Figure 1.
Two HMP frameworks. (a) In the classical HMP framework, the organ is saved in an isolated organ box and it uses the single compressor for temperature control; (b) In the modified HMP framework, oxygenated solution travels in the artery loop and the vein loop. Two peristaltic entrance pumps are used to promote the circular flow. Sensors are distributed in different parts for organ status monitoring.
Figure 1.
Two HMP frameworks. (a) In the classical HMP framework, the organ is saved in an isolated organ box and it uses the single compressor for temperature control; (b) In the modified HMP framework, oxygenated solution travels in the artery loop and the vein loop. Two peristaltic entrance pumps are used to promote the circular flow. Sensors are distributed in different parts for organ status monitoring.
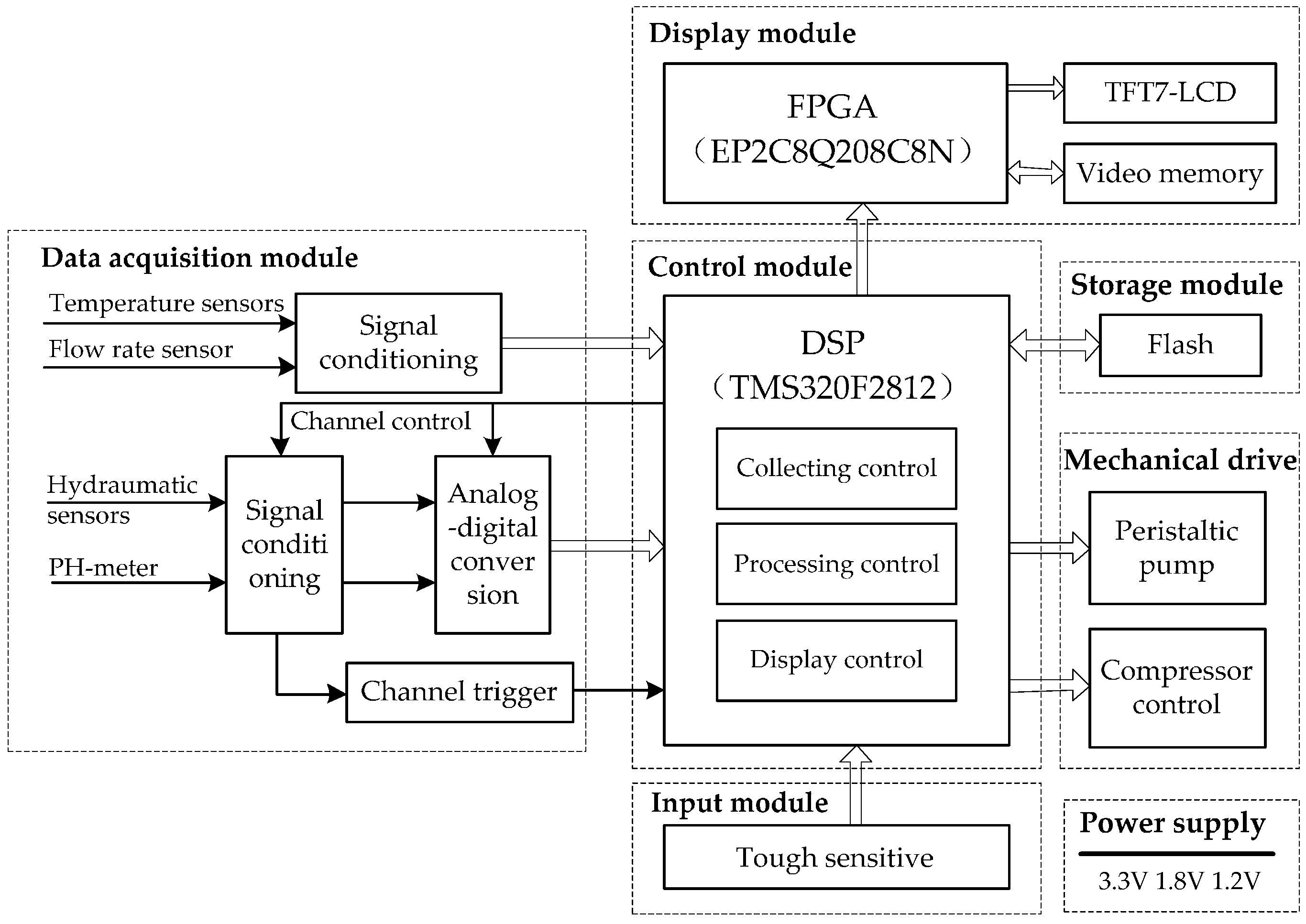
Figure 2.
The circuit diagram of the HMP system. It contains a data acquisition module, a control module, a storage module, a mechanical drive, an input and display module, and a power supply.
Figure 2.
The circuit diagram of the HMP system. It contains a data acquisition module, a control module, a storage module, a mechanical drive, an input and display module, and a power supply.
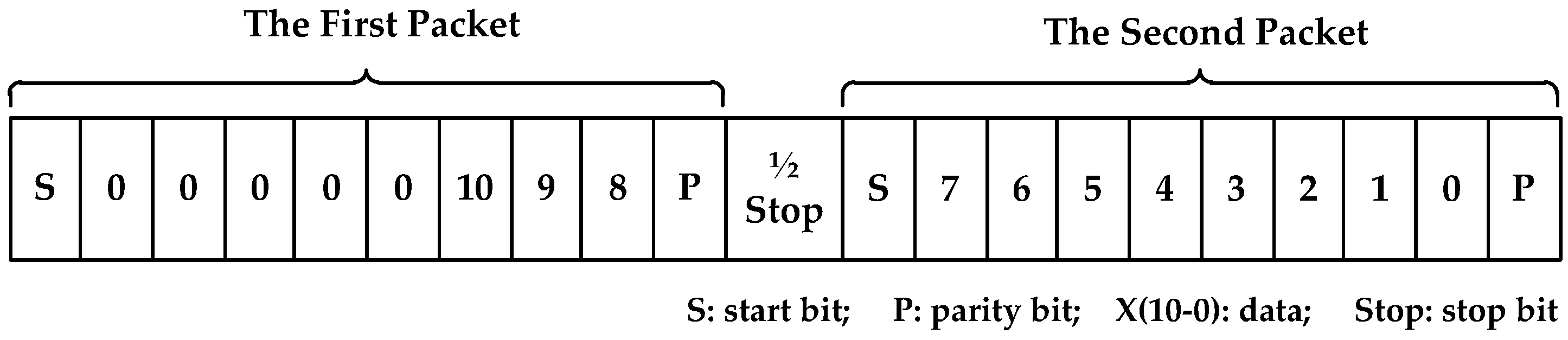
Figure 3.
The frame format of Tsic506, (1) the first packet: 1 start bit, 3 data bits, 1 parity bit; (2) the second packet: 1 start bit, 8 data bits, 1 parity bit; (3) between the first packet and the second packet: 1/2 stop bit.
Figure 3.
The frame format of Tsic506, (1) the first packet: 1 start bit, 3 data bits, 1 parity bit; (2) the second packet: 1 start bit, 8 data bits, 1 parity bit; (3) between the first packet and the second packet: 1/2 stop bit.
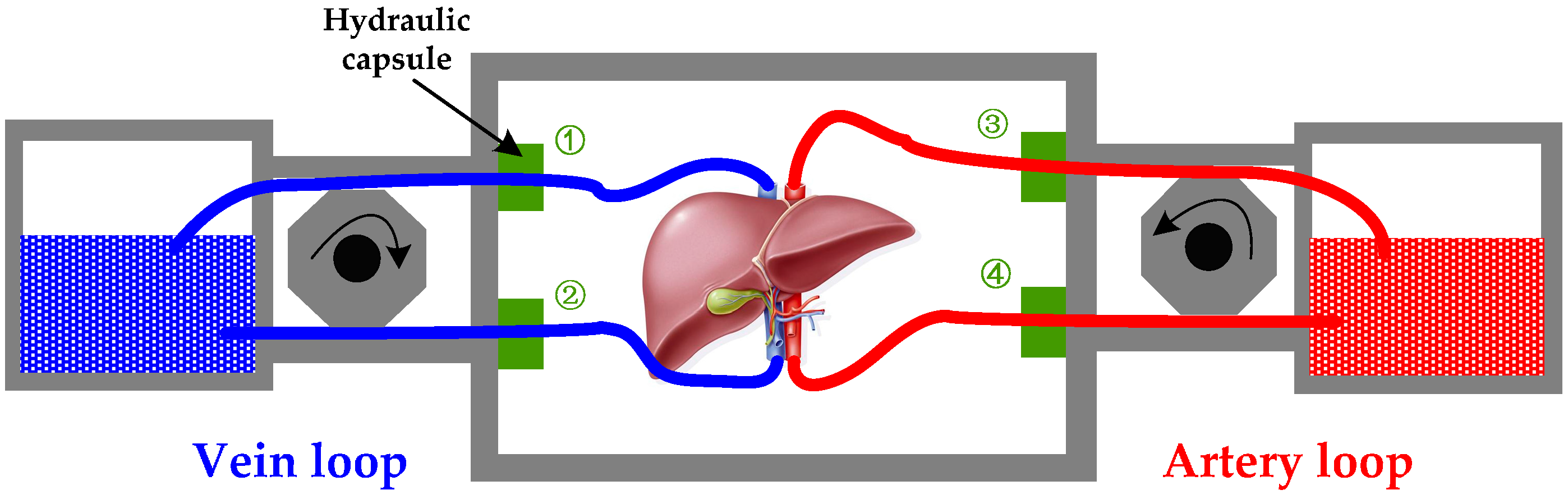
Figure 4.
The flow resistance monitoring diagram, which contains a vein loop and an artery loop. The difference between ① and ② means the pressure drop of vein; the difference between ③ and ④ means the pressure drop of artery.
Figure 4.
The flow resistance monitoring diagram, which contains a vein loop and an artery loop. The difference between ① and ② means the pressure drop of vein; the difference between ③ and ④ means the pressure drop of artery.
Figure 5.
An example of two drive models, (1) vein model: a smooth curve from 400 mL/min to 200 mL/min; (2) artery model: a fluctuating curve from 400 mL/min to 200 mL/min. The peak of artery model reflects the flow speed when the blood is systolic, while the valley reflects the flow speed when the blood is diastolic. Its fluctuating period equals to the heart pulse period.
Figure 5.
An example of two drive models, (1) vein model: a smooth curve from 400 mL/min to 200 mL/min; (2) artery model: a fluctuating curve from 400 mL/min to 200 mL/min. The peak of artery model reflects the flow speed when the blood is systolic, while the valley reflects the flow speed when the blood is diastolic. Its fluctuating period equals to the heart pulse period.
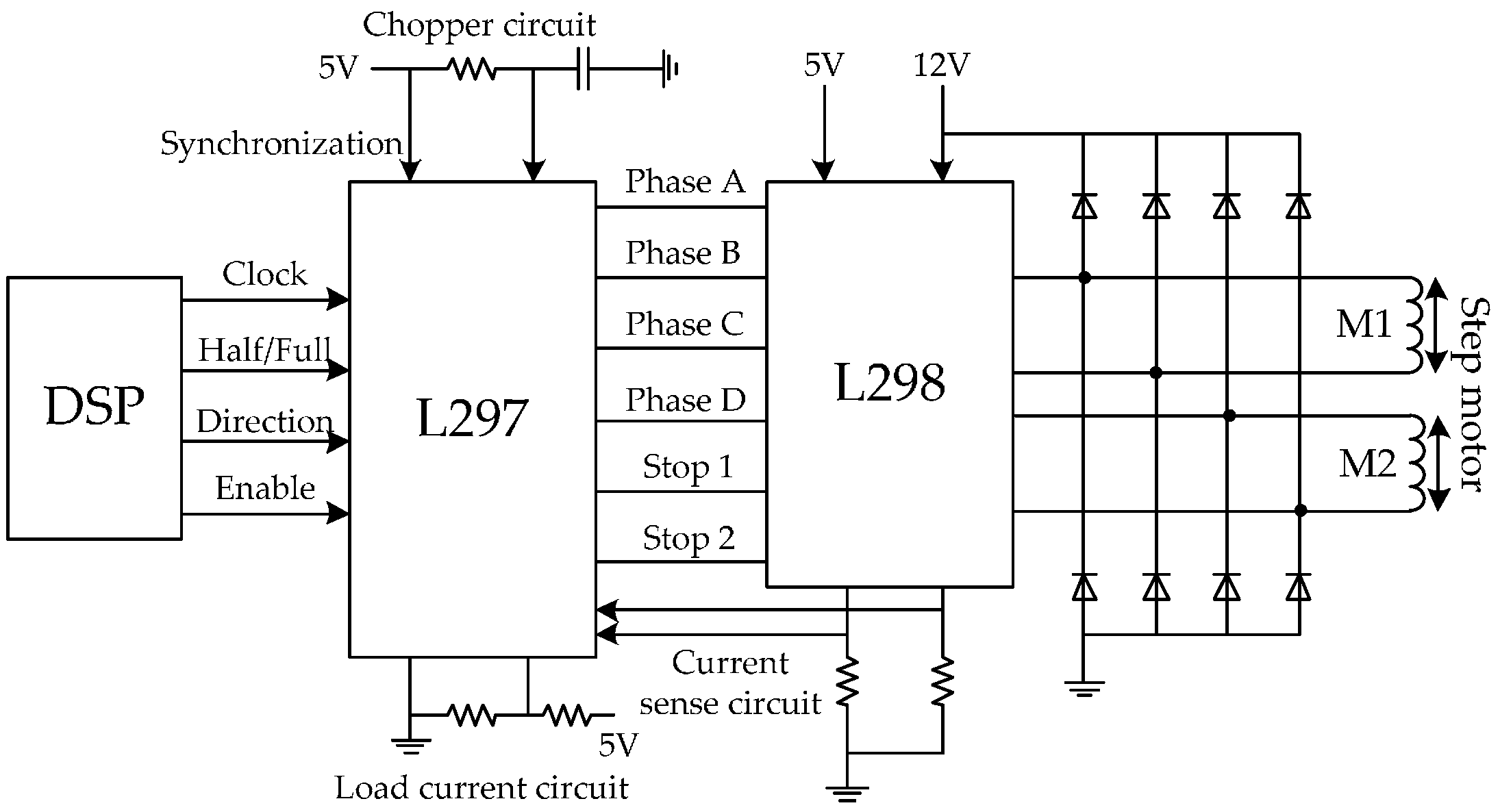
Figure 6.
The step motor drive circuit, (1) clock: high clock frequency will cause a faster rotation speed; (2) half/full: the execution efficiency of full-step mode is twice as that of half-step mode; (3) direction: 1/0 represents the positive/negative direction; (4) enable: 1/0 represents controllable/uncontrollable.
Figure 6.
The step motor drive circuit, (1) clock: high clock frequency will cause a faster rotation speed; (2) half/full: the execution efficiency of full-step mode is twice as that of half-step mode; (3) direction: 1/0 represents the positive/negative direction; (4) enable: 1/0 represents controllable/uncontrollable.
Figure 7.
The thermostatic control equipment, i.e., a compressor and a heat bar, are controlled by DSP to increase and reduce the temperature of water, respectively. After a controllable valve, the water can reach the thin aluminium alloy around the stored organ. After that, the heat of water can penetrate the metallic material and adjust the organ temperature.
Figure 7.
The thermostatic control equipment, i.e., a compressor and a heat bar, are controlled by DSP to increase and reduce the temperature of water, respectively. After a controllable valve, the water can reach the thin aluminium alloy around the stored organ. After that, the heat of water can penetrate the metallic material and adjust the organ temperature.
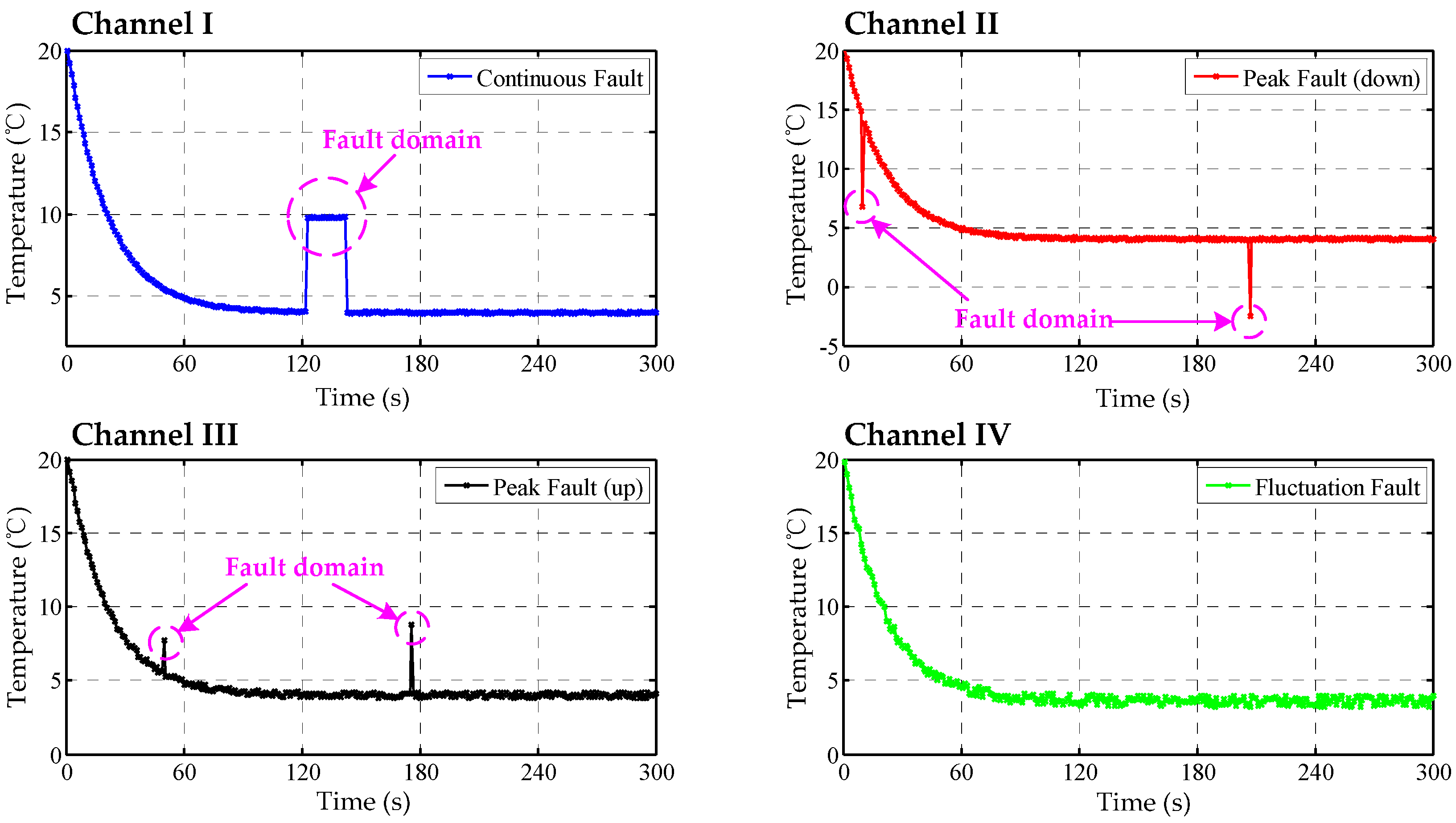
Figure 8.
The data curves of TSic506 units, channel I: continuous fault resulting from sensor fault; channel II: down peak fault resulting from accidental fault; channel III: up peak fault resulting from accidental fault; channel IV: fluctuation fault resulting from interfering noise.
Figure 8.
The data curves of TSic506 units, channel I: continuous fault resulting from sensor fault; channel II: down peak fault resulting from accidental fault; channel III: up peak fault resulting from accidental fault; channel IV: fluctuation fault resulting from interfering noise.
Figure 9.
The fused Bayes estimation curve.
Figure 9.
The fused Bayes estimation curve.
Figure 10.
The fused curves of the modified Bayes estimation.
Figure 10.
The fused curves of the modified Bayes estimation.
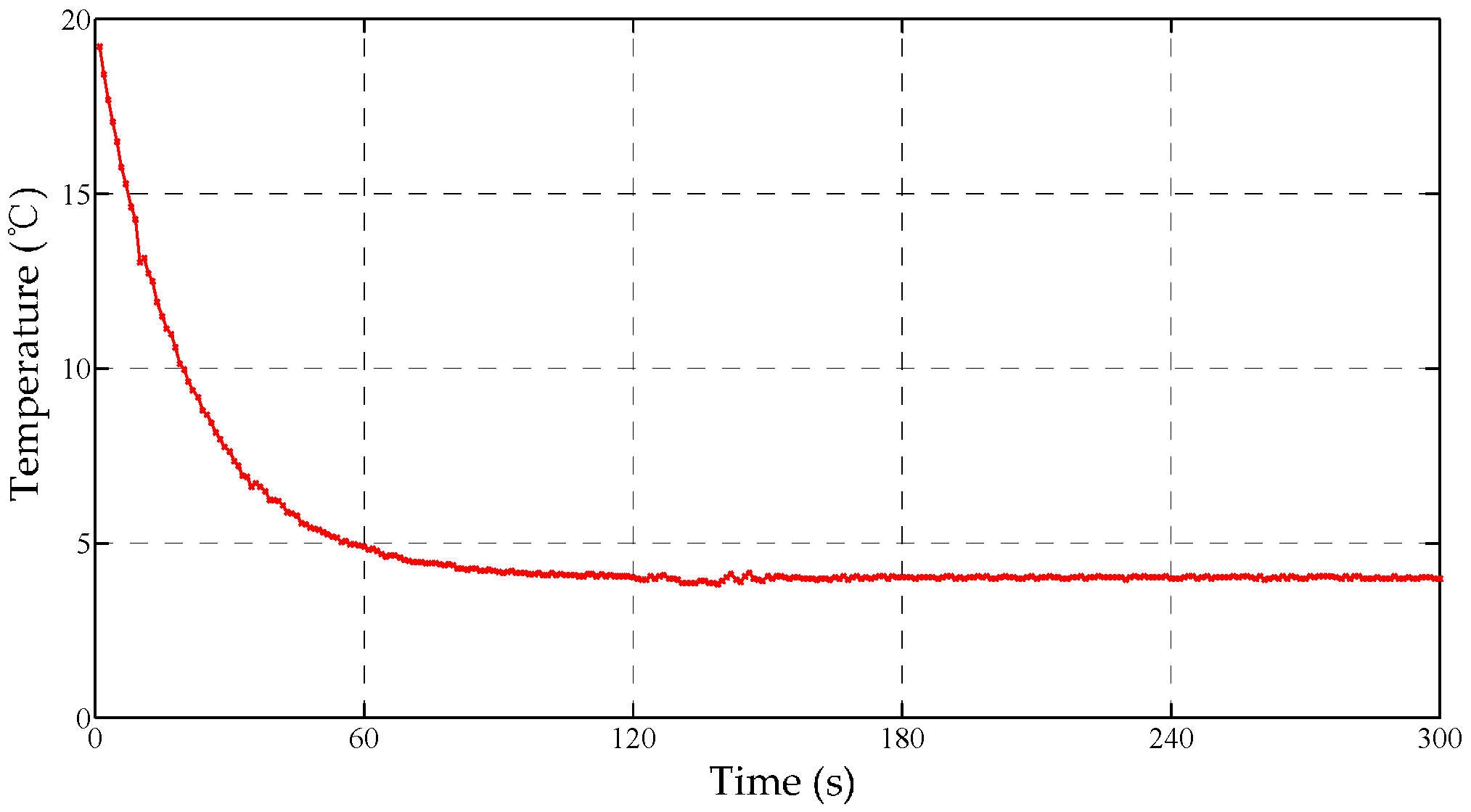
Figure 11.
The fused curve of the cascaded Bayes estimation.
Figure 11.
The fused curve of the cascaded Bayes estimation.
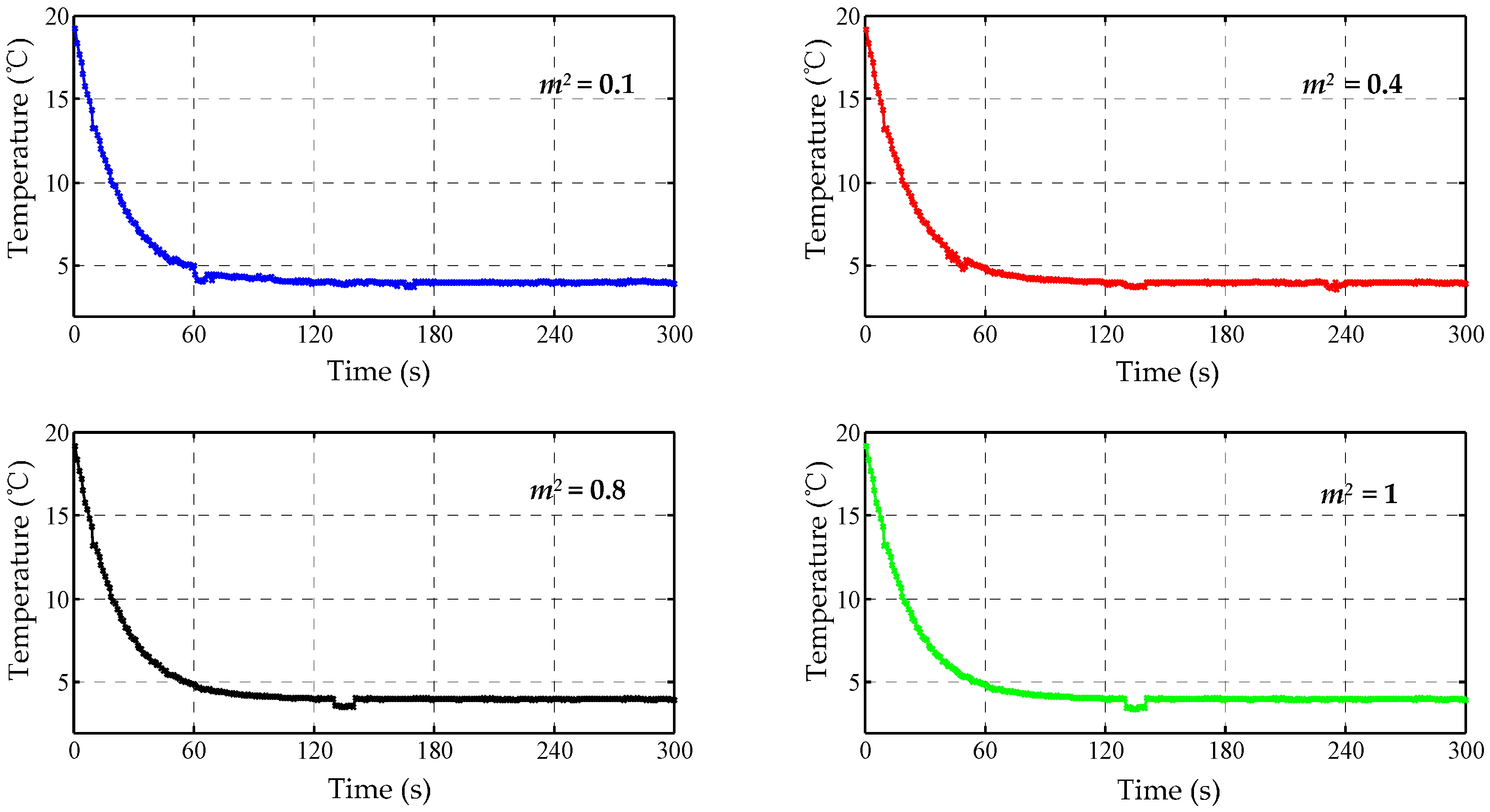
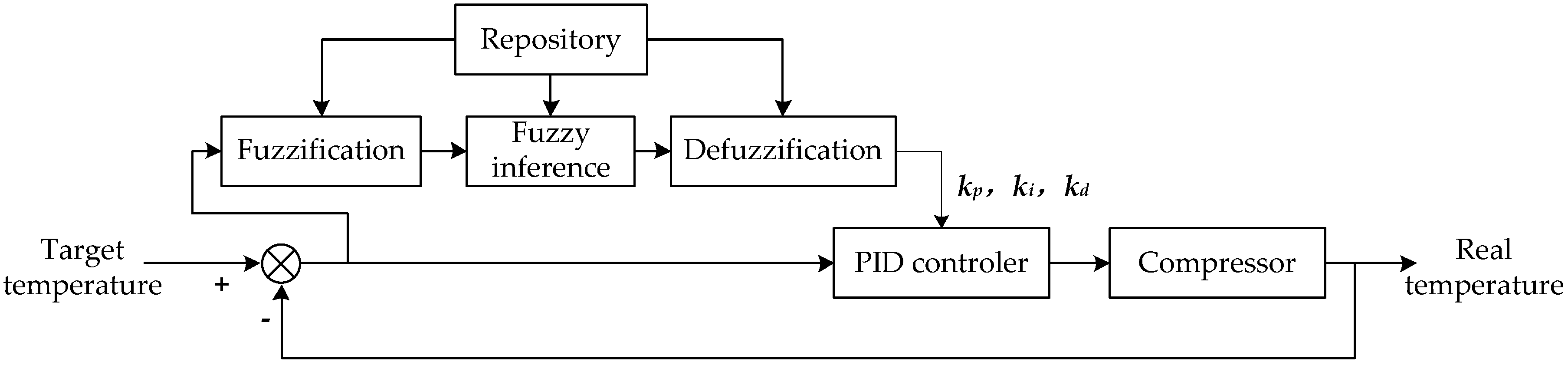
Figure 12.
The schematic diagram of thermal control. If the temperature deviation is large, the single fuzzy controller will be adopted to improve the response speed and reduce the negative overshoot; Otherwise, a fuzzy-PID controller will be carried out to improve the accuracy.
Figure 12.
The schematic diagram of thermal control. If the temperature deviation is large, the single fuzzy controller will be adopted to improve the response speed and reduce the negative overshoot; Otherwise, a fuzzy-PID controller will be carried out to improve the accuracy.
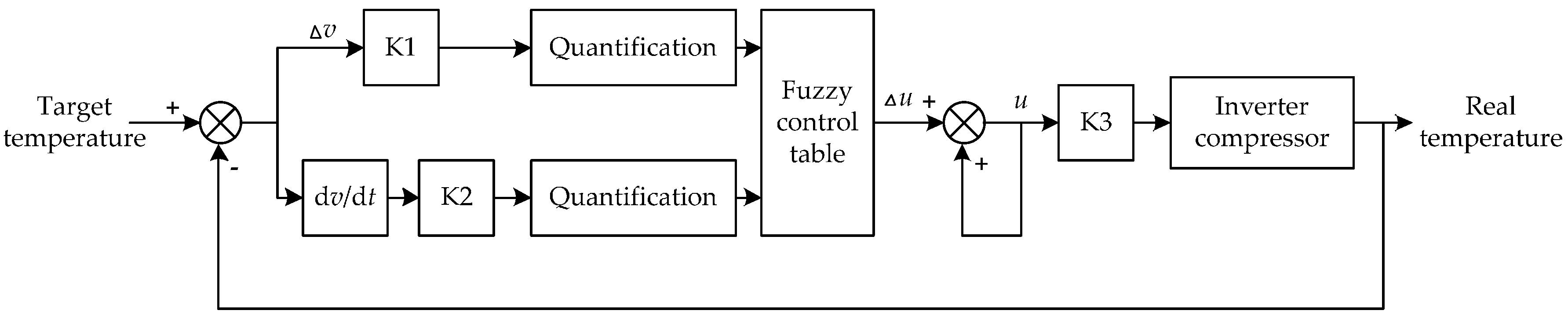
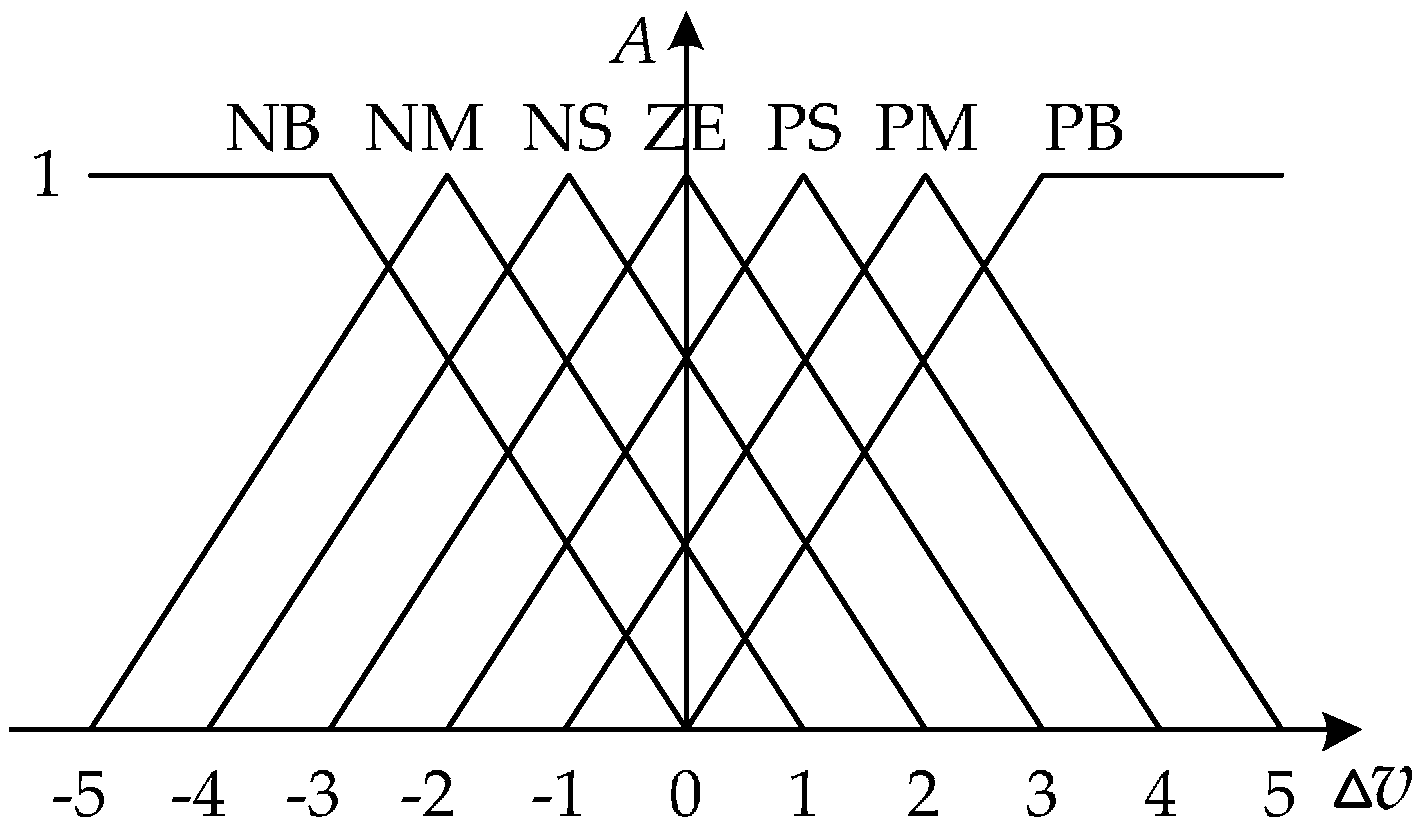
Figure 13.
The schematic diagram of fuzzy control. Here ∆u is the control quantity; ∆v is the motor speed; cv = dv⁄dt is the change rate of speed; K1 & K2 are the quantification factors; K3 is the scale factor. Where ∆u, ∆v, ∆cv are three domains of discourse of fuzzy controller.
Figure 13.
The schematic diagram of fuzzy control. Here ∆u is the control quantity; ∆v is the motor speed; cv = dv⁄dt is the change rate of speed; K1 & K2 are the quantification factors; K3 is the scale factor. Where ∆u, ∆v, ∆cv are three domains of discourse of fuzzy controller.
Figure 14.
The membership function [0, 1] ({, , }), the higher value indicates the more possibility that is classified into a certain related segment.
Figure 14.
The membership function [0, 1] ({, , }), the higher value indicates the more possibility that is classified into a certain related segment.
Figure 15.
The flow diagram of fuzzy-PID method. Here three parameters , and are adjusted using the fuzzy controller, then they are set as the proportion, integral and derivative coefficients of the PID controller.
Figure 15.
The flow diagram of fuzzy-PID method. Here three parameters , and are adjusted using the fuzzy controller, then they are set as the proportion, integral and derivative coefficients of the PID controller.
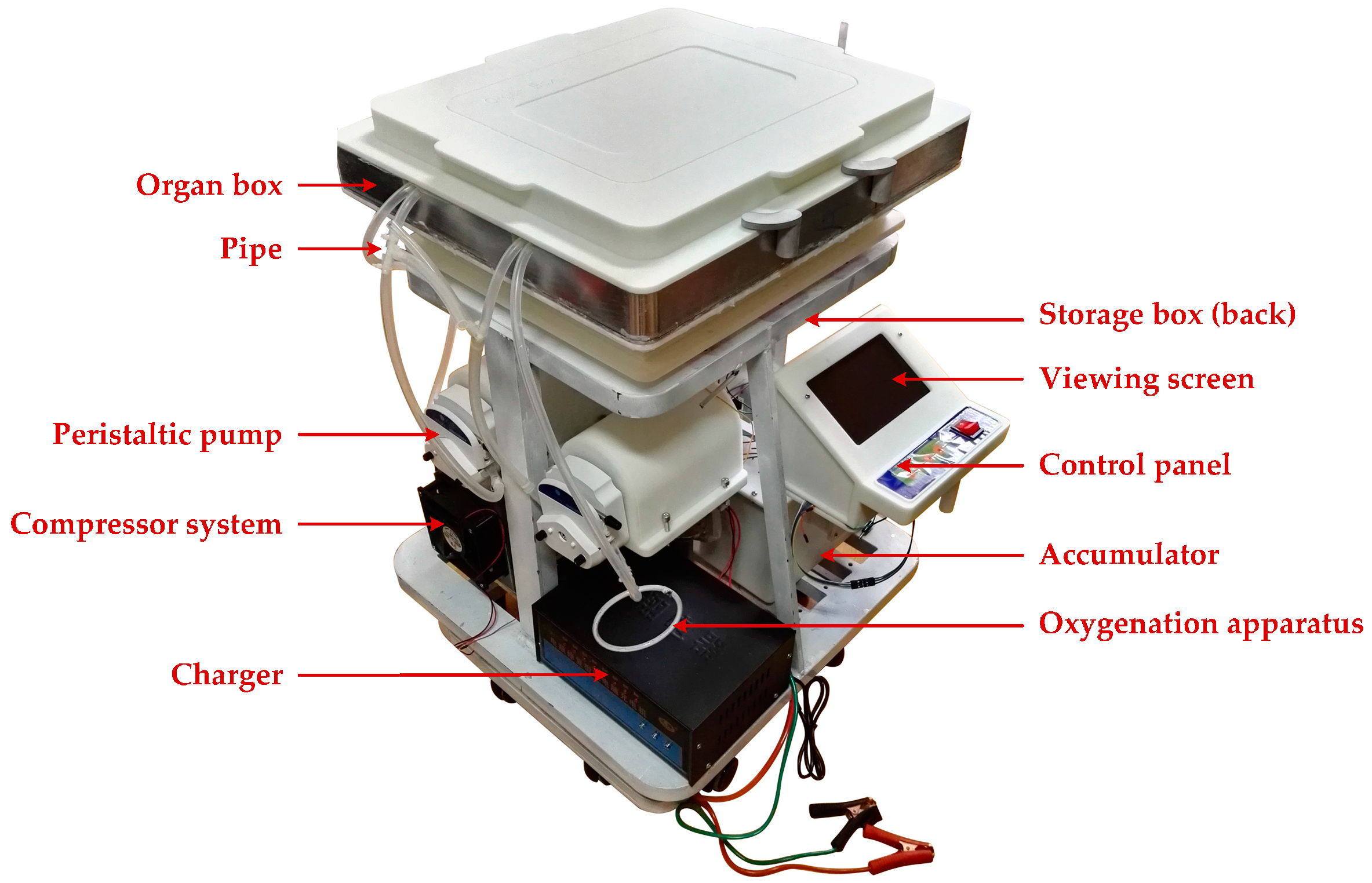
Figure 16.
The hypothermic machine perfusion (HMP) device.
Figure 16.
The hypothermic machine perfusion (HMP) device.
Figure 17.
The dynamic performance trial curves.
Figure 17.
The dynamic performance trial curves.
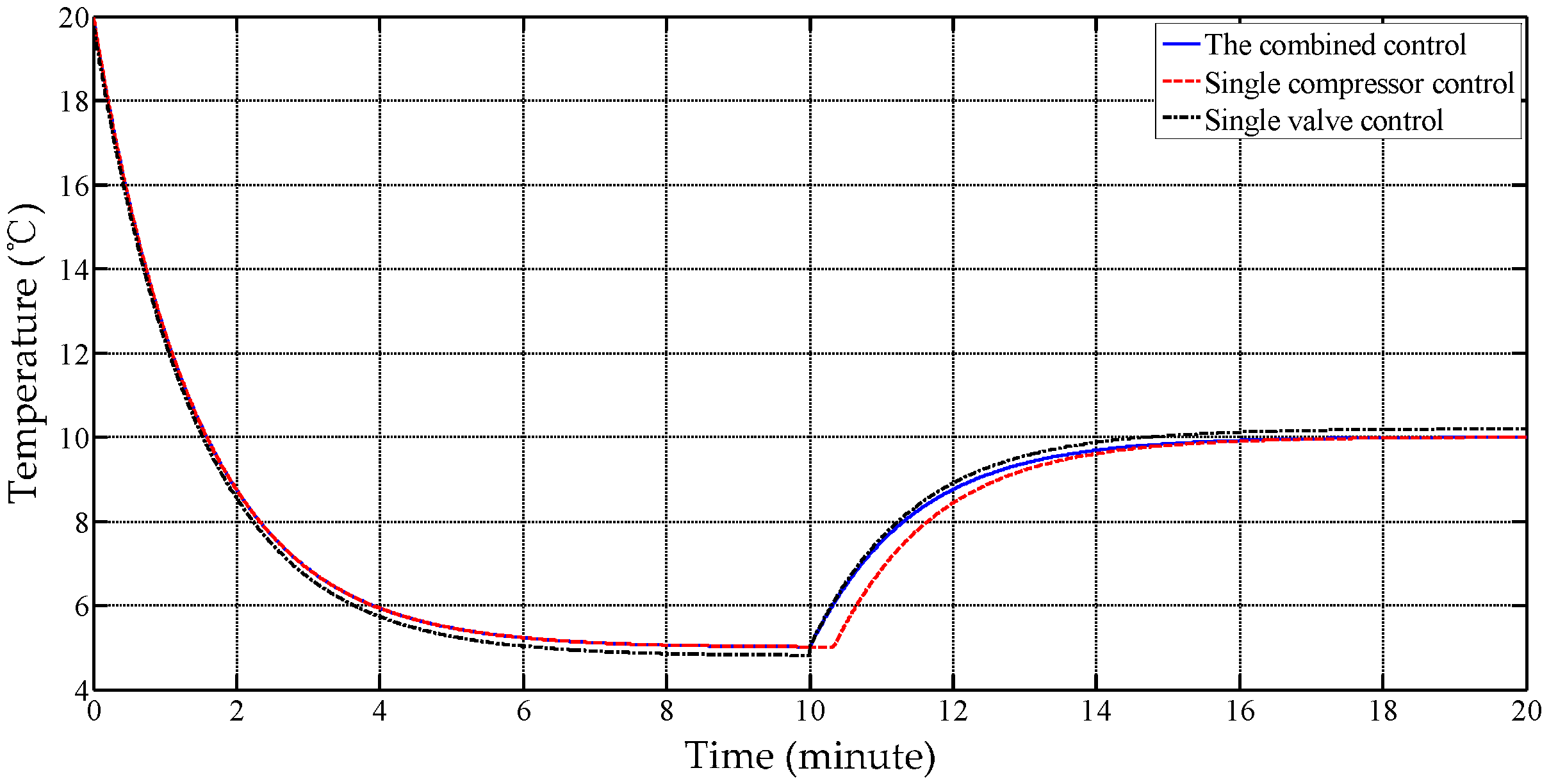
Figure 18.
The trial curves of different working modes.
Figure 18.
The trial curves of different working modes.
Table 1.
A comparison between the three strategies for kidney perfusion [
8,
9,
10,
11].
Table 1.
A comparison between the three strategies for kidney perfusion [
8,
9,
10,
11].
| Feature | SCS | NMP | HMP |
|---|
| The average preservation time | 9.5 h | 12 h | 13 h |
| Organ survival rate after 15 months | 85.0% | 87.7% | 89.2% |
| Organ survival rate after 5 years | 54.2% | 68.7% | 70.5% |
| Facility cost | inexpensive | expensive | expensive |
Table 2.
A comparison between artery and vein loops.
Table 2.
A comparison between artery and vein loops.
| | Pipe Wall | Lumen | Flow Speed |
|---|
| Artery | Thick and elastic | Rather small | Rather fast |
| Vein | Rather thick and weak elastic | Rather big | Rather slow |
Table 3.
The data format between DSP and compressor.
Table 3.
The data format between DSP and compressor.
| TXD (0x) | 1 | 2 | 3–4 | 5–13 | 14 |
| Address | On/Off | Speed | 00 | Check |
| RXD (0x) | 1 | 2–3 | 4–5 | 6 | 7–13 | 14 |
| Address | Speed | Voltage | Fault | 00 | Check |
Table 4.
The lists of fuzzy rules (∆u)/references.
Table 4.
The lists of fuzzy rules (∆u)/references.
| | | NB/−3 | NM/−2 | NS/−1 | ZE/0 | PS/1 | PM/2 | PB/3 |
|---|
| |
|---|
| NB | NB/3 | NB/3 | NM/2 | NM/2 | NS/1 | NS/1 | ZE/0 |
| NM | NB/3 | NM/2 | NM/2 | NS/1 | NS/1 | ZE/0 | PS/−1 |
| NS | NM/2 | NM/2 | NS/1 | NS/1 | ZE/0 | PS/−1 | PS/−1 |
| ZE | NM/2 | NS/1 | NS/1 | ZE/0 | PS/−1 | PS/−1 | PM/−2 |
| PS | NS/1 | NS/1 | ZE/0 | PS/−1 | PS/−1 | PM/−2 | PM/−2 |
| PM | NS/1 | ZE/0 | PS/−1 | PS/−1 | PM/−2 | PM/−2 | PB/−3 |
| PB | ZE/0 | PS/−1 | PS/−1 | PM/−2 | PM/−2 | PB/−3 | PB/−3 |
Table 5.
The lists of fuzzy rules/ references.
Table 5.
The lists of fuzzy rules/ references.
| Input | | NM/−2 | NS/−1 | ZE/0 | PS/1 | PM/2 |
|---|
| Output | | PM/0.03 | PS/0.025 | ZE/0.02 | NS/0.015 | NM/0.01 |
| PM/0.035 | PS/0.031 | ZE/0.027 | NS/0.023 | NM/0.02 |
| NM/0 | NM/0 | NS/0.005 | ZE/0.010 | PM/0.02 |
Table 6.
The standard deviations of error.
Table 6.
The standard deviations of error.
| Flow Speed (mL/min) | Setting Temperature () | Maximum Temperature () | Minimum Temperature () | Standard Deviation () |
|---|
| 40 | +5 | +5.17 | +4.85 | 0.1091 |
| +15 | +15.19 | +14.82 | 0.1056 |
| 60 | +5 | +5.29 | +4.77 | 0.1613 |
| +15 | +15.25 | +14.72 | 0.1642 |
| 80 | +5 | +5.41 | +4.66 | 0.2364 |
| +15 | +15.38 | +14.57 | 0.2348 |
Table 7.
The static performance of different modes.
Table 7.
The static performance of different modes.
| Mode | Setting Temperature () | Standard Deviation () | Power Consumption |
|---|
| Single compressor | +5 | 0.8331 | Minimum |
| +15 | 0.6517 |
| Single valve | +5 | 0.4920 | Maximum |
| +15 | 0.4501 |
| Combination | +5 | 0.0813 | Middle |
| +15 | 0.1073 |