Tyrosinase-Based Biosensors for Selective Dopamine Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. CoP-Film Preparation and Construction of Tyrosinase Biosensor
3. Results and Discussions
3.1. Characterization of the CoP-Tyr Biosensor
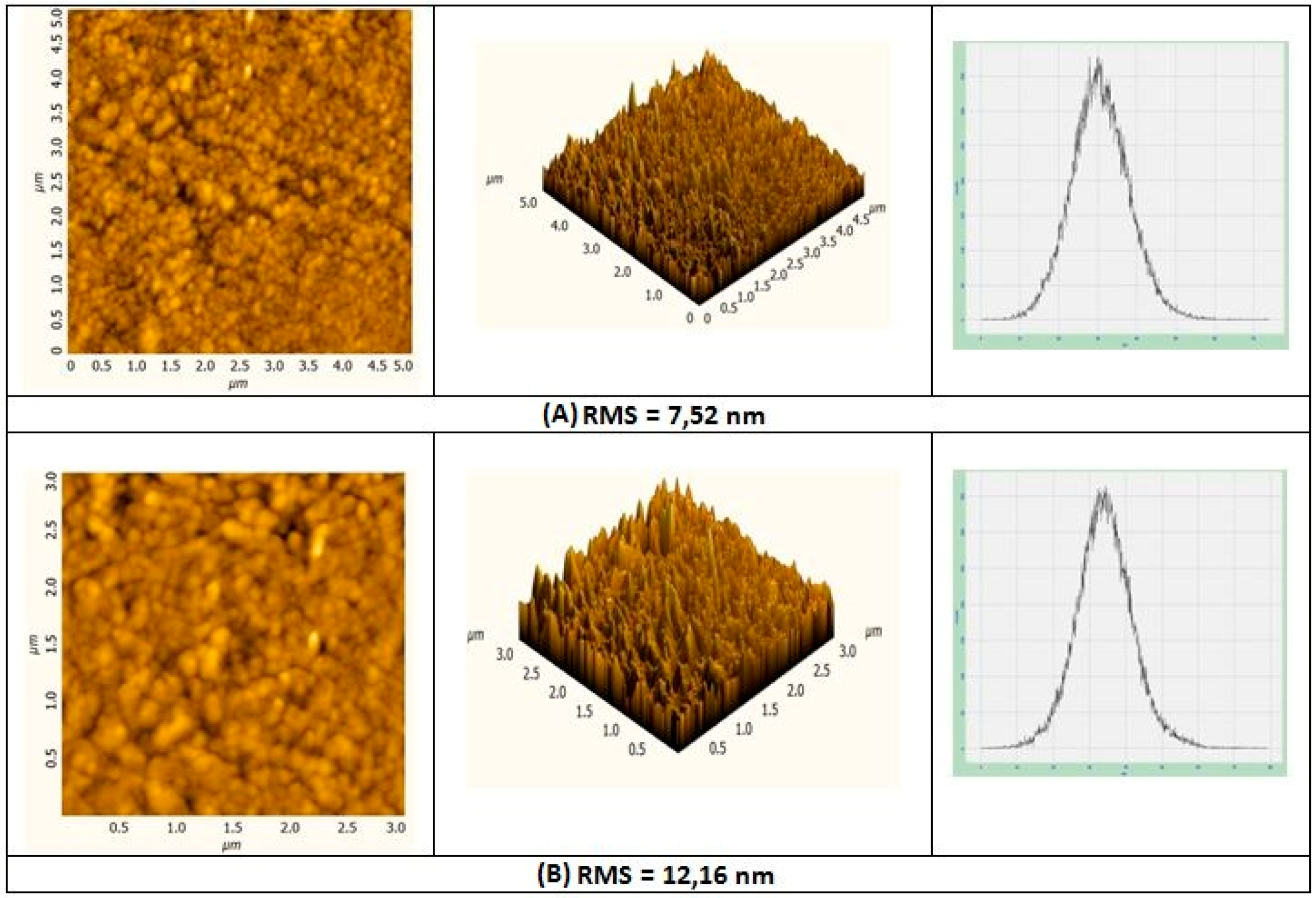
3.1.1. Surface Morphology Analysis of CoP Film
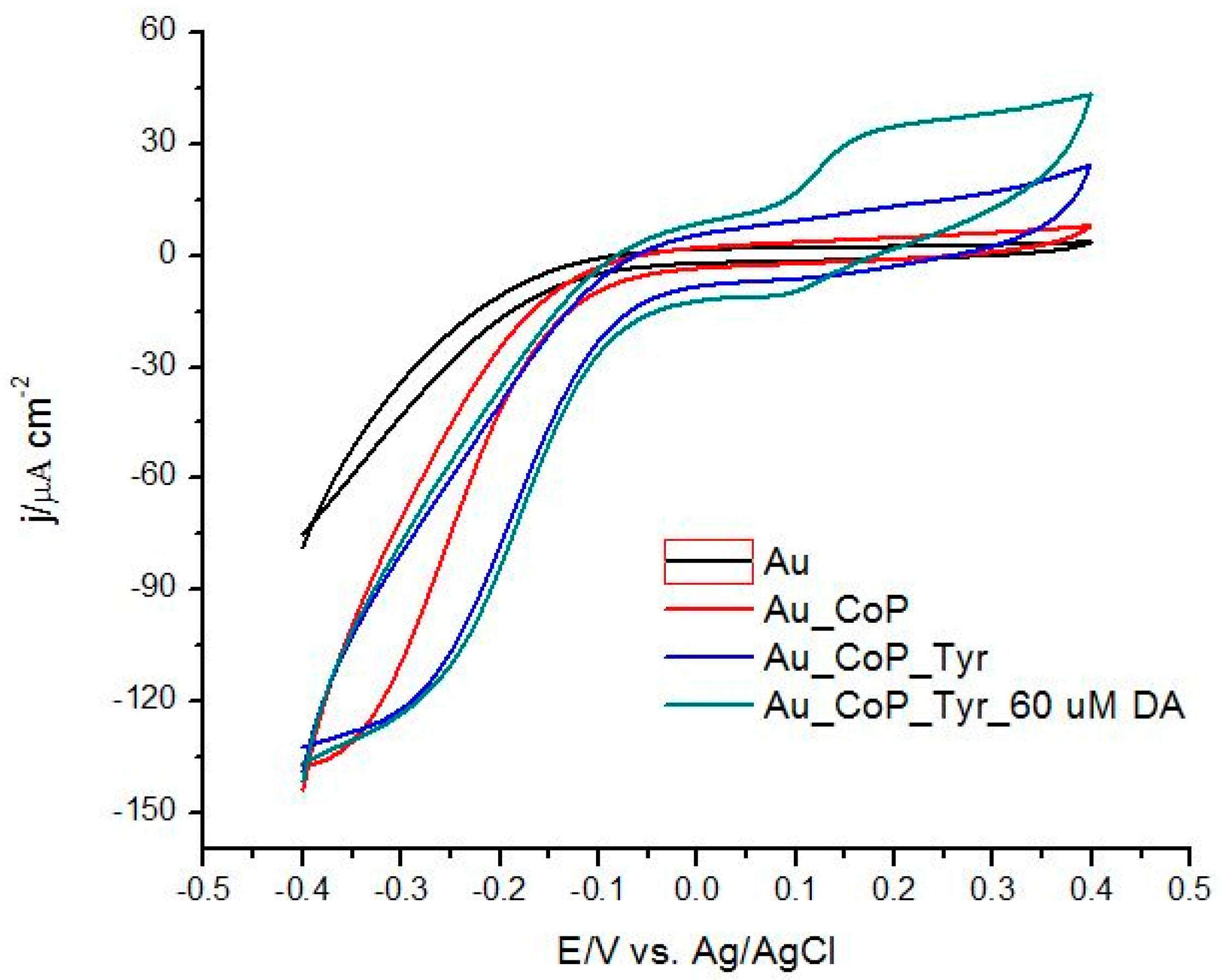
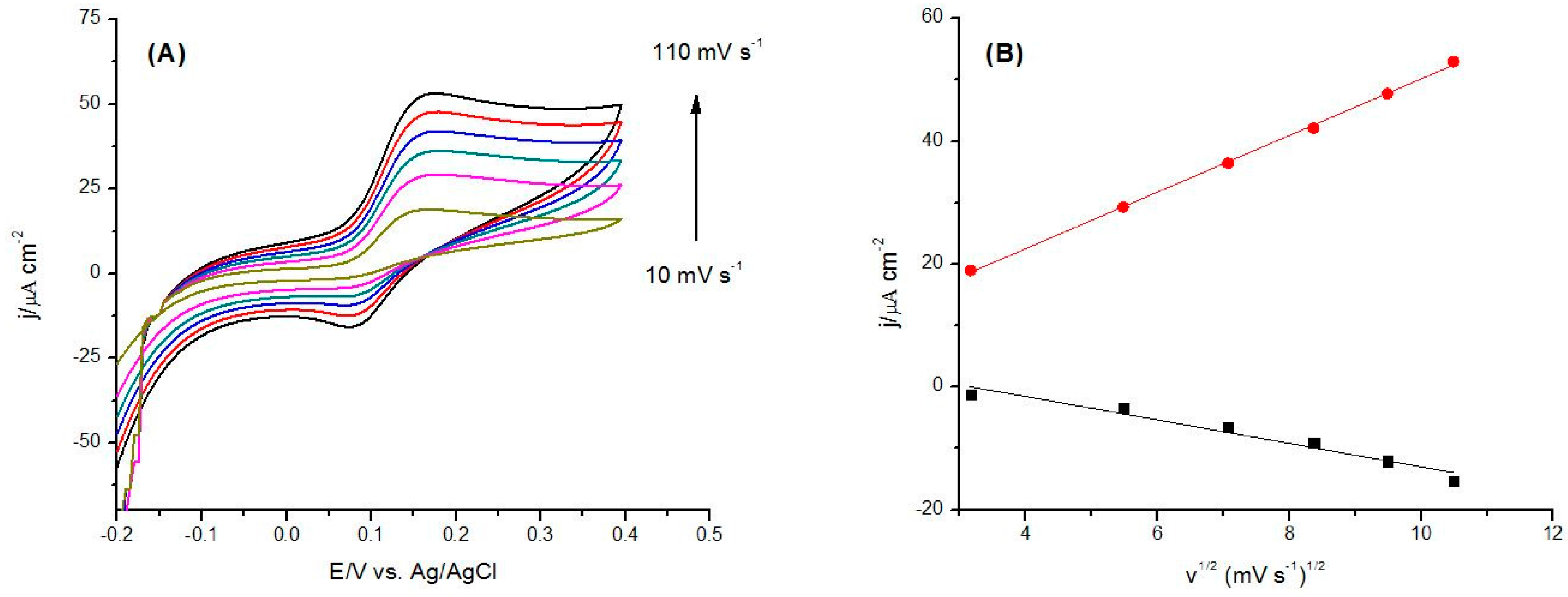
3.1.2. Electrochemical Characterization of CoP Film
3.1.3. Electrochemical Impedance Spectroscopy
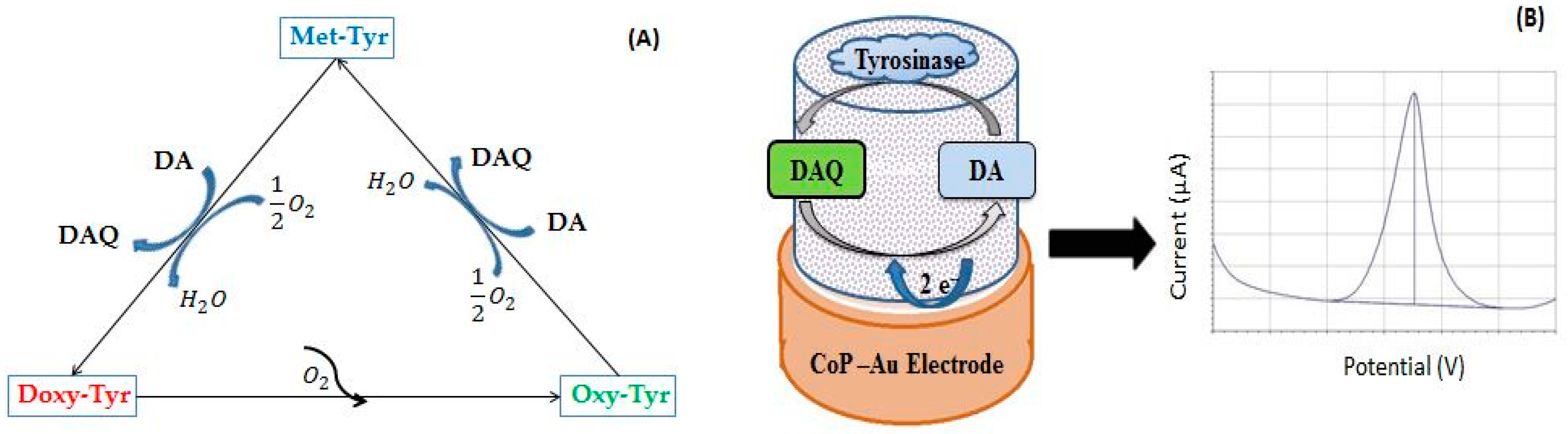
3.1.4. The Role of CoP in Dopamine Oxidation
- Electrocatalytic activity toward dopamine oxidation (enhancing the electronic conductivity and promoting electron transfer rate between the DA and electrode surface) in electrochemical sensor development, and
- Electrochemical mediator activity during enzyme-catalyzed oxidation of dopamine (enhancing electronic conductivity and acting as charge carriers) and
- Support for enzyme immobilization for biosensor development.
3.2. Optimization of Experimental Conditions for DA Detection
3.2.1. Influence of pH
3.2.2. Influence of CoP Concentration
3.2.3. Parameters of the Detection Method
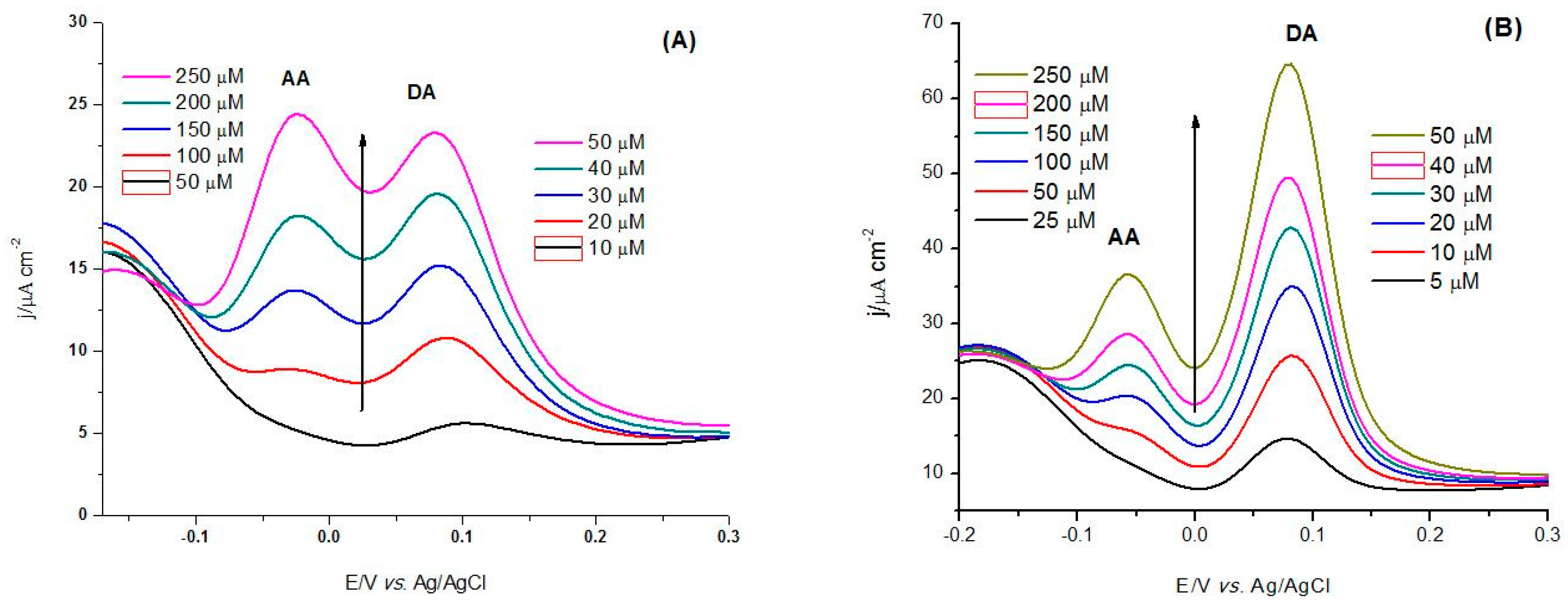
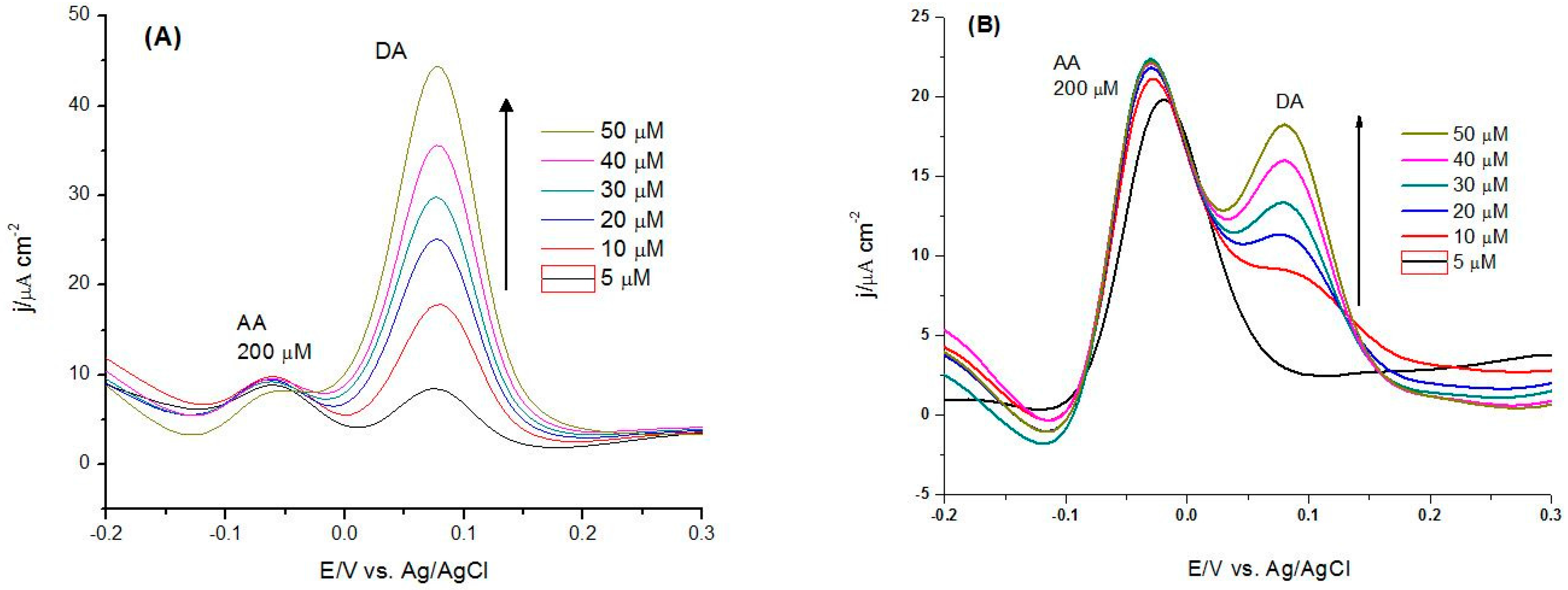
3.3. Electrocatalytic Oxidation of Dopamine
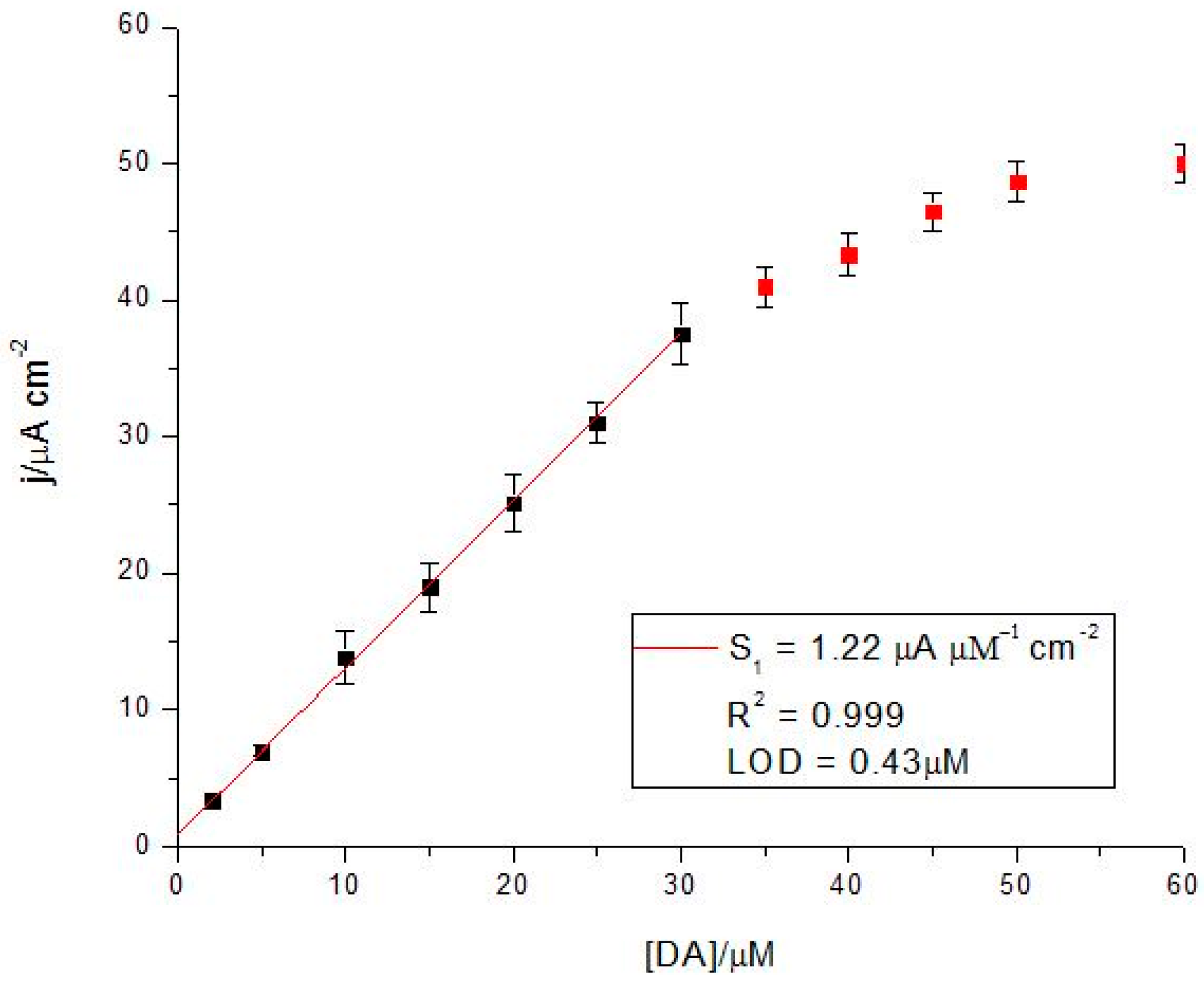
3.3.1. Analytical Parameters of the CoP-Tyr-Biosensor vs. the CoP-Sensor
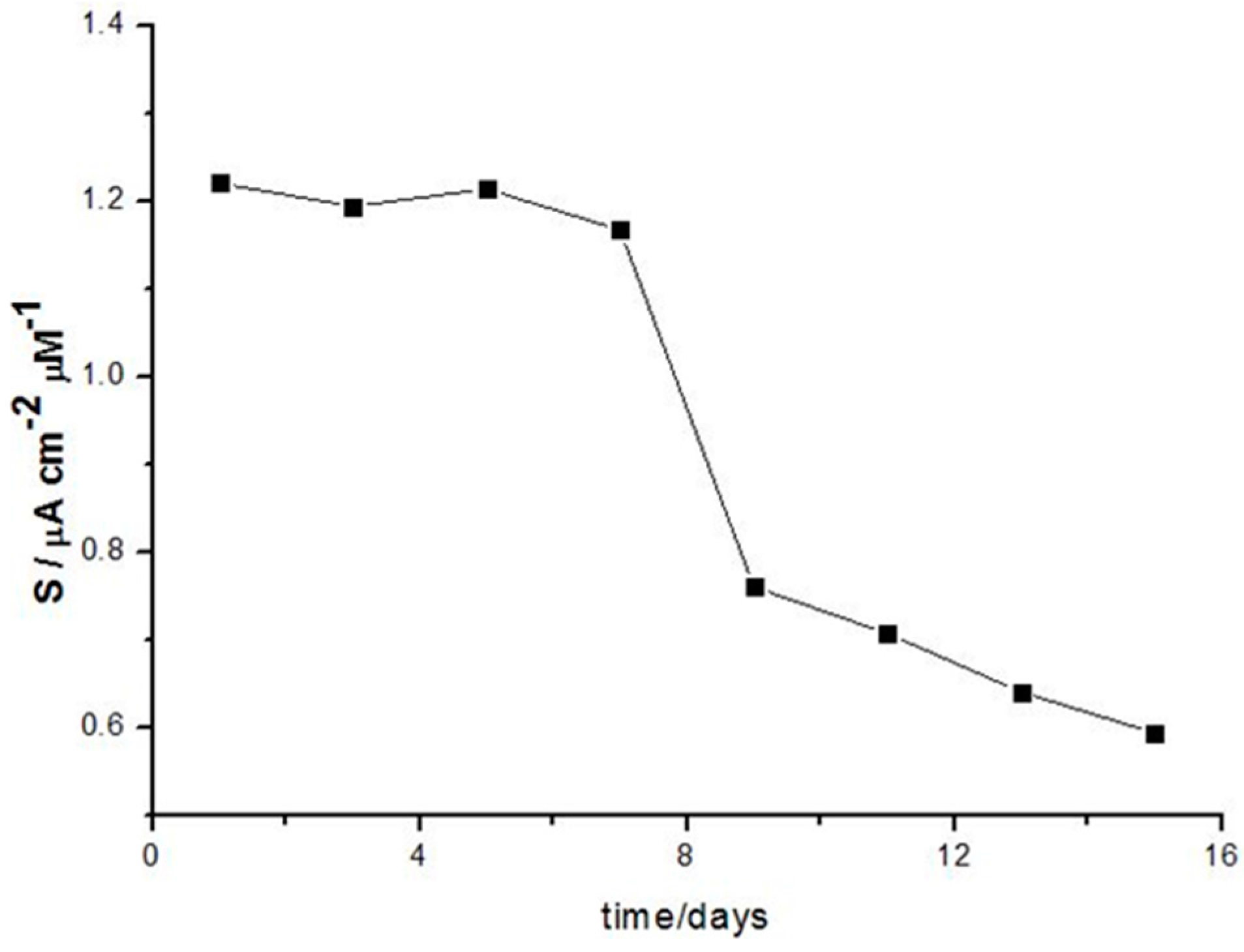
3.3.2. Biosensor Stability
3.3.3. Real Sample Detection
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paris, I.; Cardenas, S.; Lozano, J.; Perez-Pastene, C.; Graumann, R.; Riveros, A.; Caviedes, P.; Segura-Aguilar, J. Aminochrome as a preclinical experimental model to study degeneration of dopaminergic neurons in Parkinson’s disease. Neurotox. Res. 2007, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lunard, G.; Galat, S.; Tropepi, D.; Moschella, V.; Brusa, L.; Pierantozzi, M.; Stefani, A.; Rossi, S.; Fornai, F.; Fedele, E.; et al. Correlation between changes in CSF dopamine turnover and development of dyskinesia in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Crow, T.J.; Periy, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Reduced dopamine-4-hydroxylase activity in Alzheimer’s disease. Br. Med. J. 1981, 282, 93–94. [Google Scholar] [CrossRef]
- John, H.; Ferguson, M.D. National Institutes of Health Consensus Development Conference Statement: Diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 182–193. [Google Scholar]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.A.; Baker, G.B.; Bateson, A.N. In Vivo Neuromethods. In Neuromethods (34) Animal Models of Anxiety and Depression; Treit, D., Menard, J., Eds.; Human Press Inc.: Totowa, NJ, USA, 1998; pp. 89–148. [Google Scholar]
- Zhuang, Z.; Li, J.; Xu, R.; Xiao, D. Electrochemical detection of dopamine in the presence of ascorbic acid using overoxidized polypyrrole/graphene modified electrodes. Int. J. Electrochem. Sci. 2011, 6, 2149–2161. [Google Scholar]
- Beninger, R.J. The role of dopamine in locomotor activity and learning. Brain Res. Rev. 1983, 6, 173–196. [Google Scholar] [CrossRef]
- Rand, E.; Periyakaruppan, A.; Tanaka, Z.; Zhang, D.; Marsh, M.P.; Andrews, R.J.; Lee, K.H.; Chen, B.; Meyyappan, M.; Koehne, J.E. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbic acid. Biosens. Bioelectron. 2013, 42, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Armando, I.; Villar, V.A.; Jose, P.A. Dopamine and renal function and blood pressure regulation. Compr. Physiol. 2011, 1, 1075–1117. [Google Scholar] [CrossRef] [PubMed]
- Jerkovich, A.D.; Mellors, J.S.; Jorgenson, J.W. The Use of Micrometer-Sized Particles in Ultrahigh Pressure Liquid Chromatography. In The Column Watch; Majors, R.E., Ed.; LCGC Asia Pacific: Iselin, NJ, USA, 2003; Volume 6. [Google Scholar]
- Ohta, N.; Robertson, A. Colorimetry: Fundamentals and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005; ISBN 0-470-09472-9. [Google Scholar]
- Spencer, T.J.; Biederman, J.; Ciccone, P.E.; Madras, B.K.; Dougherty, D.D.; Bonab, A.A.; Livni, E.; Parasrampuria, D.A.; Fischman, A.J. PET Study Examining Pharmacokinetics, Detection and Likeability, and Dopamine Transporter Receptor Occupancy of Short- and Long-Acting Oral Methylphenidate. Am. J. Psychiatry 2006, 163, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Han, Q.; Wang, Y.; Wu, J.; Wen, T.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Amperometric detection of dopamine in human serumby electrochemical sensor based on gold nanoparticles doped molecularly imprinted polymers. Biosens. Bioelectron. 2013, 49, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Venton, B.J.; Kennedy, R.T. In Vivo Measurements of Neurotransmitters by Microdialysis Sampling. Anal. Chem. 2006, 78, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Foord, J.S.; Jiang, X. Diamond electrochemistry at the nanoscale: A review. Carbon 2016, 99, 90–110. [Google Scholar] [CrossRef]
- Pandikumar, A.; How, G.T.S.; See, T.P.; Omar, F.S.; Jayabal, S.; Kamali, K.Z.; Yusoff, N.; Jamil, A.; Ramaraj, R.; John, S.A.; et al. Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. RCS Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Stoytcheva, M.; Zlatev, R.; Velkova, Z.; Gochev, V.; Montero, G.; Toscano, L.; Olivas, A. Advances in the Electrochemical Analysis of Dopamine. Curr. Anal. Chem. 2017, 13, 89–103. [Google Scholar] [CrossRef]
- Armengaud, C.; Moisy, P.; Bedioui, F.; Devynck, J. Electrochemistry of conducting polypyrrole films containing cobalt porphyrin. J. Electroanal. Chem. 1990, 277, 197–211. [Google Scholar] [CrossRef]
- Iordache, S.; Ducu, A.M.; Cucu, A.; Andronie, A.; Stamatin, S.; Ceaus, C.; Popescu, A.; Fagadar-Cosma, E.; Cristescu, R.; Stamatin, I. The characterization of immobilized active substrates on screen-printed electrodes designed for response to the biochemical agents. J. Optoelectron. Adv. Mat. 2010, 12, 1946–1951. [Google Scholar]
- Lu, L.; Zhang, L.; Zhang, X.; Huan, S.; Shen, G.; Yu, R. A novel Tyrosinase biosensor based on hydroxyapatite-chitosan nanocomposite for the detection of phenolic compounds. Anal. Chim. Acta. 2010, 665, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Sevilla, E.; Ramirez-Silva, M.T.; Romero-Romo, M.; Ibarra-Escutia, P.; Palomar-Pardave, M. Electrochemical Quantification of the Antioxidant Capacity of Medicinal Plants Using Biosensors. Sensors 2014, 14, 14423–14439. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Huang, S.; Yue, Z.; Peng, B.; Li, M.; Zhang, J. Sensitive mediator-free Tyrosinase biosensor for the determination of 2,4-dichlorophenol. Microchim. Acta 2009, 165, 203–209. [Google Scholar] [CrossRef]
- Wu, L.; Deng, D.; Jin, J.; Lu, X.; Chen, J. Nanographene-based Tyrosinase biosensor for rapid detection of bisphenol A. Biosens. Bioelectron. 2012, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mangombo, Z.A.; Key, D.; Iwuoha, E.I.; Baker, P.G.L. Development of L-phenylalanine biosensor and its application to real sample analysis. Insci. J. 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Dincer, C.; Ktaich, R.; Laubender, E.; Hees, J.J.; Kieninger, J.; Nebel, C.E.; Heinze, J.; Urban, G.A. Nanocrystalline boron-doped diamond nanoelectrode arrays for ultrasensitive dopamine detection. Electrochim. Acta 2015, 185, 101–106. [Google Scholar] [CrossRef]
- Jiang, L.; Nelson, G.W.; Abda, J.; Foord, J.S. Novel Modifications to Carbon-Based Electrodes to Improve the Electrochemical Detection of Dopamine. ACS Appl. Mater. Interfaces 2016, 8, 28338–28348. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.F.; Min, K.; Park, S.-H.; Park, J.-H.; Yoo, J.C.; Park, D.-H. Selective determination of dopamine with an amperometric biosensor using electrochemically pretreated and activated carbon/tyrosinase /Nafion®-modified glassy carbon electrode. Biotechnol. Bioprocess Eng. 2016, 21, 627–633. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C. Development of Amperometric Biosensors Based on Nanostructured Tyrosinase-Conducting Polymer Composite Electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed]
- Lupu, S.; Lete, C.; Marin, M.; Totir, N.; Balaure, P.C. Electrochemical sensors based on platinum electrodes modified with hybrid inorganic–organic coatings for determination of 4-nitrophenol and dopamine. Electrochim. Acta 2009, 54, 1932–1938. [Google Scholar] [CrossRef]
- Maciejewska, J.; Pisarek, K.; Bartosiewicz, I.; Krysinski, P.; Jackowska, K.; Biegunski, A.T. Selective detection of dopamine on poly(indole-5-carboxylic acid)/Tyrosinase Electrode. Electrochim. Acta 2011, 56, 3700–3706. [Google Scholar] [CrossRef]
- “AS-IS” and Free for Noncommercial Use. Copyright: Aliaksandr Bandarenka and Genady Ragoisha. Last Update: 16 July 2013. Available online: http://www.abc.chemistry.bsu.by/vi/analyser (accessed on 7 June 2017).
- Brittle, S.A.; Richardson, T.H.; Hutchinson, J.; Hunter, C.A. Comparing zinc and manganese porphyrin LB films as amine vapour sensing materials. Colloid Surf. A 2008, 321, 29–33. [Google Scholar] [CrossRef]
- Raposo, M.; Ferreira, Q.; Ribeiro, P.A. A Guide for Atomic Force Microscopy Analysis of Soft-Condensed Matter. In Modern Research and Educational Topics in Microscopy; Mendez-Vilas, A., Diaz, A.J., Eds.; Formatex Research Center: Badajoz, Spain, 2007. [Google Scholar]
- Brezeanu, M.; Cristurean, E.; Antoniu, A.; Marinescu, D.; Andruh, M. Chimia Metalelor; Academiei Române: Bucureşti, Romania, 1990. [Google Scholar]
- Giancane, G.; Valli, L. State of art in porphyrin Langmuir–Blodgett films as chemical sensors. Adv. Colloid Interface Sci. 2012, 171–172, 17–35. [Google Scholar] [CrossRef] [PubMed]
- D’amico, A.; Di Natale, C.; Paolesse, R.; Macagnano, A.; Mantini, A. Metalloporphyrins as basic material for volatile sensitive sensors. Sens. Actuators B 2000, 65, 209–215. [Google Scholar] [CrossRef]
- Xiang, L.; Lin, Y.; Yu, P.; Su, L.; Mao, L. Laccase-catalyzed oxidation and intramolecular cyclization of dopamine: A new method for selective determination of dopamine with laccase/carbon nanotube-based electrochemical biosensors. Electrochim. Acta 2007, 52, 4144–4152. [Google Scholar] [CrossRef]
- Stamford, J.A.; Justice, J.G. Voltammetry has been solving the mysteries of the brain and its functions for 21 years. Anal. Chem. 1996, 68, 359A–363A. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feng, L.; Ren, J.; Qu, X. Electrochemical detection of dopamine using porphyrin-functionalized grapheme. Biosens. Bioelectron. 2012, 34, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Chen, Y.; Xu, H.; Tan, Y.; Wang, S. Detection of Dopamine Based on Tyrosinase-Fe3O4 Nanoparticles-chitosan Nanocomposite Biosensor. Am. J. Biomed. Sci. 2010, 2, 209–216. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R.; Chen, S.-M.; Kohilarani, K.; Ramachandran, R. A Sensitive Amperometric Sensor for the Determination of Dopamine at Graphene and Bismuth Nanocomposite Film Modified Electrode. Int. J. Electrochem. Sci. 2015, 10, 1199–1207. [Google Scholar]
- Palanisamy, S.; Ku, S.; Chen, S.-M. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim. Acta 2013, 180, 1037–1042. [Google Scholar] [CrossRef]
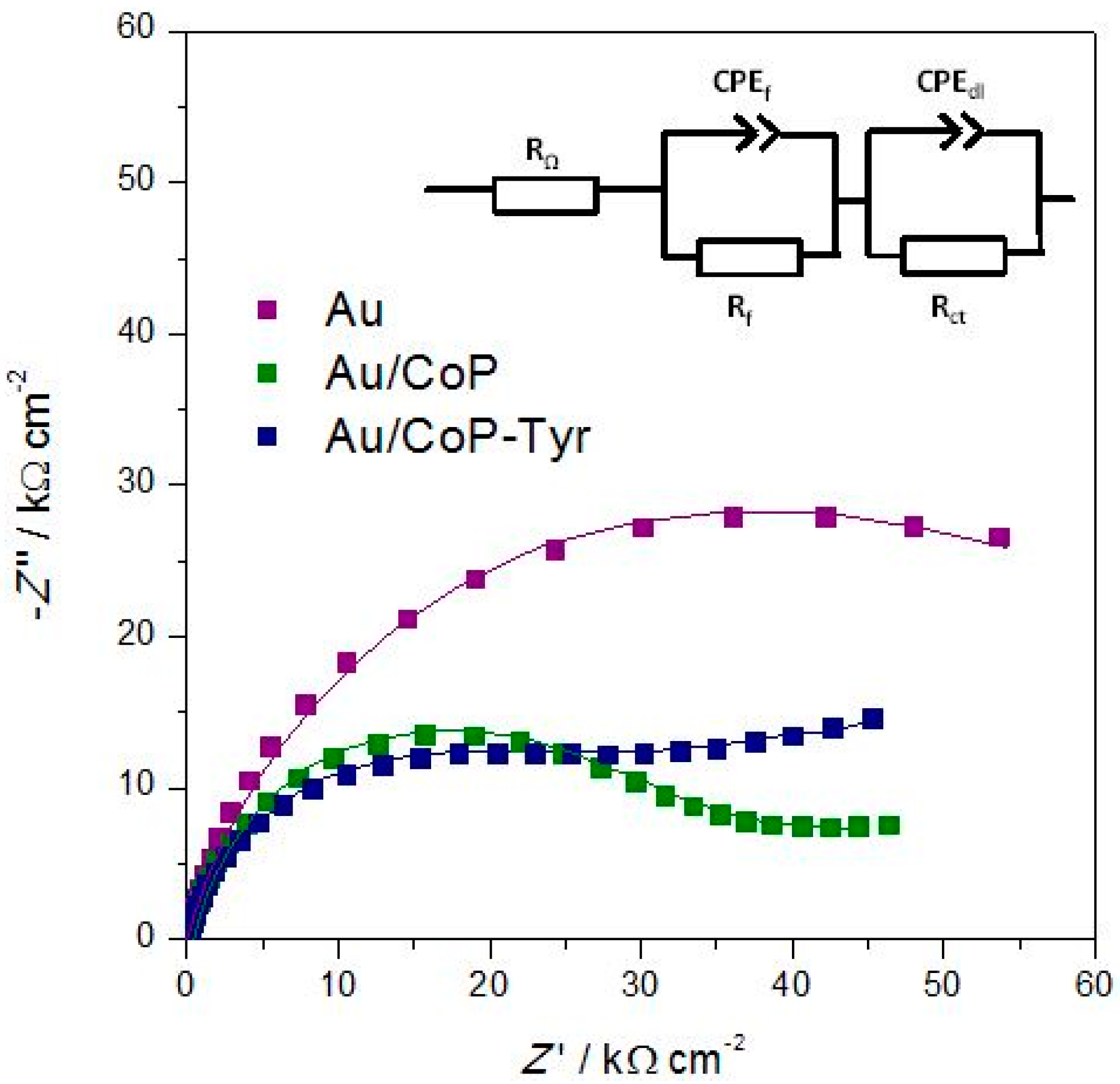
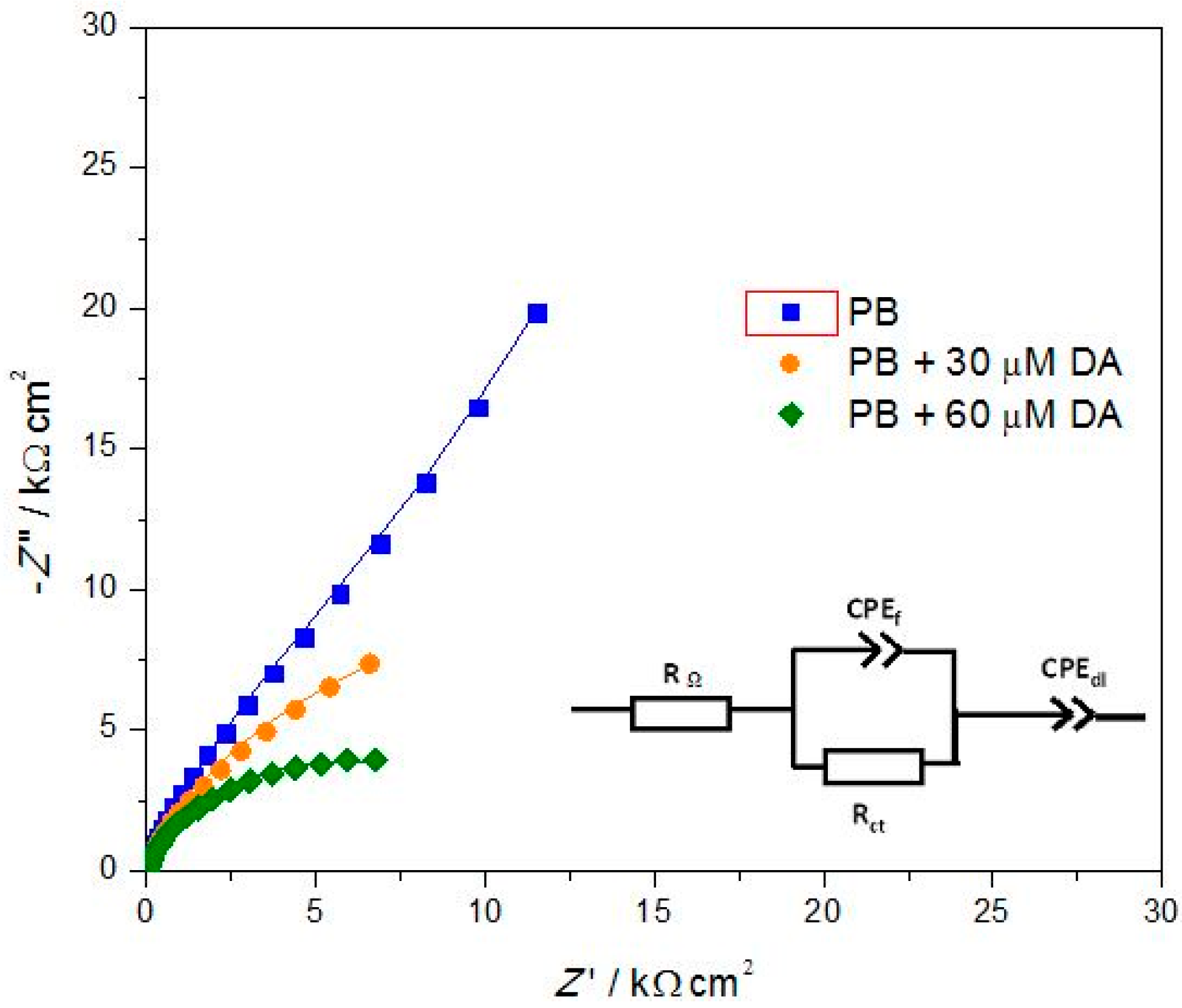
| Electrode | Rf/kΩ·cm2 | CPEf/μF·cm−2·sα−1 | αf | Rct/kΩ·cm2 | CPEdl/μF·cm−2·sα−1 | αdl |
|---|---|---|---|---|---|---|
| Au | - | - | - | 4.64 | 29.40 | 0.82 |
| Au/CoP | 0.785 | 956.3 | 0.98 | 2.09 | 30.07 | 0.87 |
| Au/CoP-Tyr | 1.420 | 901.6 | 0.96 | 2.00 | 60.43 | 0.80 |
| Electrode | Rct/kΩ·cm2 | CPEf/μF·cm−2·sα−1 | αdl | CPEdl/μF·cm−2·sα−1 | αf |
|---|---|---|---|---|---|
| Au/CoP-Tyr | 58.57 | 61.07 | 0.71 | 300.63 | 0.98 |
| Au/CoP-Tyr 30 µM DA | 26.12 | 90.89 | 0.74 | 620.50 | 0.65 |
| Au/CoP-Tyr 60 µM DA | 6.99 | 100.01 | 0.82 | 665.47 | 0.62 |
| Analyte | (Bio)sensor | Linearity/µM | S/µA·cm−2·mM−1 | LoD/µM |
|---|---|---|---|---|
| DA | CoP | 10–50 | 0.57 | 0.98 |
| CoP-Tyr | 2–30 | 1.22 | 0.43 | |
| DA and fixed AA | CoP | 20–50 | 0.56 | 2.46 |
| CoP-Tyr | 10–30 | 1.21 | 0.52 | |
| DA and AA | CoP | 20–50 | 0.43 | 3.13 |
| CoP-Tyr | 10–30 | 1.21 | 0.55 |
| Sensor | DA Specified/µM | DA Added/µM | DA Found/µM | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|
| CoP | 26.36 | 0 | 27.38 ± 0.83 | 5.9 | 103.0 |
| 26.36 | 10 | 36.57 ± 1.02 | 5.2 | 100.5 | |
| 26.36 | 20 | 46.12 ± 0.53 | 2.1 | 99.0 | |
| 26.36 | 30 | 54.42 ± 0.97 | 3.2 | 96.0 | |
| CoP-Tyr | 3.00 | 0 | 3.17 ± 0.32 | 2.6 | 105.0 |
| 3.00 | 2 | 5.07 ± 0.28 | 4.0 | 101.0 | |
| 3.00 | 4 | 6.24 ± 0.25 | 2.7 | 89.0 | |
| 3.00 | 6 | 8.07 ± 0.59 | 4.7 | 89.0 |
| Sensor | DA Specified/µM | DA Found/µM | /µM | Recovery (%) |
|---|---|---|---|---|
| CoP | 26.36 | 29.05 | 27.50 ± 1.45 | 110 |
| 26.36 | 26.18 | 99 | ||
| 26.36 | 27.28 | 103 | ||
| CoP-Tyr | 3.00 | 3.43 | 3.10 ± 0.32 | 114 |
| 3.00 | 2.79 | 93 | ||
| 3.00 | 3.09 | 103 |
| Sensor | Limit of Detection (LoD)/μM | Linear Range/µM | Interference | Recovery (%) | RSD (%) | Ref. |
|---|---|---|---|---|---|---|
| GCE-TCPP/CCG | 0.010 | 0.1–1 | AA, UA | ~99.0 | 3.00 | [42] |
| GCE-PPyox/graphene | 0.100 | 25–1000 | AA | - | 4.30 | [6] |
| GCE-Tyr-Fe3O4-CHIT | 0.006 | 0.02–0.75 | AA | ~105.0 | 5.18 | [43] |
| GCE-GNS-Bi | 0.350 | 1–30 | - | 98.4 | 2.20 | [44] |
| GCE-RGO-Pd-NPs | 0.230 | 1–150 | AA, UA | 98.8 | - | [45] |
| Au-CoP | 0.750 | 10–50 | AA | ~99.6 | 4.10 | This work |
| Au-CoP-Tyr | 0.430 | 2–30 | AA | ~96.0 | 3.50 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florescu, M.; David, M. Tyrosinase-Based Biosensors for Selective Dopamine Detection. Sensors 2017, 17, 1314. https://doi.org/10.3390/s17061314
Florescu M, David M. Tyrosinase-Based Biosensors for Selective Dopamine Detection. Sensors. 2017; 17(6):1314. https://doi.org/10.3390/s17061314
Chicago/Turabian StyleFlorescu, Monica, and Melinda David. 2017. "Tyrosinase-Based Biosensors for Selective Dopamine Detection" Sensors 17, no. 6: 1314. https://doi.org/10.3390/s17061314
APA StyleFlorescu, M., & David, M. (2017). Tyrosinase-Based Biosensors for Selective Dopamine Detection. Sensors, 17(6), 1314. https://doi.org/10.3390/s17061314







