Submersible Spectrofluorometer for Real-Time Sensing of Water Quality
Abstract
:1. Introduction
2. Instrument Design
2.1. Light Sources and Spectrometer
| Indicator | Absorption (nm) | Emission (nm) |
|---|---|---|
| Protein-like material (tyrosine and tryptophan) | 270–290 | 350–400 |
| Oil | 280–300 | 350–400 |
| Chl-a | 400–500 | 670–690 |

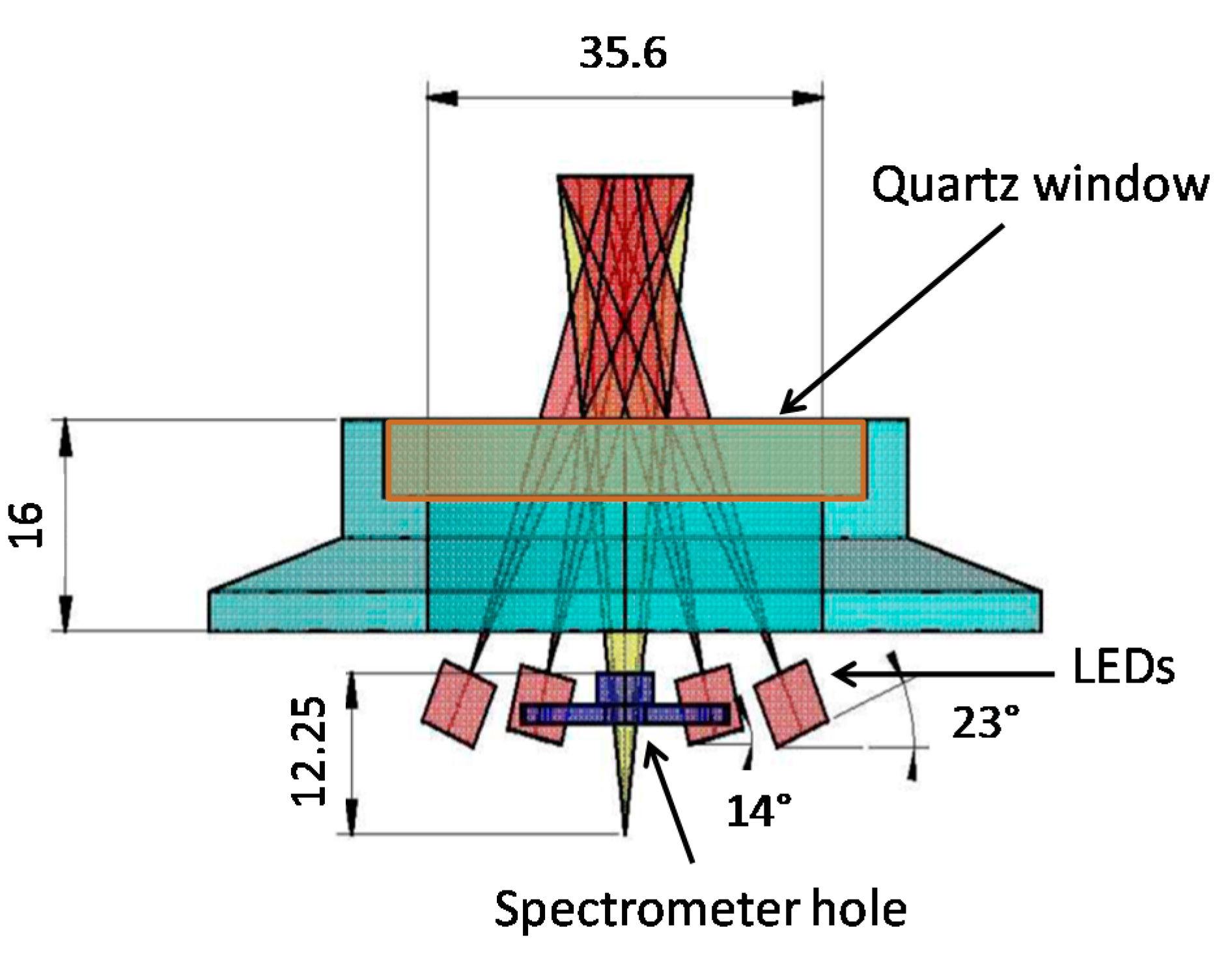
2.2. Mechanics and Electronics


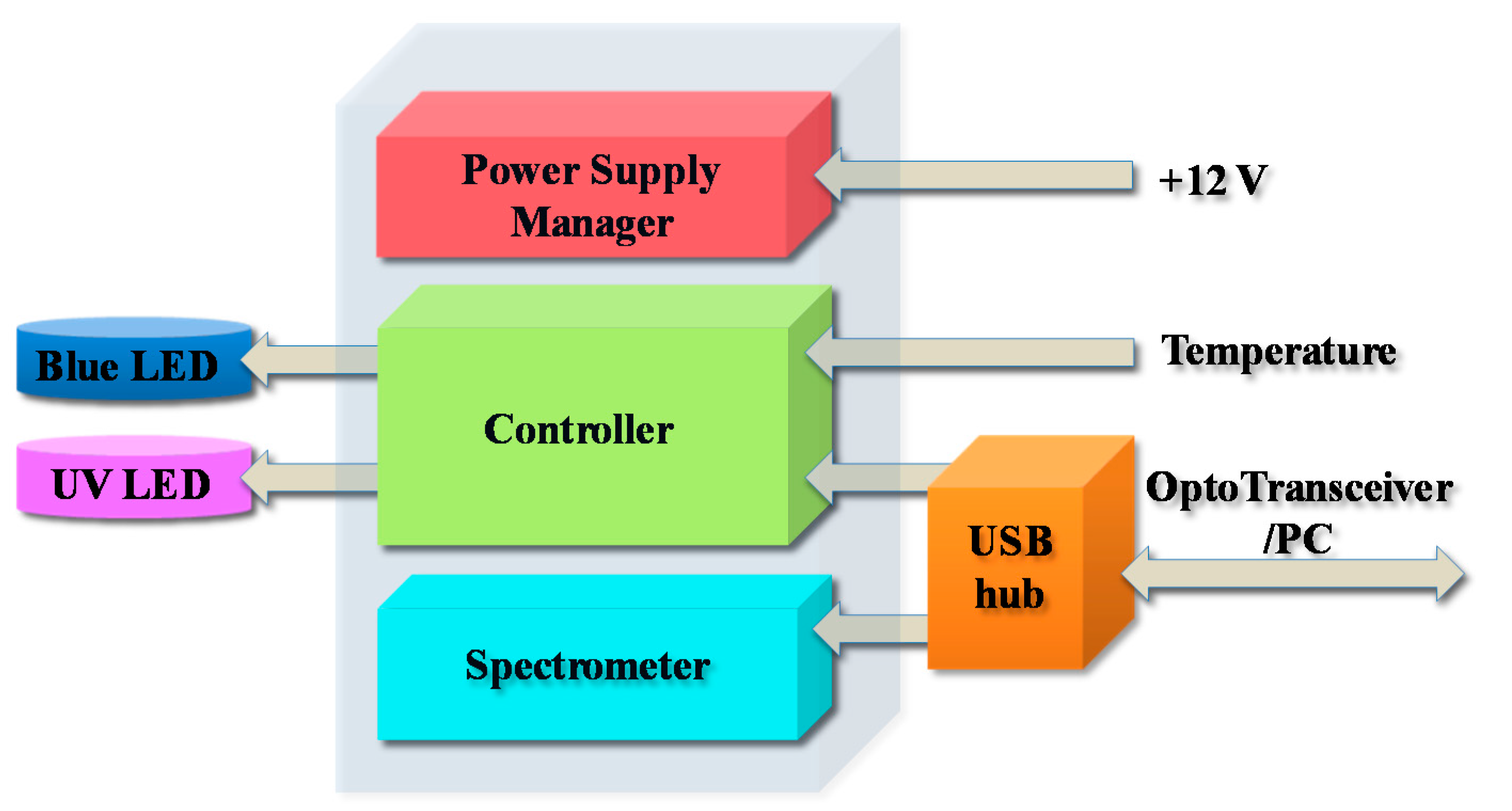
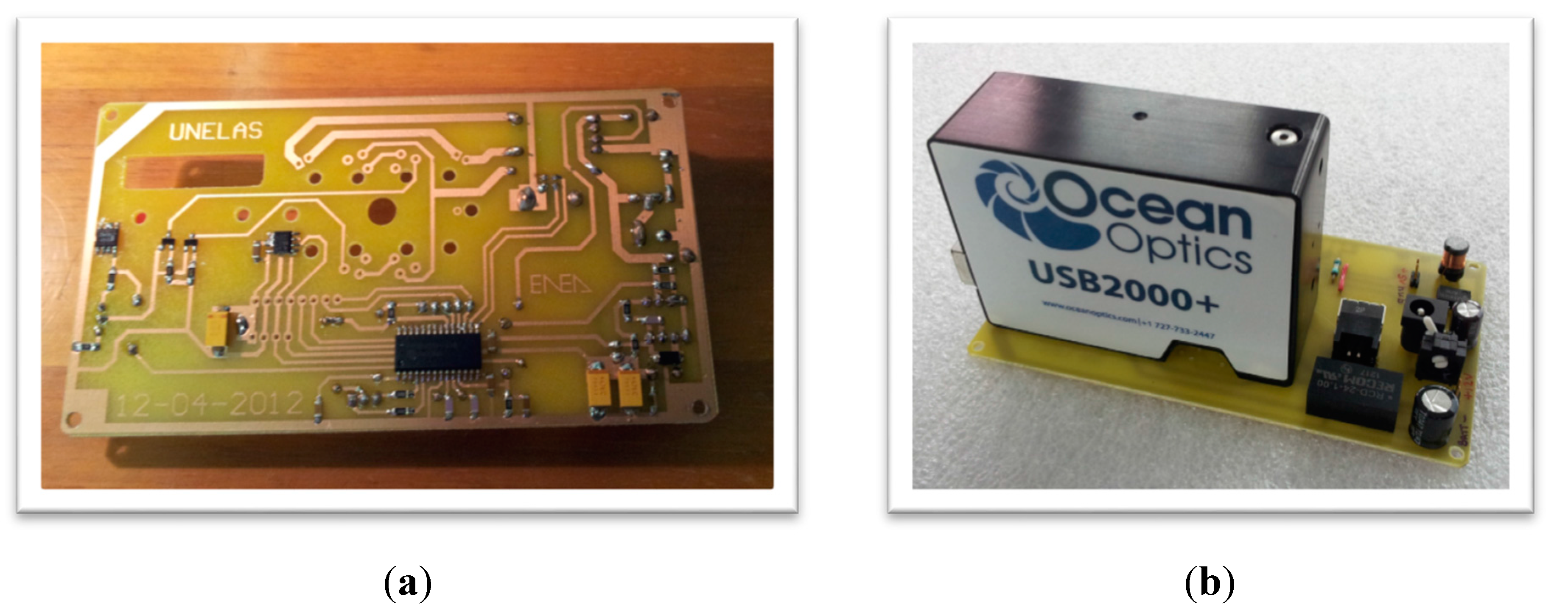
2.3. Assembled Prototype
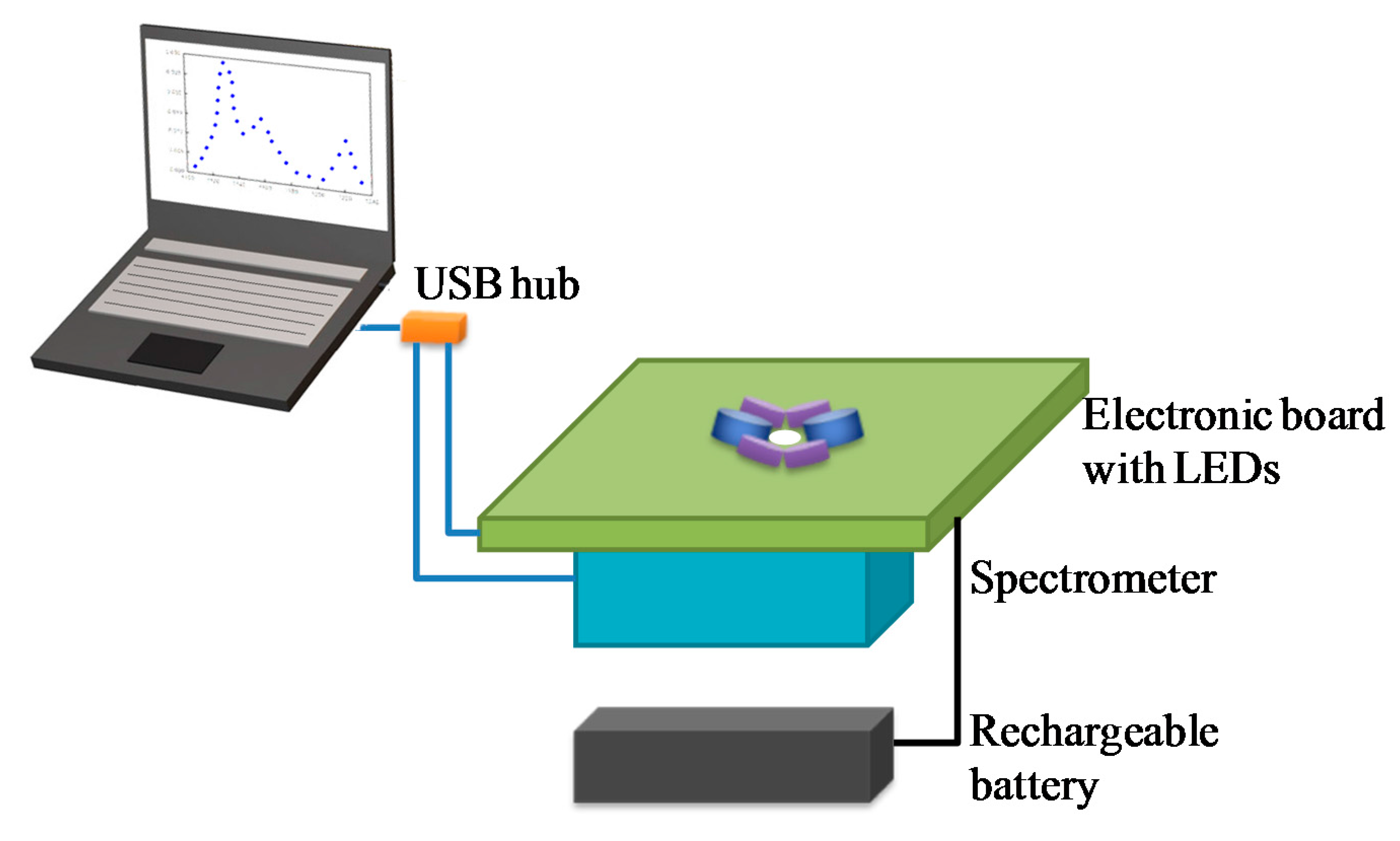

2.4. Hardware and Software for Data Acquisition and Processing
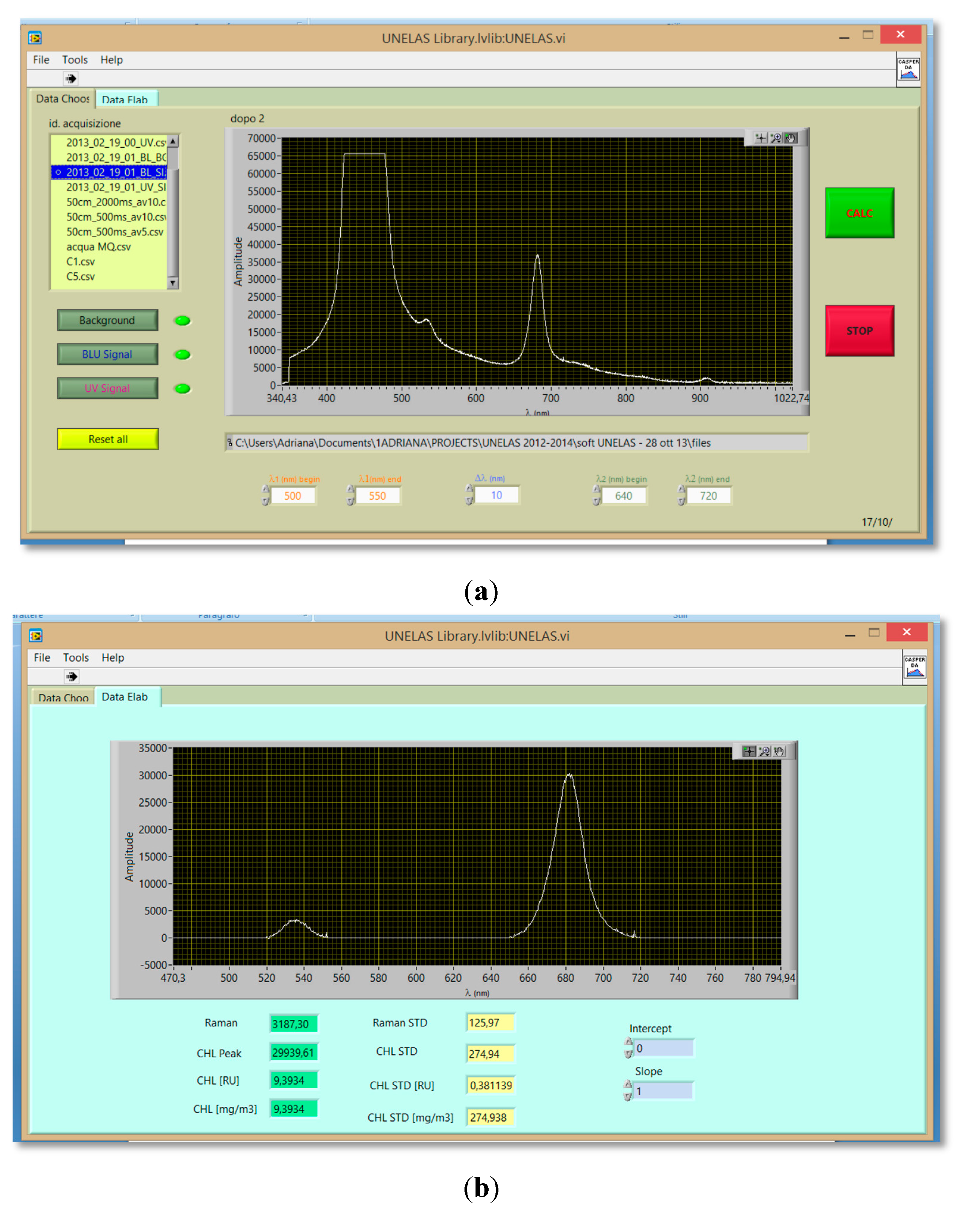
3. Materials and Procedures
3.1. Materials and Procedures for Laboratory Tests
3.2. Materials and Procedures for Field Tests

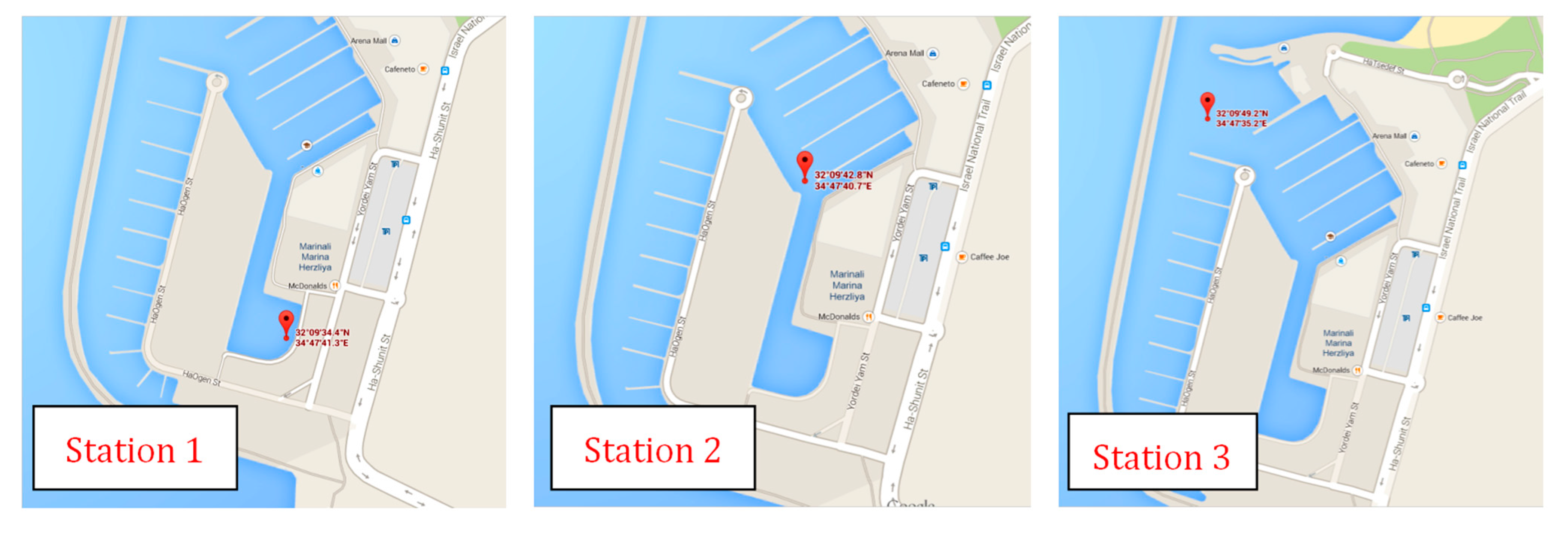
4. Results and Discussion
4.1. Sensor Calibration with Chl-a
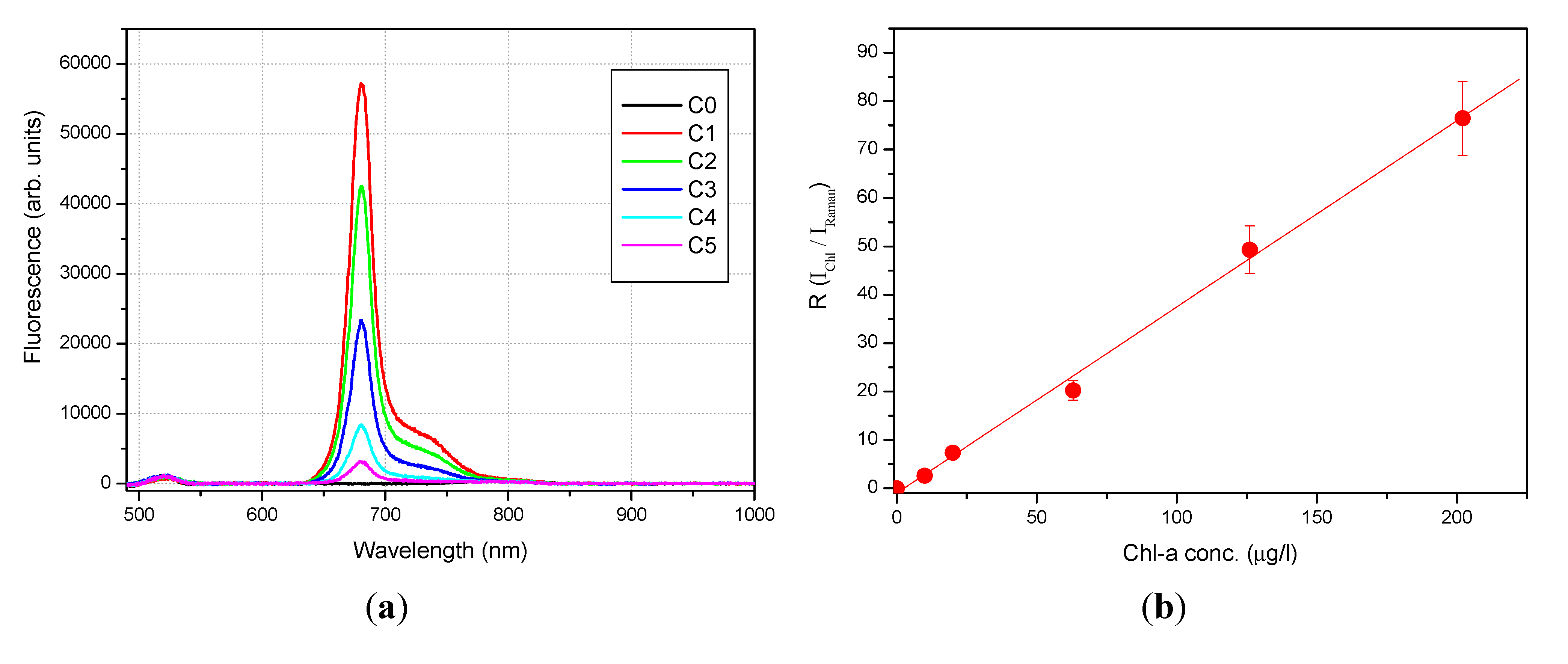
| ample | Ichl (arb. units) | IRaman (arb. units) | R = Ichl/IRaman (R.U.) | Chl-a Conc. (µg/L) |
|---|---|---|---|---|
| C0 | 0 | 1185 ± 103 | 0 | 0 |
| C1 | 56749 ± 384 | 742 ± 95 | 76.49 ± 9.81 | 202 |
| C2 | 42053 ± 323 | 853 ± 88 | 49.32 ± 5.09 | 126 |
| C3 | 22895 ± 405 | 1131 ± 121 | 20.23 ± 2.19 | 63 |
| C4 | 8250 ± 142 | 1122 ± 71 | 7.35 ± 0.48 | 20 |
| C5 | 2938 ± 97 | 1126 ± 95 | 2.61 ± 0.23 | 10 |
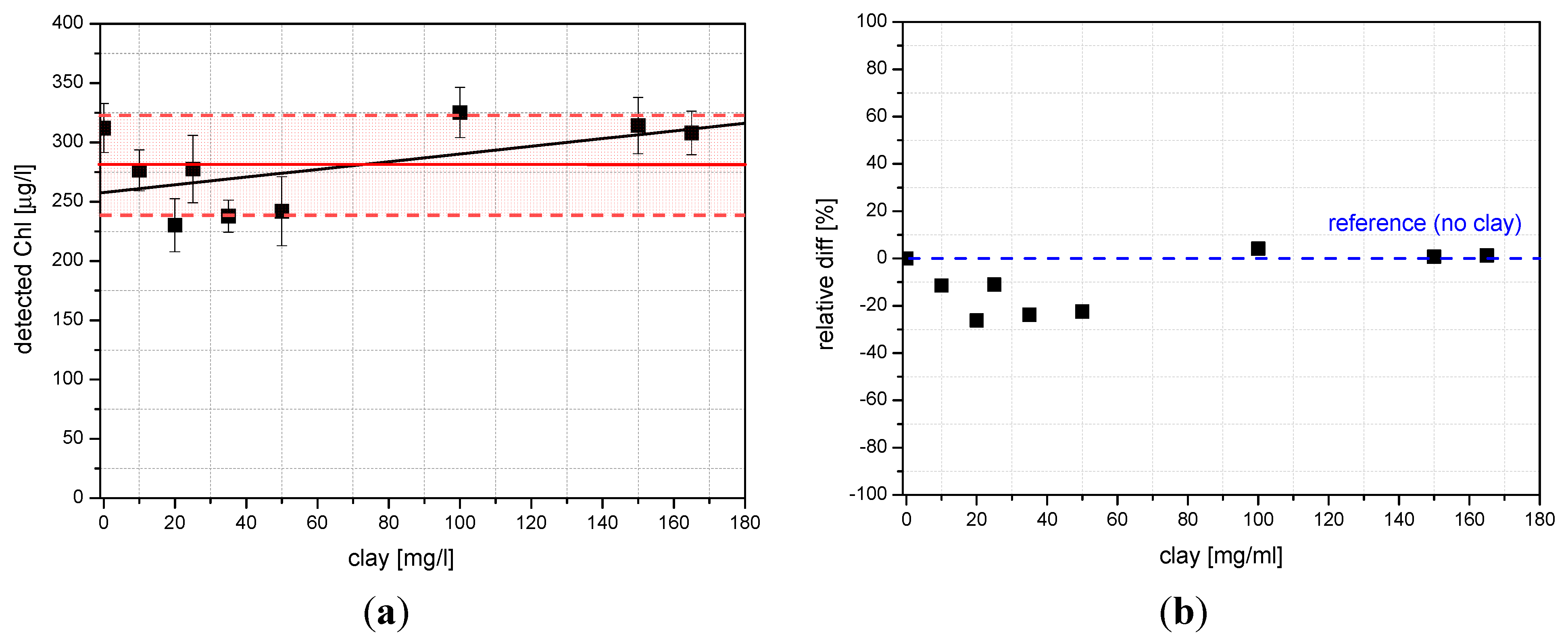
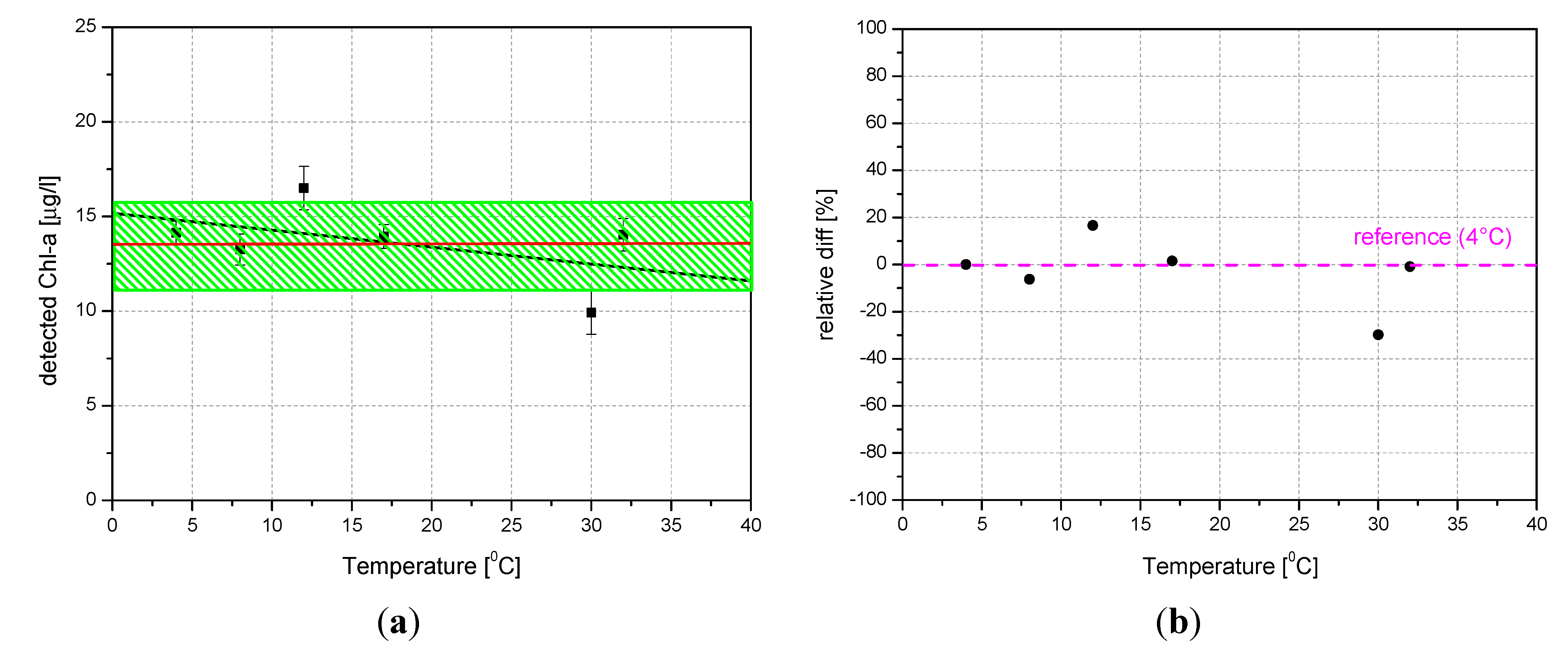
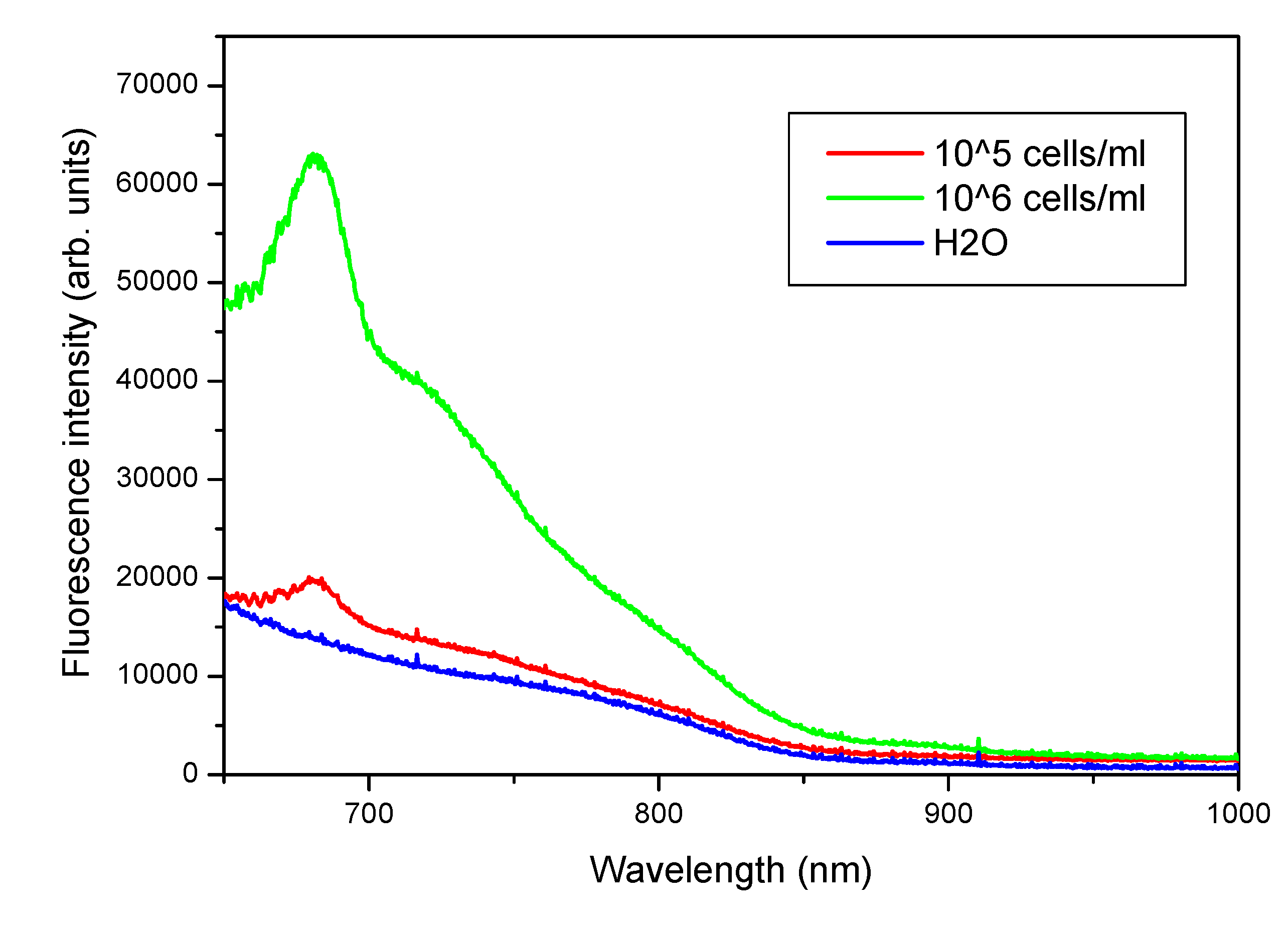
4.2. Laboratory Tests on Diesel Oil and Tryptophan

4.3. Field Results
| Parameter | Chl-a (RU) | E (RU) | ER (%) | Chl-a (µg/L) | Relative Diff. (%) |
|---|---|---|---|---|---|
| Lake surface | 0.0536 | 0.0010 | 1.9 | 0.181 | +0.03% |
| 50-cm depth | 0.0737 | 0.0021 | 2.8 | 0.249 | −0.02% |
| Station | GPS Position | Depth | Chl-a (µg/L) |
|---|---|---|---|
| 1 (AM) | N32°09′ 0.5728, E034º47′ 0.6879 | 1 m | 736 |
| 1 (PM) | N32°09′ 0.5728, E034º47′ 0.6879 | surface | 309 |
| 2 | N32°09′ 0.7129, E034º47′ 0.6784 | surface | 687 |
| 3 | N32°09′ 0.8204, E034º47′ 0.5874 | surface | 43 |
5. Conclusions and Future Developments
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pöttering, H.-G.; Lenarčič, J. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008; Official Journal of the European Union: Strasbourg, France, 2008. [Google Scholar]
- Dimas, S. Commission Directive 2009/90/EC of the European Parliament and of the Council of 31 July 2009; Official Journal of the European Union: Brussels, Belgium, 2009. [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000; Official Journal of the European Communities: Aberdeen, UK, 22 December 2000; pp. 1–73.
- Fiorani, L. Environmental monitoring by laser radar. In Lasers and Electro-Optics Research at the Cutting Edge; Larkin, S.B., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2007; pp. 123–175. [Google Scholar]
- Environmental Monitoring and Support Laboratory of the United States Environmental/Protection Agency. Handbook for Analytical Quality Control in Water and Wastewater Laboratories; EPA-600/4-79-019; U.S. Environmental Protection Agency: Washington, DC, USA, 1979. [Google Scholar]
- Pejcic, B.; Myers, M.; Ross, A. Mid-Infrared Sensing of Organic Pollutants in Aqueous Environments. Sensors 2009, 9, 6232–6253. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deen, M.J.; Kumar, S.; Selvaganapathy, P.R. Raman Spectroscopy for In-Line Water Quality Monitoring—Instrumentation and Potential. Sensors 2014, 14, 17275–17303. [Google Scholar] [CrossRef] [PubMed]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Hasan, J.; Goldbloom-Helzner, D.; Ichida, A.; Rouse, T.; Gibson, M. Technologies and Techniques for Early Warning Systems to Monitor and Evaluate Drinking Water Quality: A State-of-the-Art Review; EPA/600/R-05/156; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Lakowicz, J.R. Fluorescence Sensing. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 623–673. [Google Scholar]
- Yang, L.; Shin, H.-S.; Hur, J. Estimating the Concentration and Biodegradability of Organic Matter in 22 Wastewater Treatment Plants Using Fluorescence Excitation Emission Matrices and Parallel Factor Analysis. Sensors 2014, 14, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Palucci, A. Laser induced fluorescence. In Laser Application in Environmental Monitoring; Fiorani, L., Colao, F., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 131–154. [Google Scholar]
- Baker, A. Fluorescence properties of some farm wastes: Implications for water quality monitoring. Water Res. 2002, 36, 189–195. [Google Scholar] [CrossRef]
- Hur, J.; Hwang, S.-J.; Shin, J.-K. Using Synchronous Fluorescence Technique as a Water Quality Monitoring Tool for an Urban River. Water Air Soil Pollut. 2008, 191, 231–243. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Seredyńska-Sobecka, B.; Boe-Hansen, R.; le Tallec, N.; Waul, C.K.; Arvin, E. A potential approach for monitoring drinking water quality from groundwater systems using organic matter fluorescence as an early warning for contamination events. Water Res. 2011, 45, 6030–6038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Liu, W.Q.; Zhao, N.J.; Li, H.B.; Zhang, Y.J.; Si-Ma, W.C.; Liu, J.G. Composition analysis of colored dissolved organic matter in Taihu Lake based on three dimension excitation-emission fluorescence matrix and PARAFAC model, and the potential application in water quality monitoring. J. Environ. Sci. 2007, 19, 787–791. [Google Scholar] [CrossRef]
- MacIntyre, H.L.; Lawrenz, E.; Richardson, T.L. Taxonomic Discrimination of Phytoplankton by Spectral Fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Prasil, O., Borowitzka, M.A., Eds.; Springer: Dordrecht, Netherlands, 2010; pp. 129–170. [Google Scholar]
- Leeuw, T.; Boss, E.S.; Wright, D.L. In situ measurements of phytoplankton fluorescence using low cost electronics. Sensors 2013, 13, 7872–7883. [Google Scholar] [CrossRef] [PubMed]
- Khamis, K.; Sorensen, J.; Bradley, C.; Hannah, D.; Lapworth, D.J.; Stevens, R. In-situ tryptophan-like fluorometers: Assessing turbidity and temperature effects for freshwater applications. Environ. Sci. Processes Impacts 2015, 17, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Downing, B.D.; Pellerin, B.A.; Bergamaschi, B.A.; Saraceno, J.F.; Kraus, T.E.C. Seeing the light: The effects of particles, dissolved materials, and temperature on in situ measurements of DOM fluorescence in rivers and streams. Limnol. Oceanogr. Methods 2012, 10, 767–775. [Google Scholar] [CrossRef]
- Tedetti, M.; Guigue, C.; Goutx, M. Utilization of a submersible UV fluorometer for monitoring anthropogenic inputs in the Mediterranean coastal waters. Mar. Pollut. Bull. 2010, 60, 350–62. [Google Scholar] [CrossRef] [PubMed]
- Watras, C.J.; Hanson, P.C.; Stacy, T.L.; Morrison, K.M.; Mather, J.; Hu, Y.-H.; Milewski, P. A temperature compensation method for CDOM fluorescence sensors in freshwater. Limnol. Oceanogr. Methods 2011, 9, 296–301. [Google Scholar] [CrossRef]
- Fantoni, R.; Barbini, R.; Colao, F.; Palucci, A.; Ribezzo, S. Applications of excimer laser based remote sensing systems to problems related to water pollution. In Excimer Lasers; Laude, L.D., Ed.; Kluwer Academic Publicher: Dordrecht, The Netherlands, 1994; pp. 289–305. [Google Scholar]
- Palucci, A. Optical and morphological characterization of selected phytoplanktonic communities—Applications for remote sensing. In Optics of Biological Particles; Hoekstra, A., Maltsev, V., Videen, G., Eds.; Springer: Dordrecht, Netherlands, 2007; pp. 165–192. [Google Scholar]
- bbe Moldaenke, Germany. Available online: http://www.bbe-moldaenke.de (accessed on 19 February 2015).
- Chelsea Technologies Group, UK. Available online: http://www.chelsea.co.uk/ (accessed on 25 February 2015).
- JFE Advantech Corporation, Japan. Available online: http://www.jfe-alec.co.jp/html/english_top.htm (accessed on 4 March 2015).
- Photon Systems Instruments, Czech Republic. Available online: http://www.psi.cz/ (accessed on 9 March 2015).
- Turner Designs, US. Available online: http://www.turnerdesigns.com (accessed on 11 March 2015).
- Fiorani, L.; Palucci, A. Local and remote laser sensing of bio-optical parameters in natural waters. J. Comput. Technol. 2006, 11, 39–45. [Google Scholar]
- Sighicelli, M.; Iocola, I.; Pittalis, D.; Lugliè, A.; Padedda, B.M.; Pulina, S.; Iannett, M.; Menicucci, I.; Fiorani, L.; Palucci, A. An innovative and high-speed technology for seawater monitoring of Asinara Gulf (Sardinia-Italy). Open J. Mar. Sci. 2014, 4, 31–41. [Google Scholar] [CrossRef]
- Caputo-Rapti, D.; Fioran, L.; Palucci, A. Compact laser spectrofluorometer for water monitoring campaigns of Southern Italian regions affected by salinization and desertification processes. J. Optoelectron. Adv. Mater. 2008, 10, 461–469. [Google Scholar]
- Montes-Hugo, M.A.; Fiorani, L.; Marullo, S.; Roy, S.; Gagné, J.-P.; Borelli, R.; Demers, S.; Palucci, A. A comparison between local and global spaceborne chlorophyll indices in the St Lawrence Estuary. Remote Sens. 2012, 4, 3666–3688. [Google Scholar] [CrossRef]
- Cisek, M.; Colao, F.; Demetrio, E.; di Cicco, A.; Drozdowska, V.; Fiorani, L.; Goszczko, I.; Lazic, V.; Okladnikov, I.G.; Palucci, A.; et al. Remote and local monitoring of dissolved and suspended fluorescent organic matter off the Svalbard. J. Optoelectron. Adv. Mater. 2010, 12, 1604–1618. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puiu, A.; Fiorani, L.; Menicucci, I.; Pistilli, M.; Lai, A. Submersible Spectrofluorometer for Real-Time Sensing of Water Quality. Sensors 2015, 15, 14415-14434. https://doi.org/10.3390/s150614415
Puiu A, Fiorani L, Menicucci I, Pistilli M, Lai A. Submersible Spectrofluorometer for Real-Time Sensing of Water Quality. Sensors. 2015; 15(6):14415-14434. https://doi.org/10.3390/s150614415
Chicago/Turabian StylePuiu, Adriana, Luca Fiorani, Ivano Menicucci, Marco Pistilli, and Antonia Lai. 2015. "Submersible Spectrofluorometer for Real-Time Sensing of Water Quality" Sensors 15, no. 6: 14415-14434. https://doi.org/10.3390/s150614415
APA StylePuiu, A., Fiorani, L., Menicucci, I., Pistilli, M., & Lai, A. (2015). Submersible Spectrofluorometer for Real-Time Sensing of Water Quality. Sensors, 15(6), 14415-14434. https://doi.org/10.3390/s150614415









