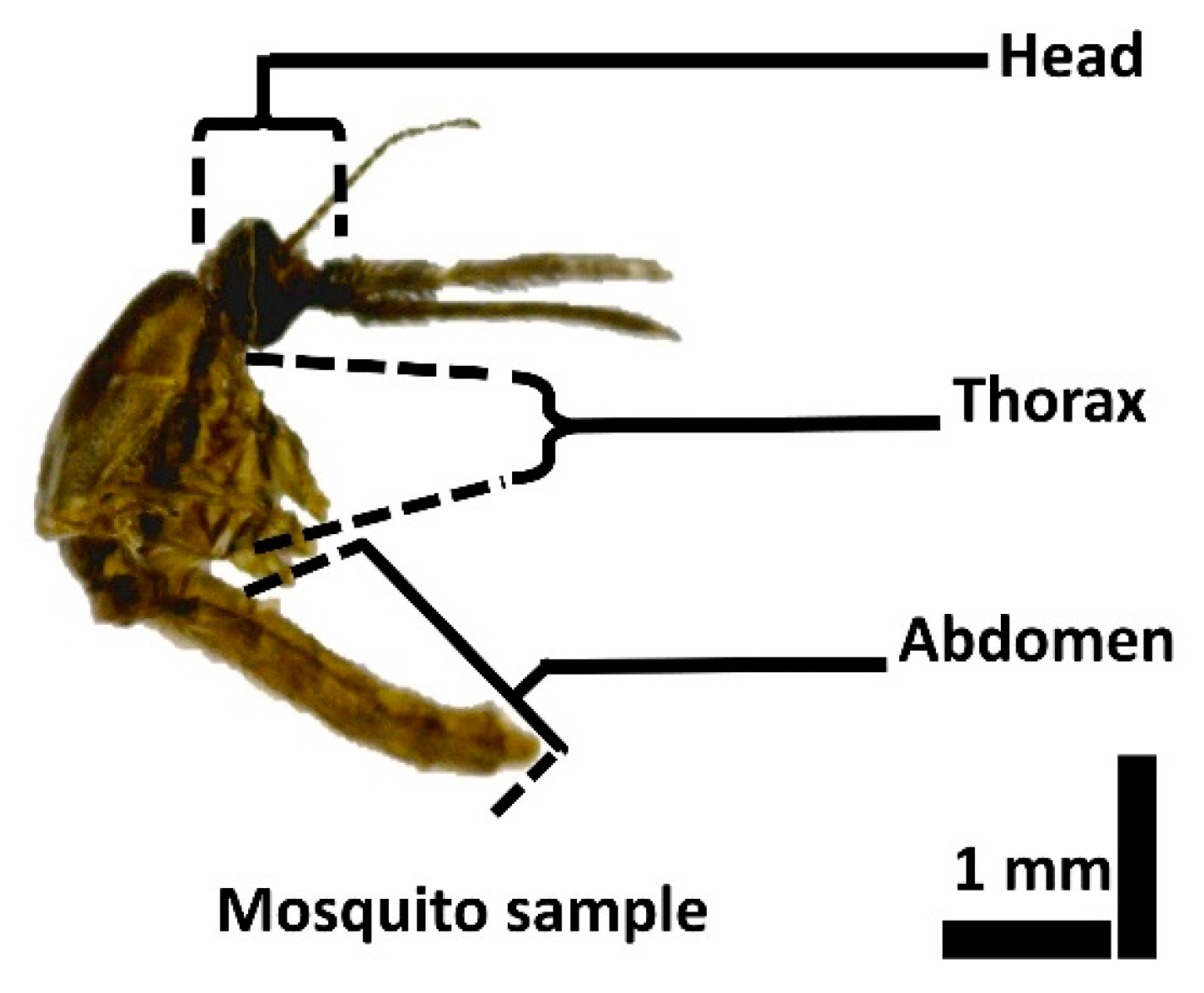
2.2. Mosquito Specimen Preparation for OCT Imaging
2.3. Specimen Preparation for Histological Analysis
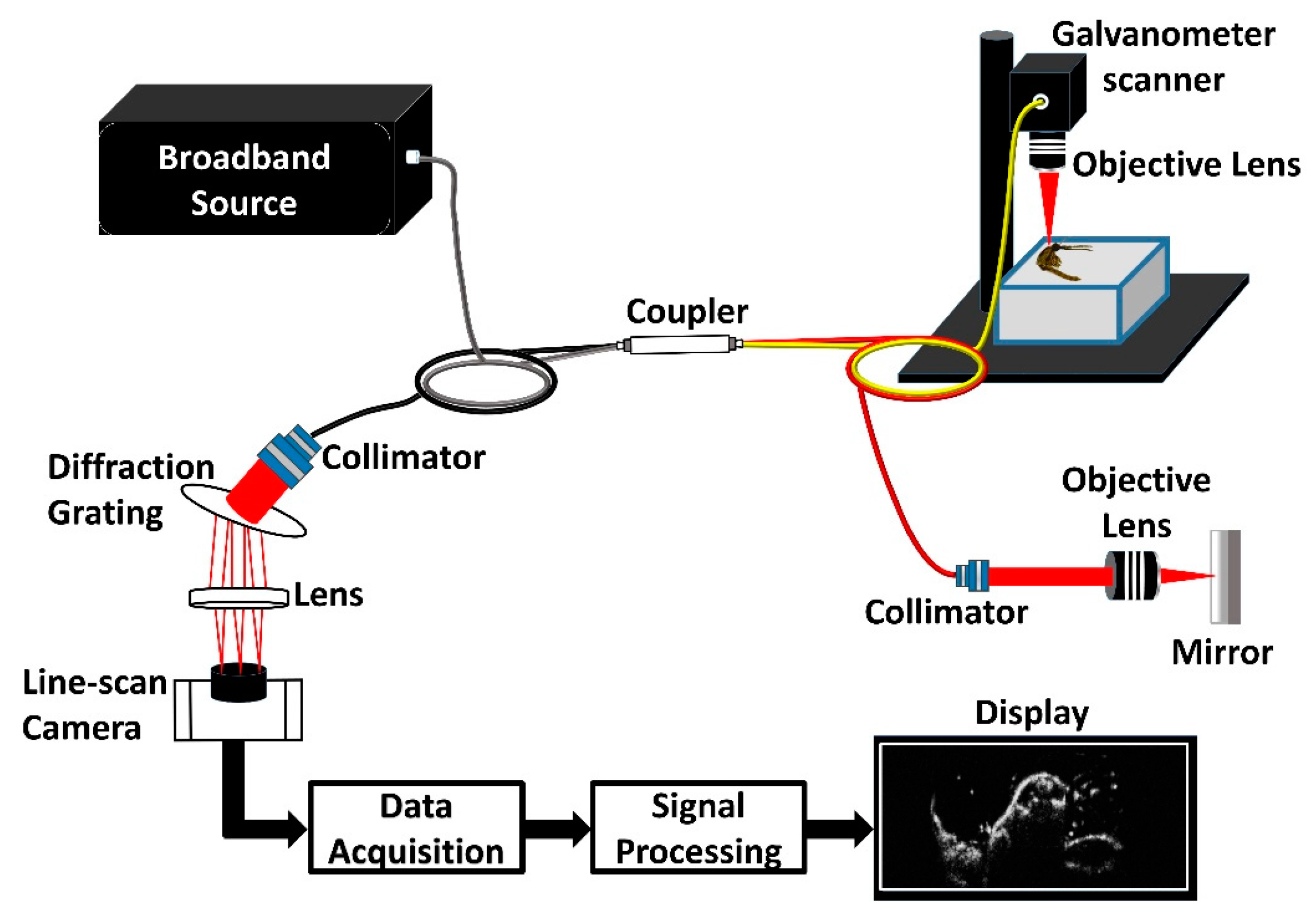
2.4. SD-OCT System Setup
3. Results and Discussion
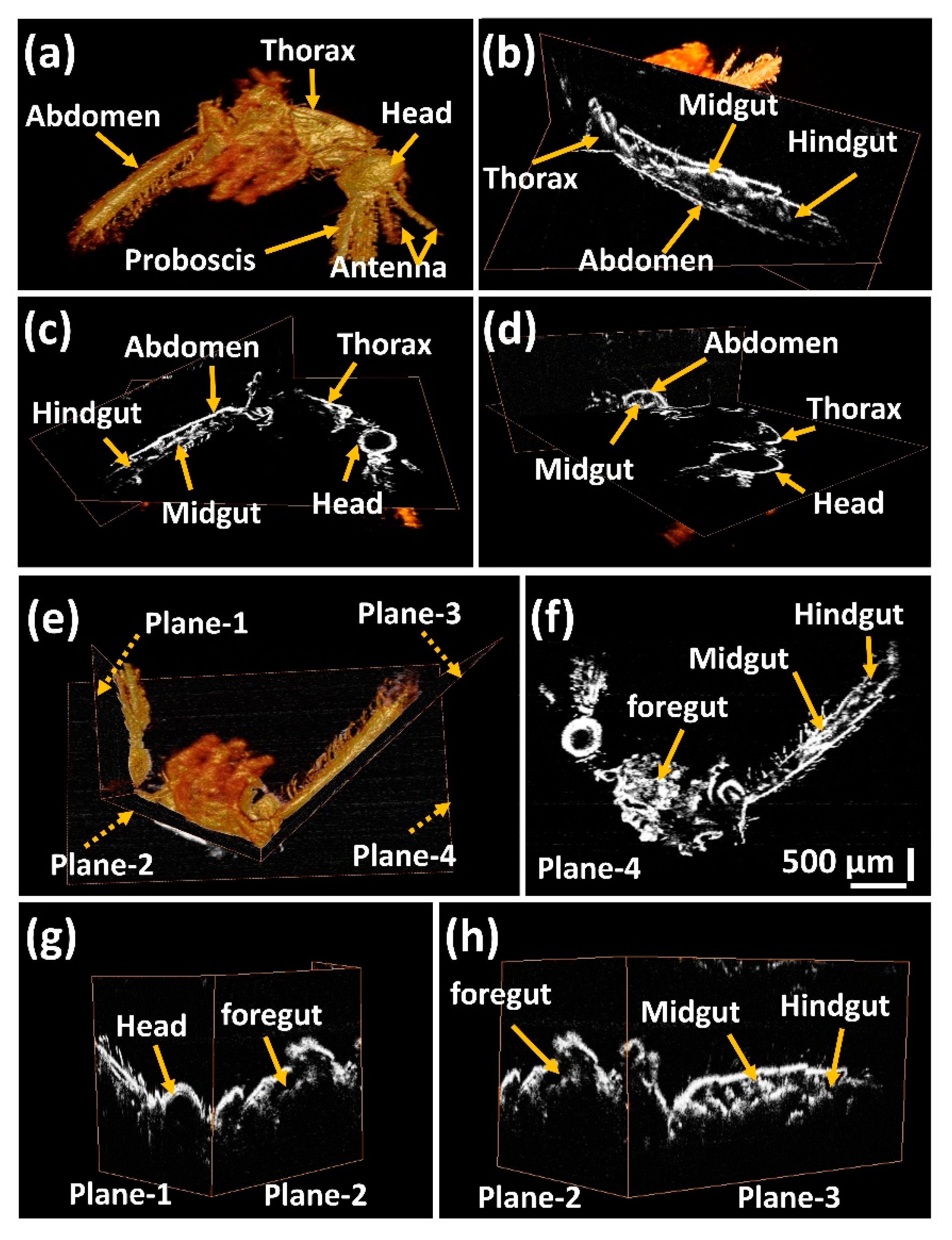
3.1. Volumetric 3D OCT Image Analysis of Mosquito Specimens
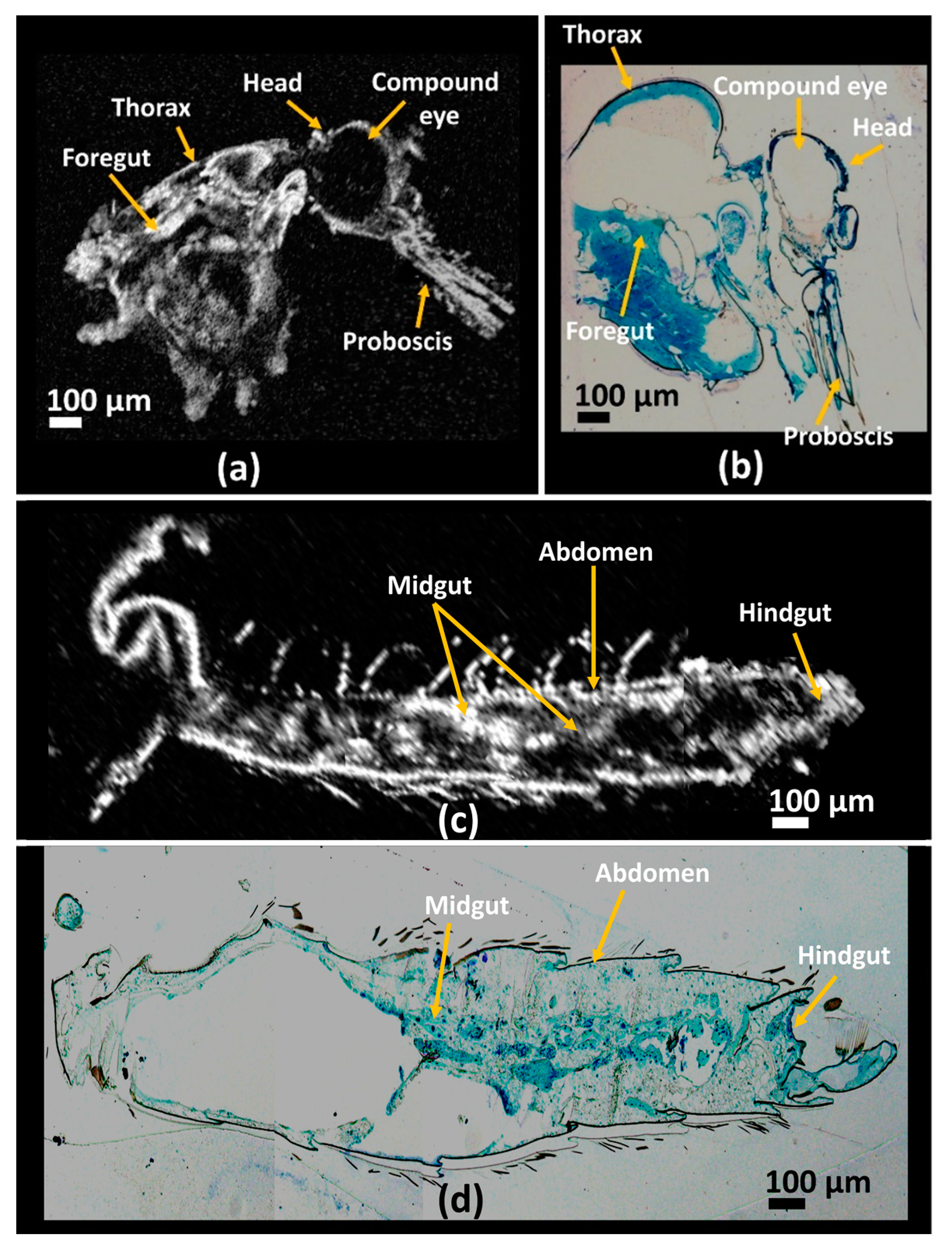
3.2. Histological Image Comparison Using OCT Images of Mosquito Specimens
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tolle, M.A. Mosquito-borne diseases. Curr. Probl. Pediatric Adolesc. Health Care 2009, 39, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.A.; Gikandi, P.W.; Tatem, A.J.; Noor, A.M.; Smith, D.L.; Hay, S.I.; Snow, R.W. The limits and intensity of Plasmodium falciparum transmission: Implications for malaria control and elimination worldwide. PLoS Med. 2008, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, D.; Montero, F. The Zika Virus and Pregnancy. Curr. Obstet. Gynecol. Rep. 2016, 3, 234–238. [Google Scholar] [CrossRef]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Apperson, C.S.; Harrison, B.A.; Unnasch, T.R.; Hassan, H.K.; Irby, W.S.; Savage, H.M.; Aspen, S.E.; Watson, D.W.; Rueda, L.M.; Engber, B.R. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J. Med. Entomol. 2002, 39, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Boorman, J. Observations on the feeding habits of the mosquito Aedes (Stegomyia) aegypti (Linnaeus): The loss of fluid after a blood-meal and the amount of blood taken during feeding. Ann. Trop. Med. Parasitol. 1960, 54, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Saig, E.; Turley, A.P.; Ribeiro, J.M.; O’Neill, S.L.; McGraw, E.A. Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef] [PubMed]
- Platt, K.B.; Linthicum, K.J.; Myint, K.S.; Innis, B.L.; Lerdthusnee, K.; Vaughn, D.W. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am. J. Trop. Med. Hyg. 1997, 57, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Crans, W.J. A classification system for mosquito life cycles: Life cycle types for mosquitoes of the northeastern United States. J. Vector Ecol. 2004, 29, 1–10. [Google Scholar] [PubMed]
- Ha, Y.-R.; Lee, S.-C.; Seo, S.-J.; Ryu, J.; Lee, D.-K.; Lee, S.-J. Comparison of the functional features of the pump organs of Anopheles sinensis and Aedes togoi. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Jiraungkoorskul, W. Larvicidal and histopathological effects of Andrographis paniculata leaf extract against Culex quinquefasciatus larva. Walailak J. Sci. Technol. 2015, 13, 133–140. [Google Scholar]
- League, G.P.; Onuh, O.C.; Hillyer, J.F. Comparative structural and functional analysis of the larval and adult dorsal vessel and its role in hemolymph circulation in the mosquito Anopheles gambiae. J. Exp. Biol. 2015, 218, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H. X-ray diffraction pattern from the flight muscle of Toxorhynchites towadensis reveals the specific phylogenic position of mosquito among Diptera. Zool. Lett. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.F.; Wijesinghe, R.E.; Ravichandran, N.K.; Kim, P.; Jeon, M.; Kim, J. Dual-path handheld system for cornea and retina imaging using optical coherence tomography. Opt. Rev. 2017, 24, 219–225. [Google Scholar] [CrossRef]
- Wijesinghe, R.E.; Park, K.; Kim, P.; Oh, J.; Kim, S.-W.; Kim, K.; Kim, B.-M.; Jeon, M.; Kim, J. Optically deviated focusing method based high-speed SD-OCT for in vivo retinal clinical applications. Opt. Rev. 2016, 23, 307–315. [Google Scholar] [CrossRef]
- Cho, N.H.; Lee, S.H.; Jung, W.; Jang, J.H.; Kim, J. Optical coherence tomography for the diagnosis and evaluation of human otitis media. J. Korean Med. Sci. 2015, 30, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.E.; Cho, N.H.; Park, K.; Jeon, M.; Kim, J. Bio-Photonic detection and quantitative evaluation method for the progression of dental caries using optical frequency-domain imaging method. Sensors 2016, 16, 2076. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.F.; Park, K.; Wijesinghe, R.E.; Jeong, H.; Han, S.; Kim, P.; Jeon, M.; Kim, J. Fast industrial inspection of optical thin film using optical coherence tomography. Sensors 2016, 16, 1598. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, N.K.; Wijesinghe, R.E.; Shirazi, M.F.; Park, K.; Lee, S.-Y.; Jung, H.-Y.; Jeon, M.; Kim, J. In Vivo Monitoring on Growth and Spread of Gray Leaf Spot Disease in Capsicum Annuum Leaf Using Spectral Domain Optical Coherence Tomography. J. Spectrosc. 2016, 2016. [Google Scholar] [CrossRef]
- Wijesinghe, R.; Lee, S.-Y.; Ravichandran, N.K.; Shirazi, M.F.; Kim, P. Optical screening of Venturianashicola caused Pyruspyrifolia (Asian pear) scab using optical coherence tomography. Int. J. Appl. Eng. Res. 2016, 11, 7728–7731. [Google Scholar]
- Ravichandran, N.K.; Wijesinghe, R.E.; Shirazi, M.F.; Park, K.; Jeon, M.; Jung, W.; Kim, J. Depth enhancement in spectral domain optical coherence tomography using bidirectional imaging modality with a single spectrometer. J. Biomed. Opt. 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Harvey, M. Optical Coherence Tomography: Age Estimation of Calliphora Vicina Pupae In Vivo? Forensic Sci. Int. 2014, 242, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-K. Tomographic three-dimensional imaging of a biological specimen using wavelength-scanning digital interference holography. Opt. Express 2000, 7, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Boppart, S.A.; Bouma, B.E.; Brezinski, M.E.; Tearney, G.J.; Fujimoto, J.G. Imaging developing neural morphology using optical coherence tomography. J. Neurosci. Methods 1996, 70, 65–72. [Google Scholar] [CrossRef]
- Choi, K.S.; Wijesinghe, R.E.; Lee, C.; Lee, S.Y.; Jung, H.Y.; Jeon, M.; Kim, J. In Vivo Observation of Metamorphosis of Plodia Interpunctella Hübner using Three-Dimensional Optical Coherence Tomography. Entomol. Res. 2017. [Google Scholar] [CrossRef]
- Bradu, A.; Ma, L.; Bloor, J.W.; Podoleanu, A. Dual optical coherence tomography/fluorescence microscopy for monitoring of Drosophila melanogaster larval heart. J. Biophotonics 2009, 2, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Bradu, A.; Podoleanu, A.G.; Bloor, J.W. Arrhythmia caused by a Drosophila tropomyosin mutation is revealed using a novel optical coherence tomography instrument. PLoS ONE 2010, 5, e14348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, D.; Park, M.; Saeung, A.; Choochote, W.; Min, G. Multiplex assay to identify Korean vectors of malaria. Mol. Ecol. Resour. 2010, 10, 748–750. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichandran, N.K.; Wijesinghe, R.E.; Lee, S.-Y.; Choi, K.S.; Jeon, M.; Jung, H.-Y.; Kim, J. Non-Destructive Analysis of the Internal Anatomical Structures of Mosquito Specimens Using Optical Coherence Tomography. Sensors 2017, 17, 1897. https://doi.org/10.3390/s17081897
Ravichandran NK, Wijesinghe RE, Lee S-Y, Choi KS, Jeon M, Jung H-Y, Kim J. Non-Destructive Analysis of the Internal Anatomical Structures of Mosquito Specimens Using Optical Coherence Tomography. Sensors. 2017; 17(8):1897. https://doi.org/10.3390/s17081897
Chicago/Turabian StyleRavichandran, Naresh Kumar, Ruchire Eranga Wijesinghe, Seung-Yeol Lee, Kwang Shik Choi, Mansik Jeon, Hee-Young Jung, and Jeehyun Kim. 2017. "Non-Destructive Analysis of the Internal Anatomical Structures of Mosquito Specimens Using Optical Coherence Tomography" Sensors 17, no. 8: 1897. https://doi.org/10.3390/s17081897
APA StyleRavichandran, N. K., Wijesinghe, R. E., Lee, S.-Y., Choi, K. S., Jeon, M., Jung, H.-Y., & Kim, J. (2017). Non-Destructive Analysis of the Internal Anatomical Structures of Mosquito Specimens Using Optical Coherence Tomography. Sensors, 17(8), 1897. https://doi.org/10.3390/s17081897








