Enhanced Sensitivity of Gas Sensor Based on Poly(3-hexylthiophene) Thin-Film Transistors for Disease Diagnosis and Environment Monitoring
Abstract
:1. Introduction
2. Experimental Section
2.1. Sensors Preparation
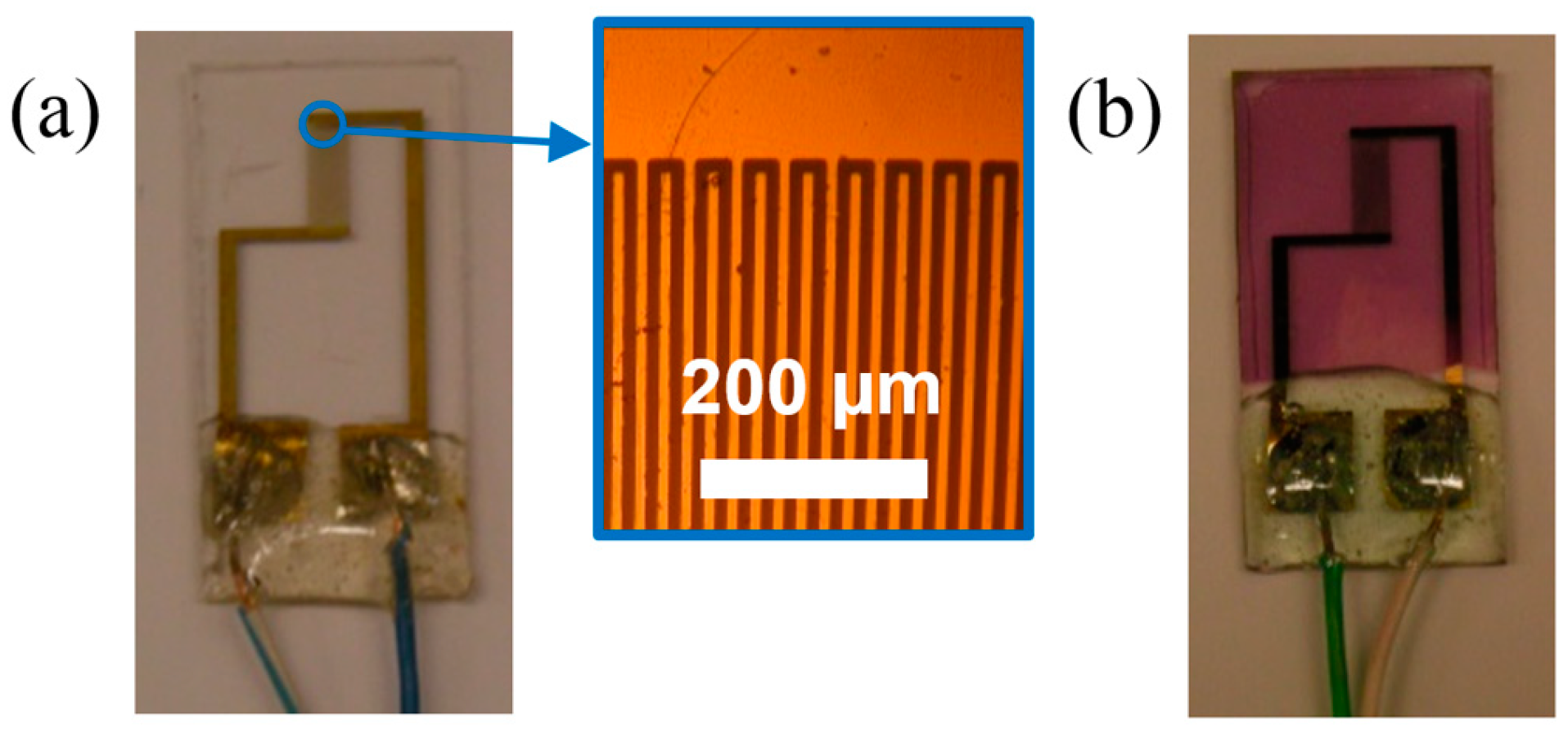
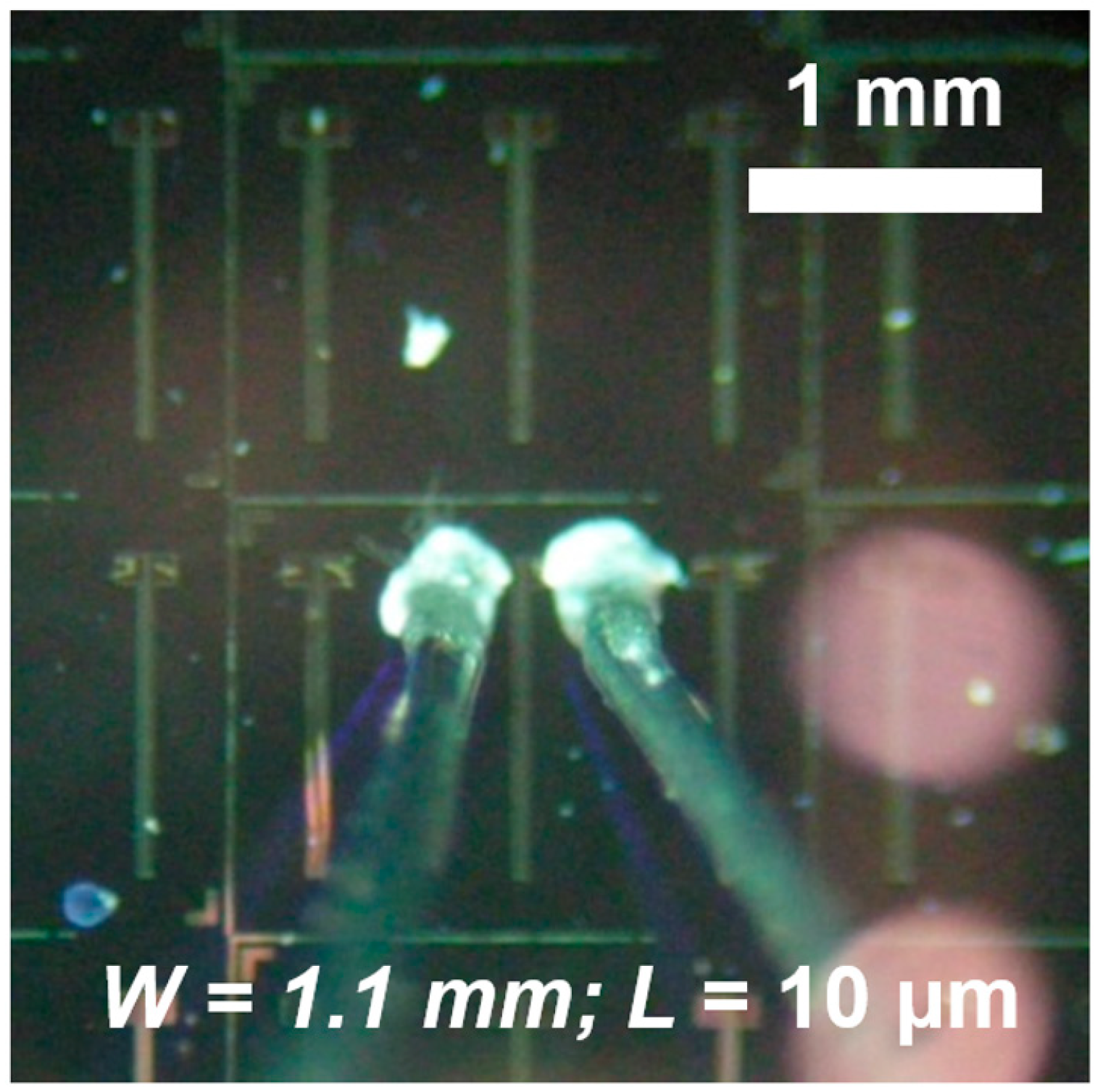
2.2. Gaseous Samples
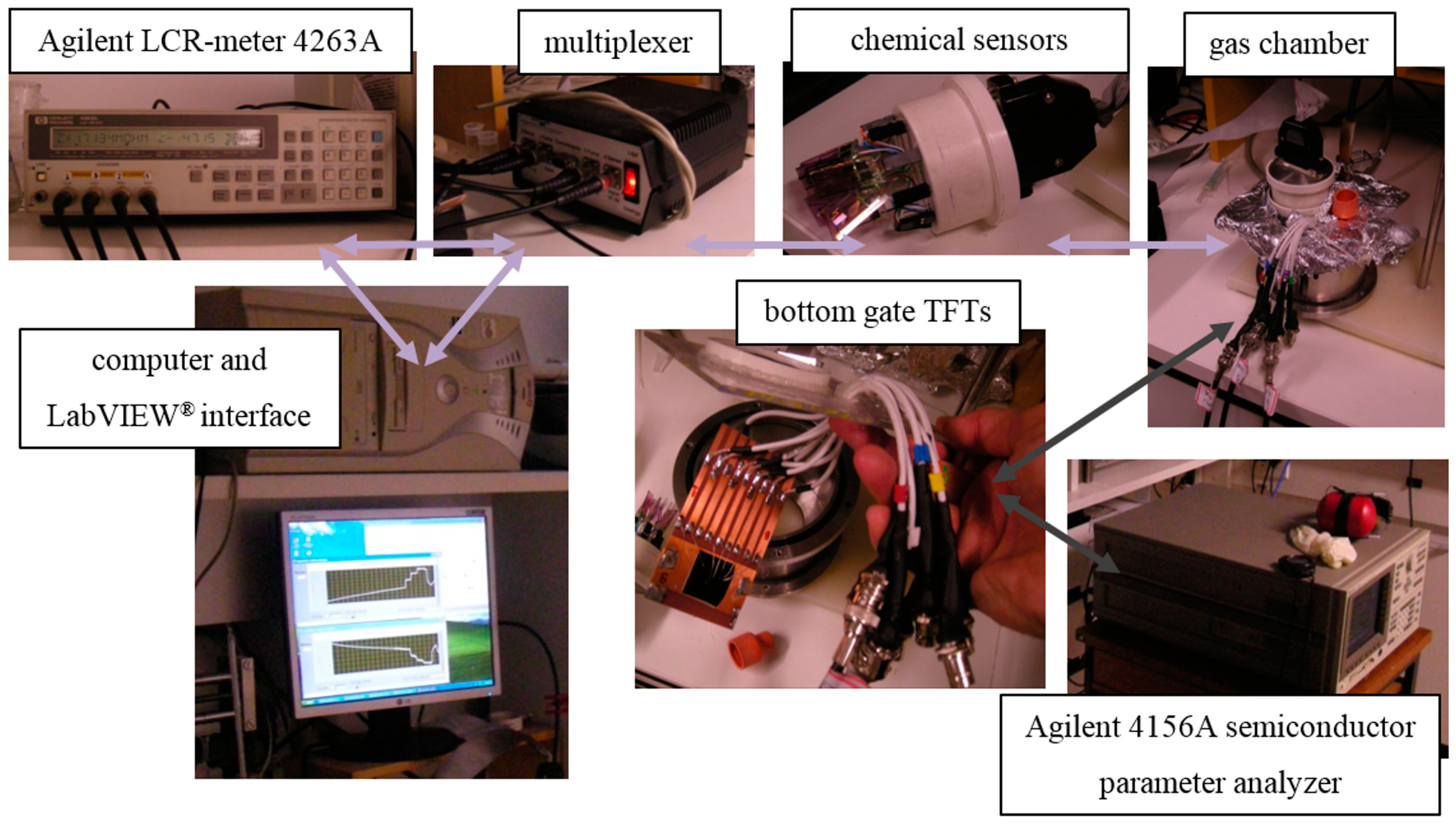
2.3. Electronic Nose System and Sample Measurement
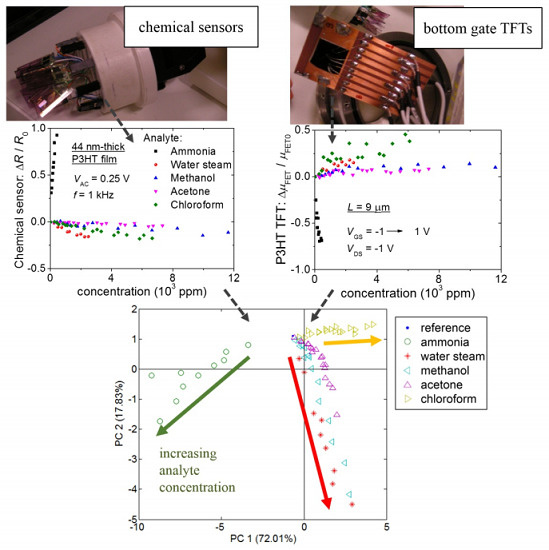
2.4. Data Analysis
3. Results
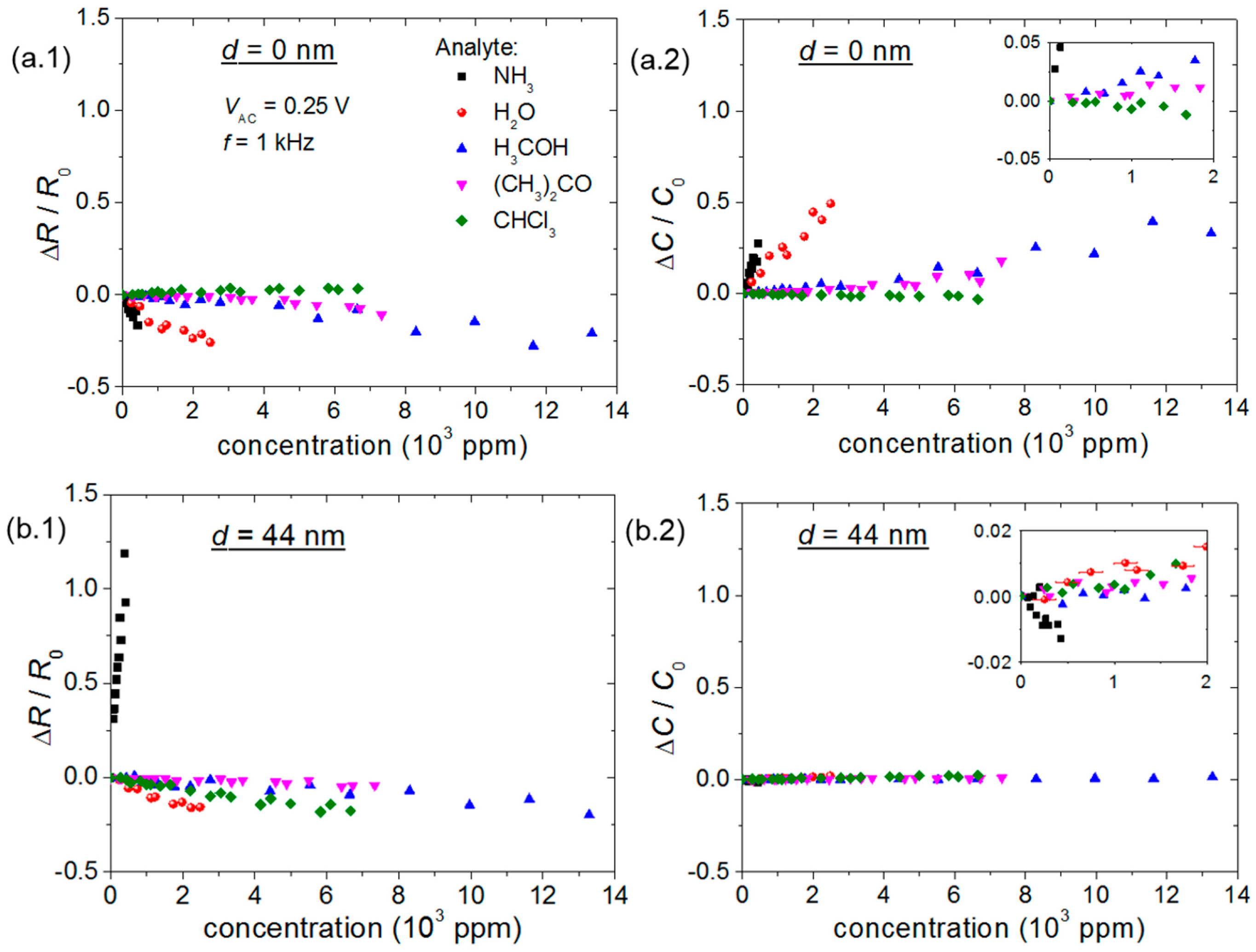
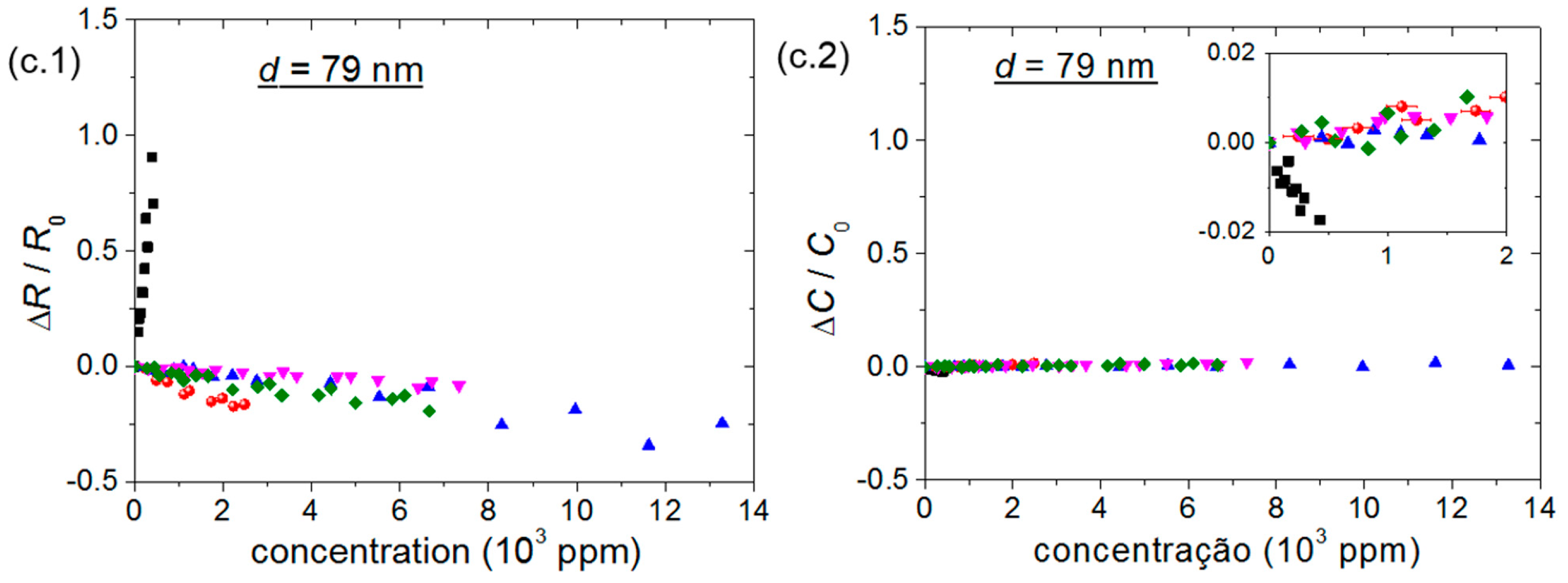
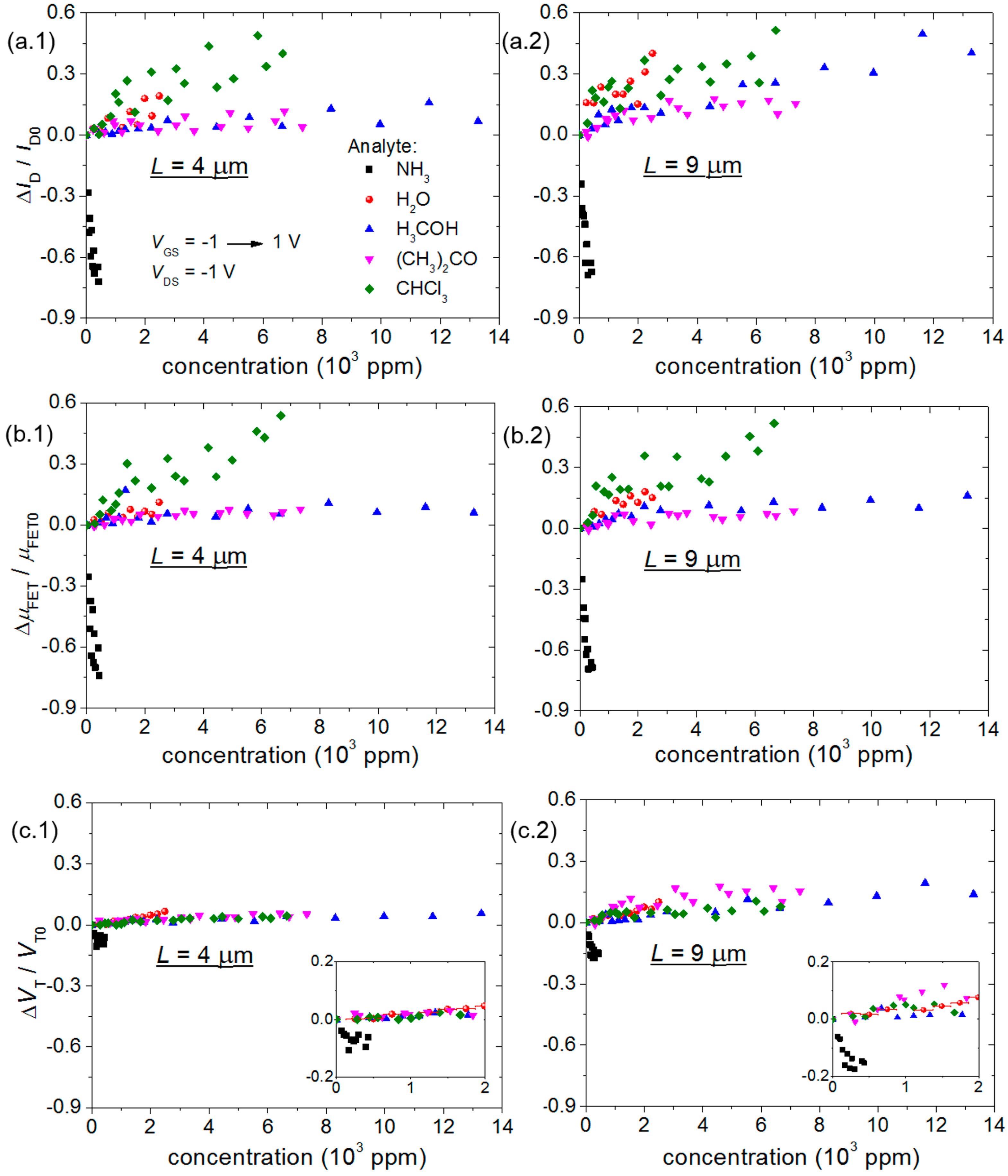
- (i)
- The R and C changes of chemical sensors have opposite signs;
- (ii)
- The highest ΔC/C0 of chemical sensors occurs for the bare sensor;
- (iii)
- ID and μFET variations have the opposite sign of a change in R;
- (iv)
- Differently from other gaseous analytes investigated herein, an increase in ammonia concentration increases R of P3HT chemical sensors and decreases ID, μFET and VT of transistors.
| Analyte | Chemical Sensors | P3HT TFTs | |||||
|---|---|---|---|---|---|---|---|
| d (nm) | L (μm) | ||||||
| NH3 * | 0 | −104 ± 52 | 150 ± 68 | ||||
| 44 ± 3 | 2480 ± 240 | −45.8 ± 7.5 | 4 ± 1 | −2260 ± 60 | −2190 ± 40 | −303 ± 24 | |
| 79 ± 4 | 2080 ± 200 | −53.1 ± 6.8 | 9 ± 1 | −2310 ± 150 | −2270 ± 80 | −555 ± 35 | |
| H2O | 0 | −95.1 ± 11.6 | 187 ± 14 | ||||
| 44 ± 3 | −64.9 ± 5.7 | 7.1 ± 1.3 | 4 ± 1 | 62.9 ± 8.1 | 35.7 ± 4.2 | 24.0 ± 0.9 | |
| 79 ± 4 | −69.4 ± 7.0 | 4.7 ± 1.3 | 9 ± 1 | 145 ± 17 | 76.9 ± 5.8 | 35.0 ± 1.9 | |
| H3COH | 0 | −18.5 ± 1.8 | 27.9 ± 2.1 | ||||
| 44 ± 3 | −12.1 ± 1.4 | 0.9 ± 0.1 | 4 ± 1 | 17.1 ± 1.7 | 48.3 ± 18.3 | 12.1 ± 1.2 | |
| 79 ± 4 | −22.9 ± 2.3 | 0.8 ± 0.2 | 9 ± 1 | 79.4 ± 10.9 | 44.2 ± 3.3 | 16.9 ± 1.8 | |
| (CH3)2CO | 0 | −11.7 ± 1.1 | 17.1 ± 2.1 | ||||
| 44 ± 3 | −5.2 ± 0.7 | 1.0 ± 0.2 | 4 ± 1 | 36.8 ± 7.1 | 22.0 ± 3.6 | 21.1 ± 2.8 | |
| 79 ± 4 | −10.8 ± 0.8 | 2.0 ± 0.2 | 9 ± 1 | 75.0 ± 5.5 | 38.7 ± 5.8 | 41.8 ± 3.8 | |
| CHCl3 | 0 | 4.7 ± 0.8 | −2.9 ± 0.5 | ||||
| 44 ± 3 | −27.0 ± 1.5 | 3.4 ± 0.3 | 4 ± 1 | 143 ± 21 | 164 ± 26 | 10.8 ± 1.9 | |
| 79 ± 4 | −24.7 ± 2.2 | 1.6 ± 0.4 | 9 ± 1 | 169 ± 22 | 197 ± 26 | 43.0 ± 4.5 | |
- (i)
- Further distinction of acetone from water vapor and methanol;
- (ii)
- Increased distance among the lowest concentration points of chloroform, methanol and acetone.
- (i)
- R0 from C0 along PC 2 for bare sensors and {R1, R2} from {C1, C2} along PC 1 for P3HT chemical sensors;
- (ii)
- {R0, C0} from {R1, R2} and {C1, C2} along PC 1.

4. Discussion
4.1. P3HT Role in Chemical Sensors
- (i)
- The main sensing mechanism is through thin-film resistivity changes, as |ΔR/R0| ≫ |ΔC/C0|;
- (ii)
- The highest sensitivity is in response to ammonia, the only analyte responsible for increasing R;
- (iii)
- All analytes can be quantified if injected separately in the concentration range of the herein reported experiments.
4.2. E-Nose Performance Improvement with TFTs
| Analyte | P3HT Chemical Sensor | P3HT TFT | ||||
|---|---|---|---|---|---|---|
| d (nm) | L (μm) | |||||
| NH3 * | 44 ± 3 | 4.94 ± 0.48 | 4 ± 1 | −824 ± 23 | −797 ± 13 | −110 ± 9 |
| 79 ± 4 | 4.14 ± 0.39 | 9 ± 1 | −1890 ± 120 | −1860 ± 60 | −454 ± 29 | |
| H2O | 44 ± 3 | −0.129 ± 0.011 | 4 ± 1 | 22.9 ± 2.9 | 13.0 ± 1.5 | 8.7 ± 0.3 |
| 79 ± 4 | −0.138 ± 0.014 | 9 ± 1 | 118 ± 14 | 62.9 ± 4.7 | 28.6 ± 1.6 | |
| H3COH | 44 ± 3 | −0.024 ± 0.003 | 4 ± 1 | 6.2 ± 0.6 | 17.6 ± 6.7 | 4.4 ± 0.4 |
| 79 ± 4 | −0.046 ± 0.005 | 9 ± 1 | 65.0 ± 8.9 | 36.2 ± 2.7 | 13.8 ± 1.5 | |
| (CH3)2CO | 44 ± 3 | −0.010 ± 0.001 | 4 ± 1 | 13.4 ± 2.6 | 8.0 ± 1.3 | 7.7 ± 1.0 |
| 79 ± 4 | −0.022 ± 0.002 | 9 ± 1 | 61.4 ± 4.5 | 31.7 ± 4.7 | 34.2 ± 3.1 | |
| CHCl3 | 44 ± 3 | −0.054 ± 0.003 | 4 ± 1 | 52.1 ± 7.7 | 59.6 ± 9.5 | 3.9 ± 0.7 |
| 79 ± 4 | −0.049 ± 0.004 | 9 ± 1 | 138 ± 18 | 161 ± 21 | 35.2 ± 3.7 | |
5. Conclusions
Acknowledgments
Supplementary Files
Supplementary File 1Author Contributions
Conflicts of Interest
References
- Mukhopadhyay, R. Don’t waste your breath. Anal. Chem. 2004, 76, 273A–276A. [Google Scholar] [PubMed]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A. The diagnostic potential of breath analysis. Clin. Chem. 1983, 29, 5–15. [Google Scholar] [PubMed]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Chang, J.B.; Liu, V.; Subramanian, V.; Sivula, K.; Luscombe, C.; Murphy, A.; Liu, J.; Fréchet, J.M.J. Printable polythiophene gas sensor array for low-cost electronic noses. J. Appl. Phys. 2006, 100, 014506. [Google Scholar] [CrossRef]
- Bartic, C.; Borghs, G. Organic thin-film transistors as transducers for (bio)analytical applications. Anal. Bioanal. Chem. 2006, 384, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.; Gustafsson, G.; Willander, M.; Svensson, C.; Inganäs, O. Determination of field-effect mobility of poly(3-hexylthiophene) upon exposure to NH3 gas. Synth. Met. 1990, 37, 123–130. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, Y.D.; Kim, Y.M.; Park, Y.W.; Choi, J.H.; Park, T.H.; Soo, C.D.; Won, S.M.; Han, I.K.; Ju, B.K. The response characteristics of a gas sensor based on poly-3-hexylithiophene thin-film transistors. Sens. Actuators B Chem. 2010, 146, 40–45. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, A.K.; Joshi, L.; Chakrabarti, P.; Takashima, W.; Kaneto, K.; Prakash, R. Poly-3-hexylthiophene based organic field-effect transistor: Detection of low concentration of ammonia. Sens. Actuators B Chem. 2012, 171–172, 962–968. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Tai, C.-F.; Lin, R.S.; Yang, S.-C.; Chen, C.-J.; Shih, T.-S.; Liou, S.-H. A proportionate cancer morbidity ratio study of workers exposed to chlorinated organic solvents in Taiwan. Ind. Health 2003, 41, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.-I.; Wang, J.-D.; Chen, P.-C. Increased risk of cancer in the offspring of female electronics workers. Reprod. Toxicol. 2008, 25, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lynge, E.; Anttila, A.; Hemminki, K. Organic solvents and cancer. Cancer Causes Control 1997, 8, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.T.; Wantz, G.; Bejbouji, H.; Urien, M.; Dautel, O.J.; Vignau, L.; Hirsch, L. Polymeric solar cells based on P3HT:PCBM: Role of the casting solvent. Sol. Energy Mater. Sol. Cells 2011, 95, 3408–3418. [Google Scholar] [CrossRef]
- Arias, A.C.; MacKenzie, J.D.; McCulloch, I.; Rivnay, J.; Salleo, A. Materials and applications for large area electronics: Solution-based approaches. Chem. Rev. 2010, 110, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, K.; Zhou, J.; Liu, Y.; Peng, J.; Cao, Y. Poly(3-hexylthiophene) Thin-film transistors with dual insulator layers. Jpn. J. Appl. Phys. 2007, 46, 913–916. [Google Scholar] [CrossRef]
- Tanase, C.; Meijer, E.J.; Blom, P.W.M.; de Leeuw, D.M. Local charge carrier mobility in disordered organic field-effect transistors. Org. Electron. 2003, 4, 33–37. [Google Scholar] [CrossRef]
- Itoh, E.; Miyairi, K. Interfacial charge phenomena at the semiconductor/gate insulator interface in organic field effect transistors. Thin Solid Films 2006, 499, 95–103. [Google Scholar] [CrossRef]
- Braga, G.S.; Paterno, L.G.; Fonseca, F.J. Performance of an electronic tongue during monitoring 2-rnethylisoborneol and geosmin in water samples. Sens. Actuators B Chem. 2012, 171–172, 181–189. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Amorim, C.A.; Santos, G.; Mergulhao, S.; Fonseca, F.J.; Andrade, A.M. Methodology of semiconductor selection for polymer thin-transistors based on charge carrier mobility. J. Mater. Sci. Eng. Adv. Technol. 2011, 4, 33–56. [Google Scholar]
- Scarpa, G.; Idzko, A.; Yadav, A.; Martin, E.; Thalhammer, S. Toward Cheap Disposable Sensing Devices for Biological Assays. IEEE Trans. Nanotechnol. 2010, 9, 527–532. [Google Scholar] [CrossRef]
- Lobo, R.F.M.; Pereira-da-Silva, M.A.; Raposo, M.; Faria, R.M.; Oliveira, O.N., Jr. In situ thickness measurements of ultra-thin multilayer polymer films by atomic force microscopy. Nanotechnology 1999, 10. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 86th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Manceau, M.; Rivaton, A.; Gardette, J.-L.; Guillerez, S.; Lemaître, N. The mechanism of photo- and thermooxidation of poly(3-hexylthiophene) (P3HT) reconsidered. Polym. Degrad. Stabil. 2009, 94, 898–907. [Google Scholar] [CrossRef]
- Alexiadis, O.; Mavrantzas, V.G. All-Atom Molecular Dynamics Simulation of Temperature Effects on the Structural, Thermodynamic, and Packing Properties of the Pure Amorphous and Pure Crystalline Phases of Regioregular P3HT. Macromolecules 2013, 46, 2450–2467. [Google Scholar] [CrossRef]
- Poelking, C.; Andrienko, D. Effect of Polymorphism, Regioregularity and Paracrystallinity on Charge Transport in Poly(3-hexylthiophene) [P3HT] Nanofibers. Macromolecules 2013, 46, 8941–8956. [Google Scholar] [CrossRef]
- Ellis, D.L.; Zakin, M.R.; Bernstein, L.S.; Rubner, M.F. Conductive polymer films as ultrasensitive chemical sensors for hydrazine and monomethylhydrazine vapor. Anal. Chem. 1996, 68, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Santhanam, S.; Schultz, L.; Jeffries-EL, M.; Iovu, M.C.; Sauvé, G.; Cooper, J.; Zhang, R.; Revelli, J.C.; Kusne, A.G.; et al. Inkjet printed chemical sensor array based on polythiophene conductive polymers. Sens. Actuators B Chem. 2007, 123, 651–660. [Google Scholar] [CrossRef]
- Im, J.; Sengupta, S.K.; Baruch, M.F.; Granz, C.D.; Ammu, S.; Manohar, S.K.; Whitten, J.E. A hybrid chemiresistive sensor system for the detection of organic vapors. Sens. Actuators B Chem. 2011, 156, 715–722. [Google Scholar] [CrossRef]
- Alberga, D.; Mangiatordi, G.F.; Torsi, L.; Lattanzi, G. Effects of Annealing and Residual Solvents on Amorphous P3HT and PBTTT Films. J. Phys. Chem. C 2014, 118, 8641–8655. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.-Q.; Liu, L.-W.; Pan, G.-B. Ammonia chemiresistor sensor based on poly(3-hexylthiophene) film oxidized by nitrosonium hexafluorophosphate. Chem. Lett. 2012, 41, 1569–1570. [Google Scholar] [CrossRef]
- Wiziacki, N.K.L.; Catini, A.; Santonico, M.; D’Amico, A.; Paolesse, R.; Paterno, L.G.; Fonseca, F.J.; di Natale, C. A sensor array based on mass and capacitance transducers for the detection of adulterated gasolines. Sens. Actuators B Chem. 2009, 140, 508–513. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. MOSFETs. In Physics of Semiconductor Devices, 3rd ed.; Wiley: New York, NY, USA, 2006; pp. 293–373. [Google Scholar]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Gburek, B.; Wagner, V. Influence of the semiconductor thickness on the charge carrier mobility in P3HT organic field-effect transistors in top-gate architecture on flexible substrates. Org. Electron. 2010, 11, 814–819. [Google Scholar] [CrossRef]
- Park, B.; Aiyar, A.; Hong, J.-I.; Reichmanis, E. Electrical Contact Properties between the Accumulation Layer and Metal Electrodes in Ultrathin Poly(3-hexylthiophene)(P3HT) Field Effect Transistors. ACS Appl. Mater. Interfaces 2011, 3, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C.; Gevorgyan, S.A.; Alstrup, J. A roll-to-roll process to flexible polymer solar cells: Model studies, manufacture and operational stability studies. J. Mater. Chem. 2009, 19, 5442–5451. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallari, M.R.; Izquierdo, J.E.E.; Braga, G.S.; Dirani, E.A.T.; Pereira-da-Silva, M.A.; Rodríguez, E.F.G.; Fonseca, F.J. Enhanced Sensitivity of Gas Sensor Based on Poly(3-hexylthiophene) Thin-Film Transistors for Disease Diagnosis and Environment Monitoring. Sensors 2015, 15, 9592-9609. https://doi.org/10.3390/s150409592
Cavallari MR, Izquierdo JEE, Braga GS, Dirani EAT, Pereira-da-Silva MA, Rodríguez EFG, Fonseca FJ. Enhanced Sensitivity of Gas Sensor Based on Poly(3-hexylthiophene) Thin-Film Transistors for Disease Diagnosis and Environment Monitoring. Sensors. 2015; 15(4):9592-9609. https://doi.org/10.3390/s150409592
Chicago/Turabian StyleCavallari, Marco R., José E. E. Izquierdo, Guilherme S. Braga, Ely A. T. Dirani, Marcelo A. Pereira-da-Silva, Estrella F. G. Rodríguez, and Fernando J. Fonseca. 2015. "Enhanced Sensitivity of Gas Sensor Based on Poly(3-hexylthiophene) Thin-Film Transistors for Disease Diagnosis and Environment Monitoring" Sensors 15, no. 4: 9592-9609. https://doi.org/10.3390/s150409592
APA StyleCavallari, M. R., Izquierdo, J. E. E., Braga, G. S., Dirani, E. A. T., Pereira-da-Silva, M. A., Rodríguez, E. F. G., & Fonseca, F. J. (2015). Enhanced Sensitivity of Gas Sensor Based on Poly(3-hexylthiophene) Thin-Film Transistors for Disease Diagnosis and Environment Monitoring. Sensors, 15(4), 9592-9609. https://doi.org/10.3390/s150409592