A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of Cu2+/PANI/WO3 Films
2.3. Fabrication of Surface Acoustic Wave Sensors
2.4. Surface Acoustic Wave Sensor Measurement
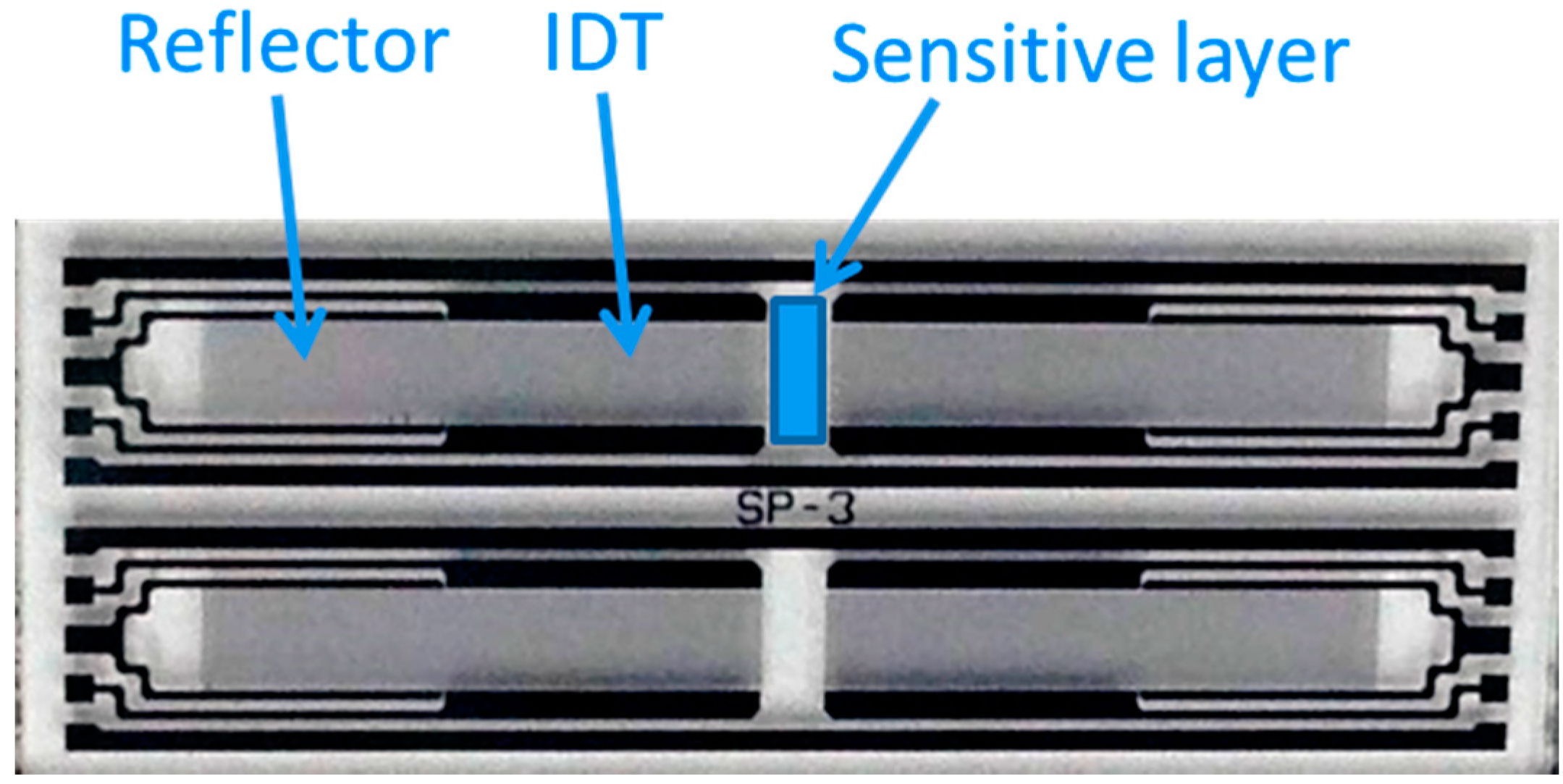
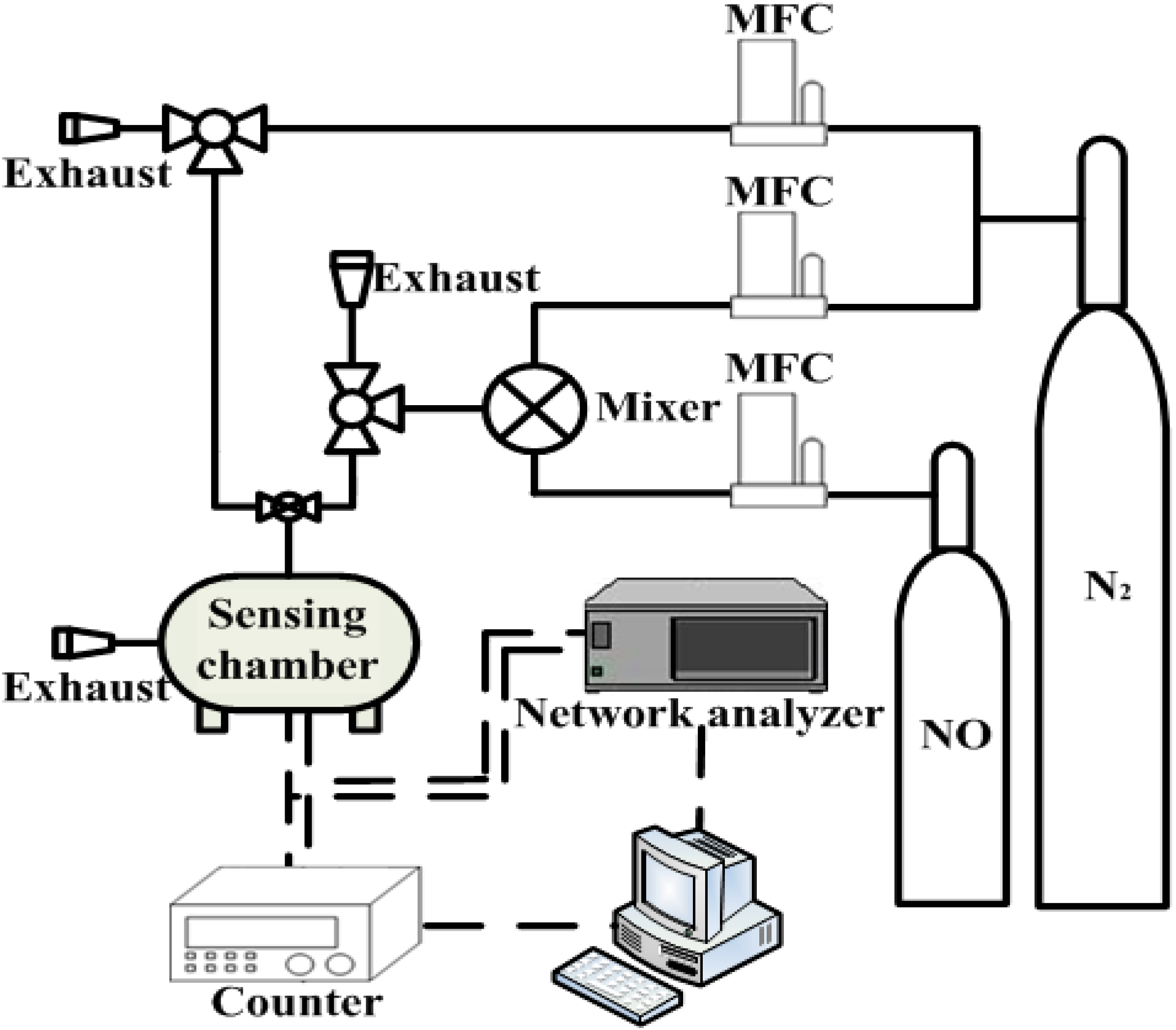
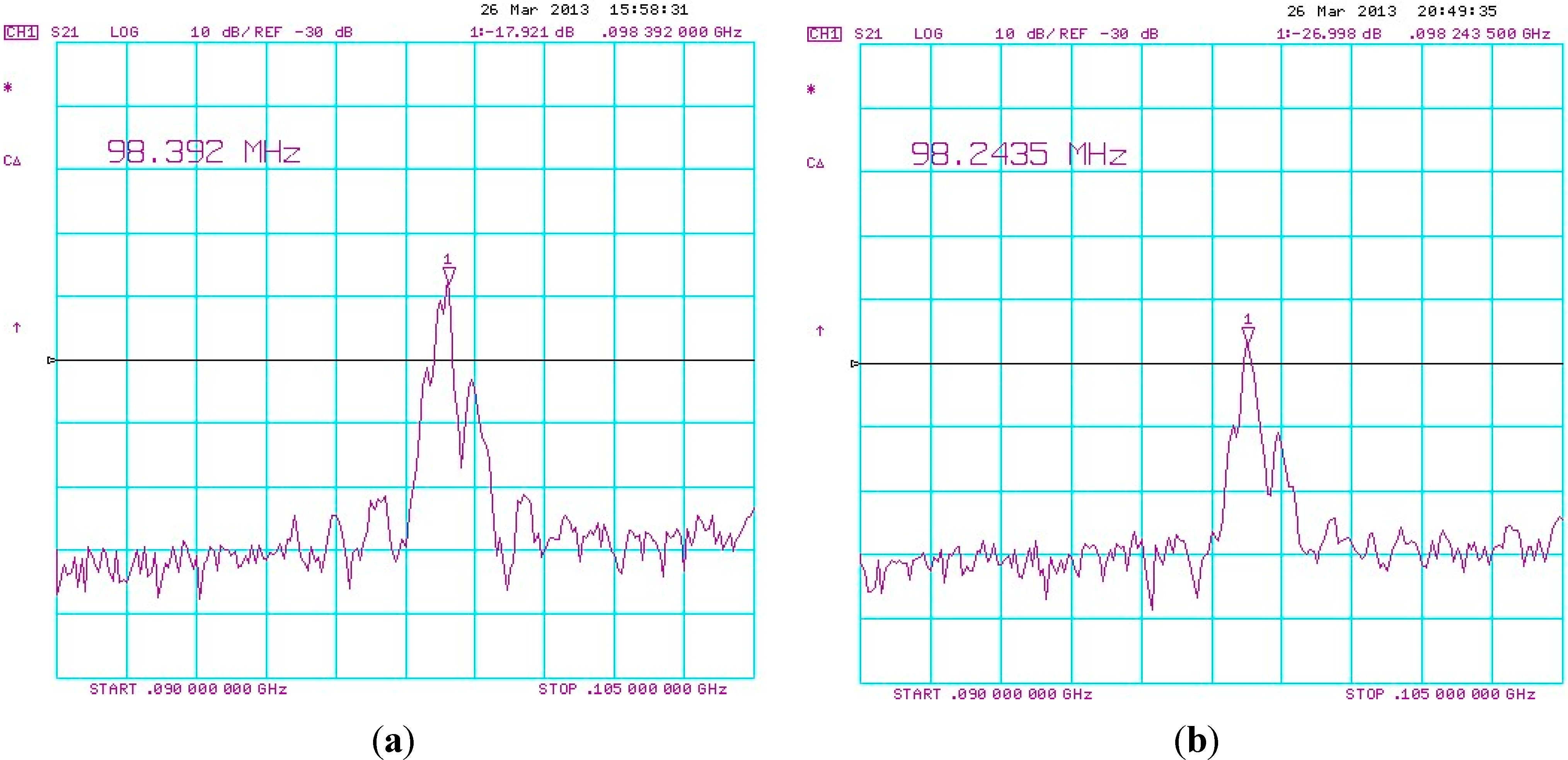
3. Results and Discussion
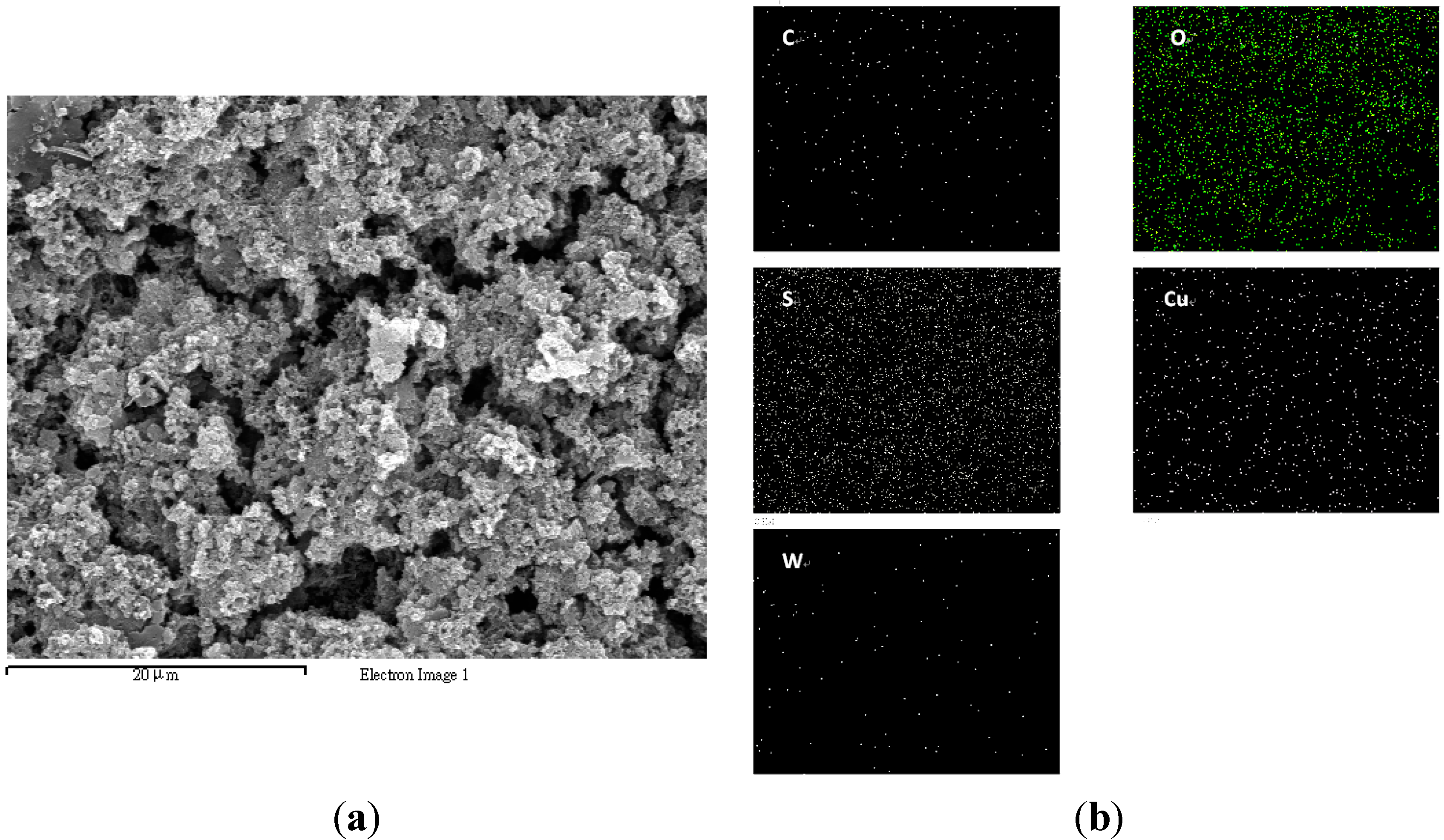
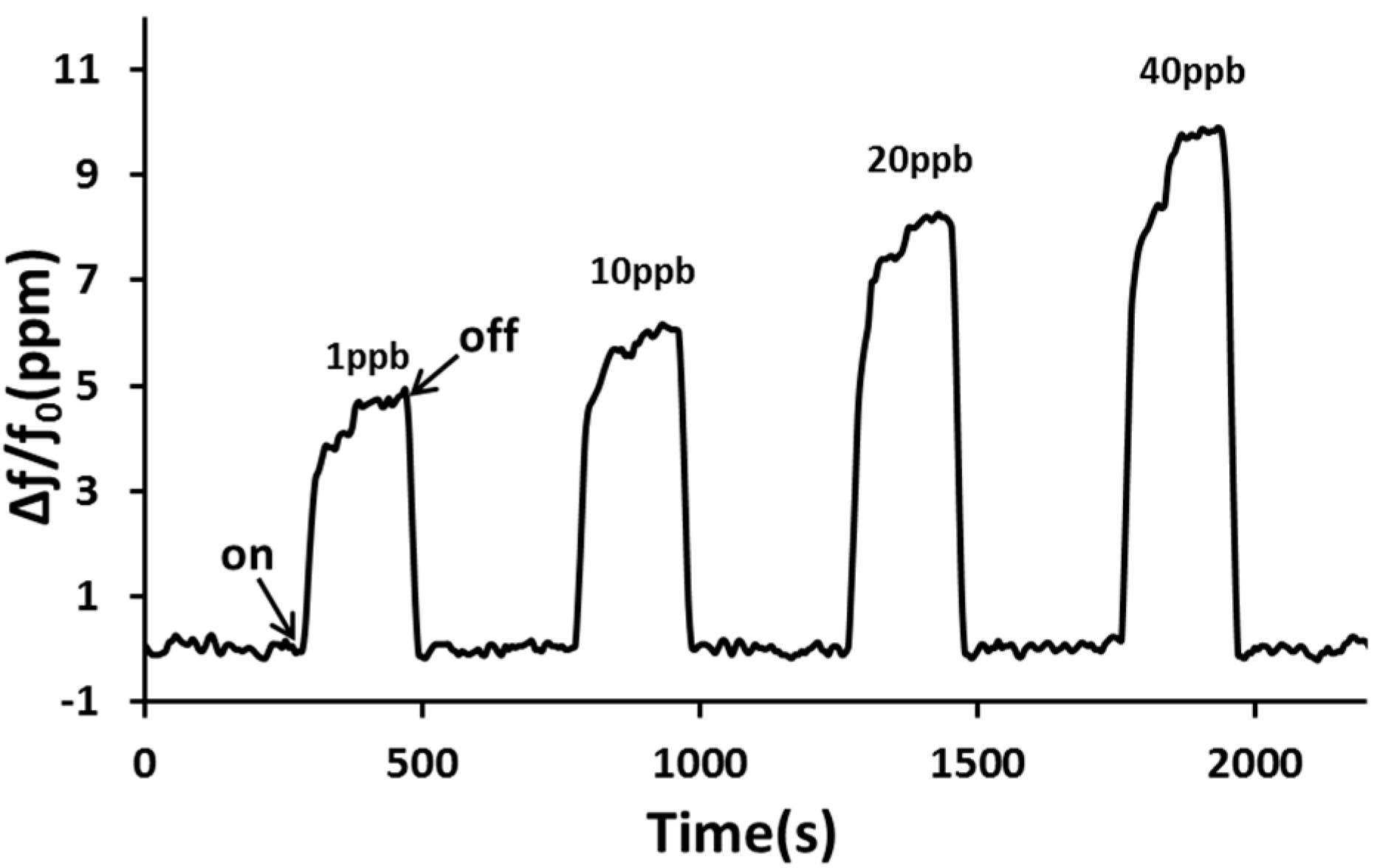

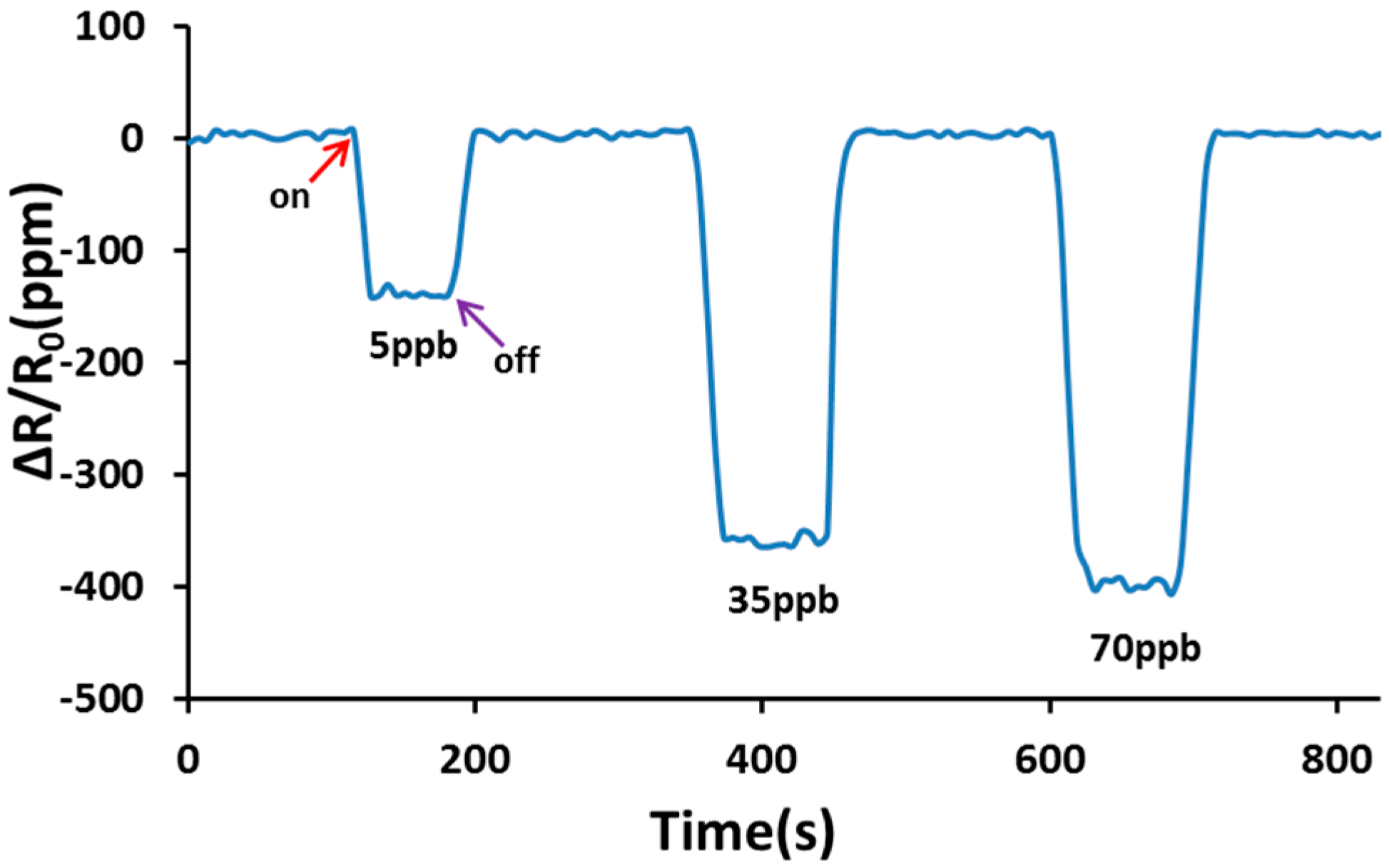
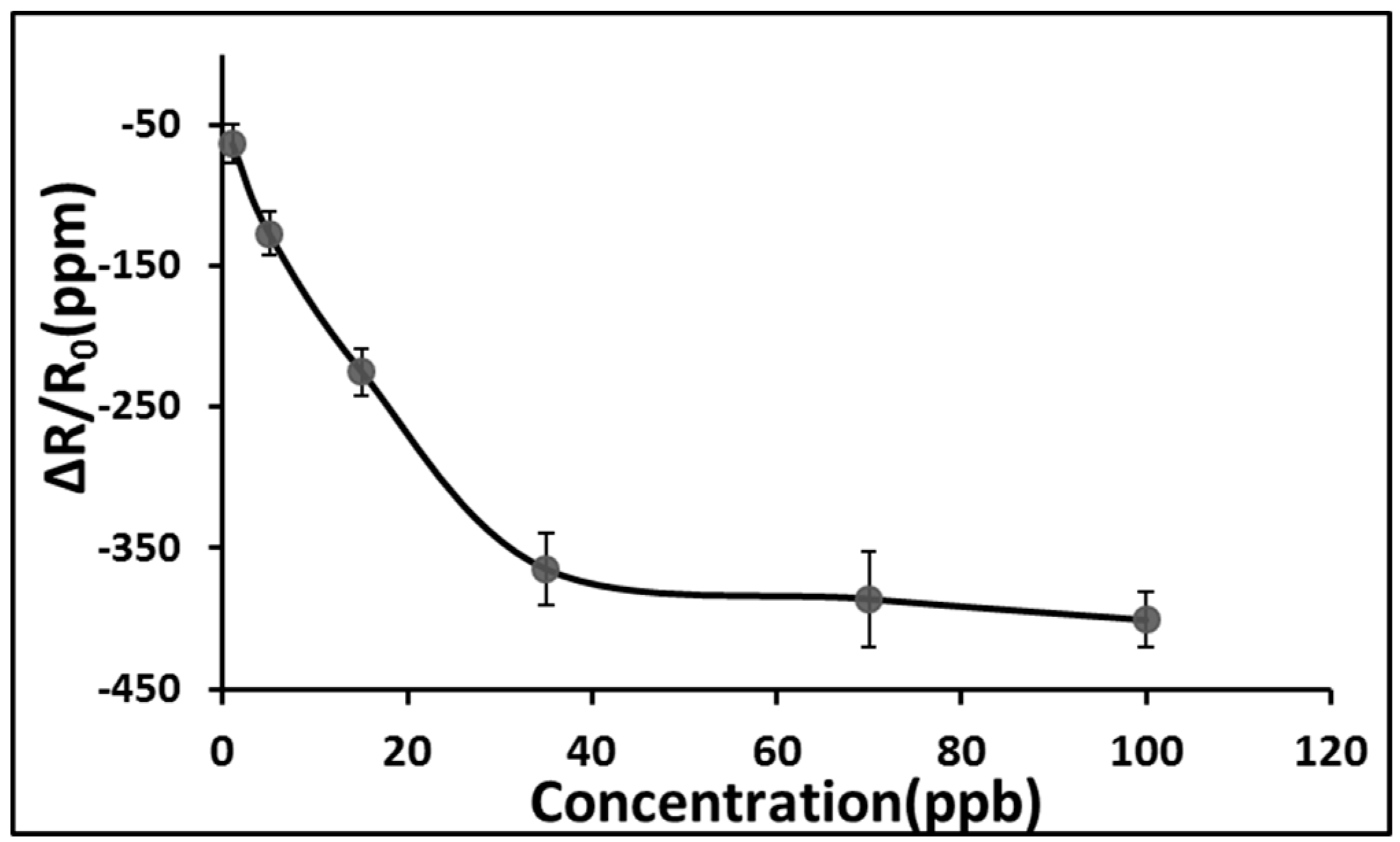
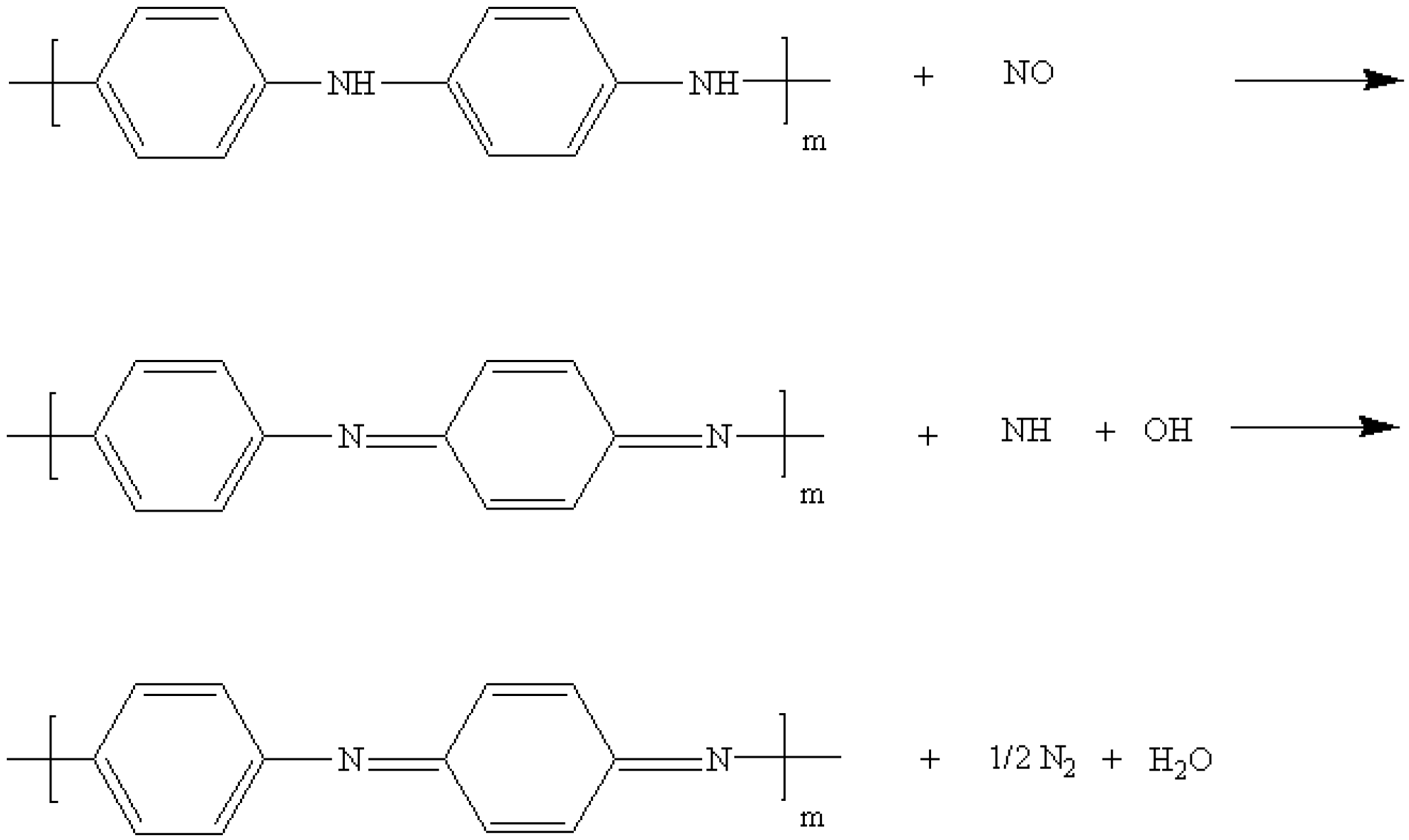
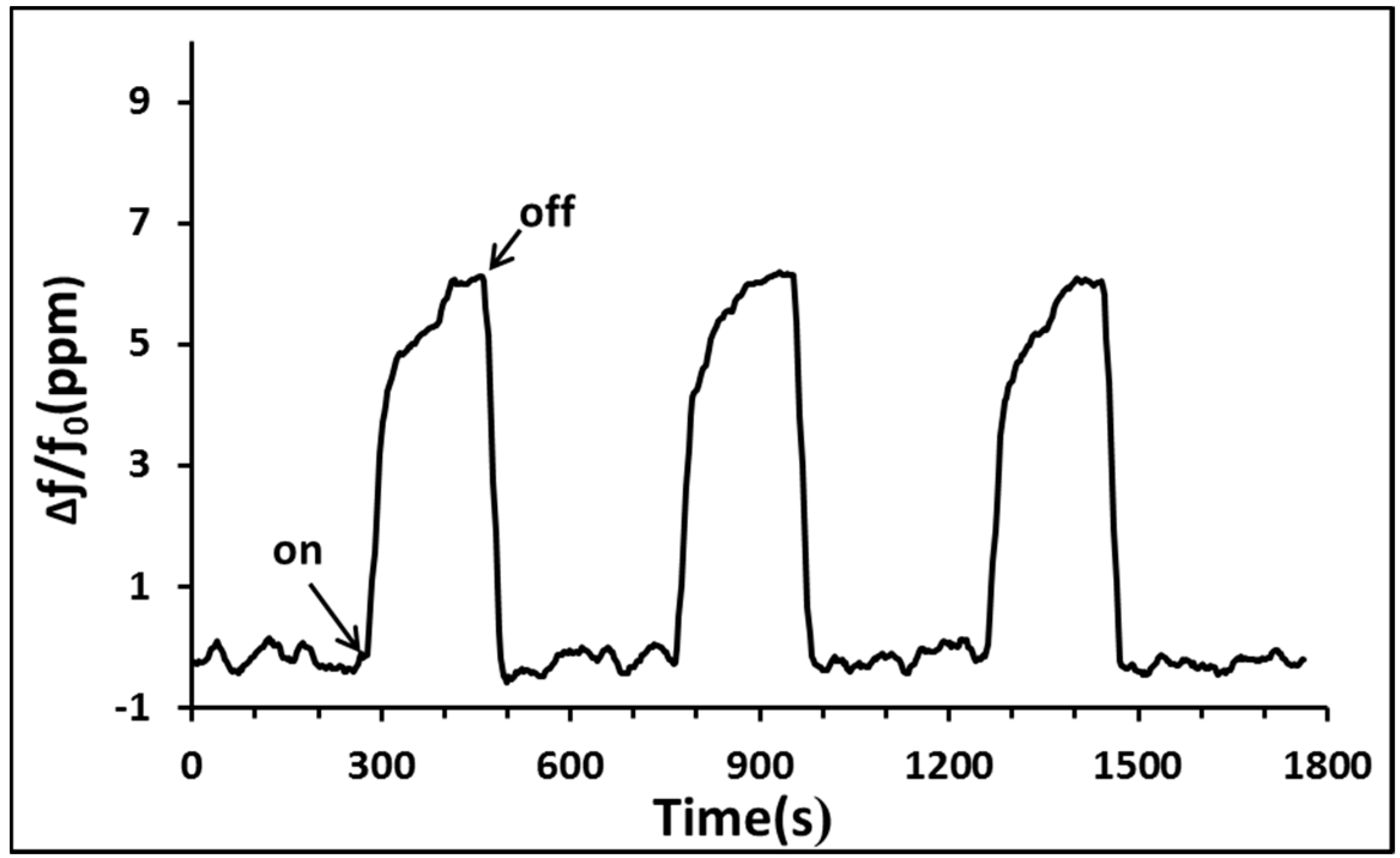
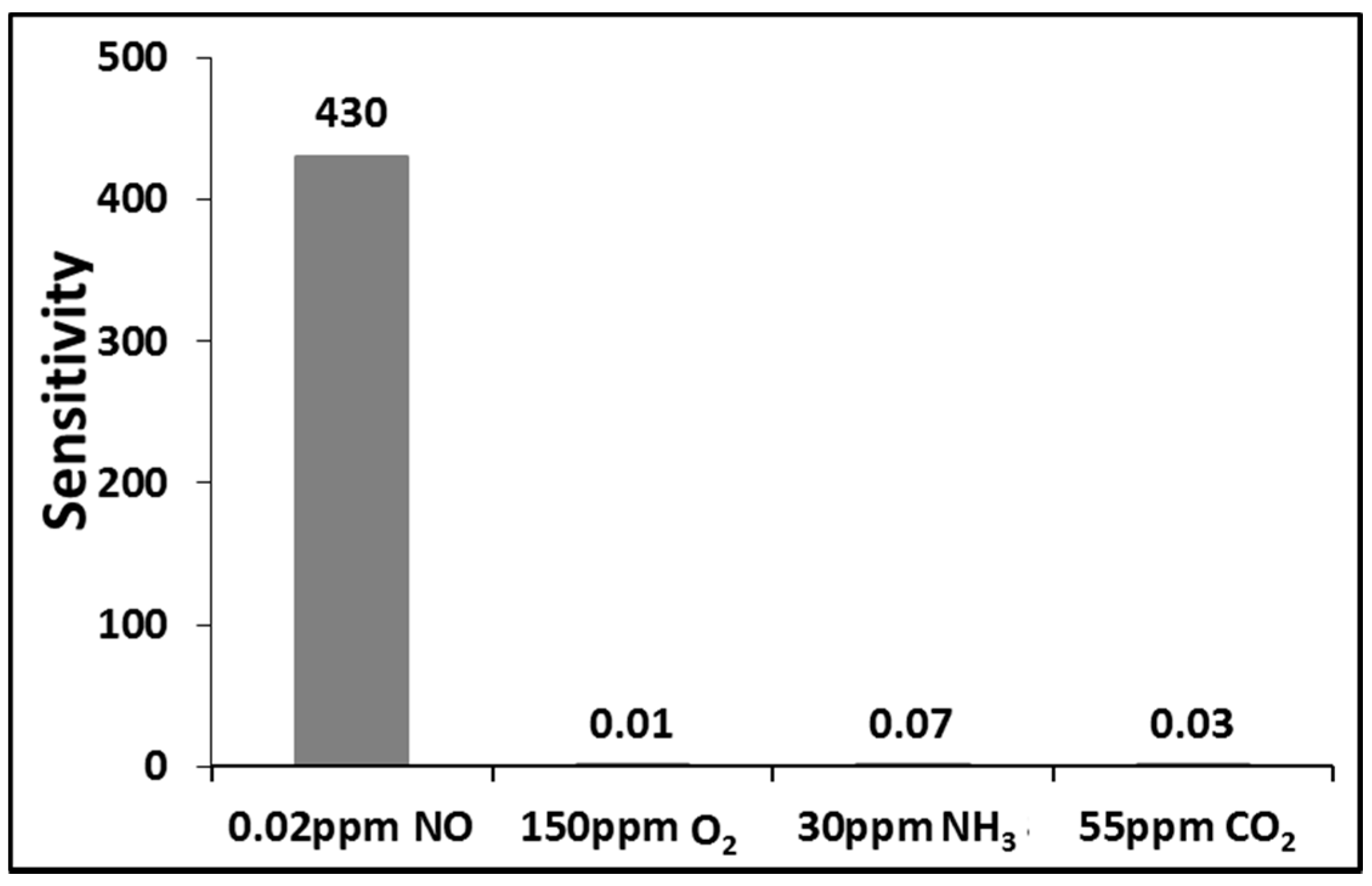
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wartelle, C.; Pereira Rodrigues, N.; Koudelka-Hep, M.; Bedioui, F. Amperometric fluidic microchip array sensing device for nitric oxide determination in solution. Mater. Sci. Eng. C 2006, 26, 534–537. [Google Scholar] [CrossRef]
- Kim, J.W.; Oh, E.G.; Cha, G.Y.; Jeong, S.H.; Huh, J.S. NO Sensing Characteristics of ZnO Nanorod Prepared by Ultrasound Radiation Method. In Proceedings of the 5th IEEE International Conference on Sensors, Daegu, South Korea, 22–25 October 2006; pp. 239–242.
- Rathgeb, F.; Gauglitz, G. Optical gas sensors in analytical chemistry: Applications, trends and general comments. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: New York, NY, USA, 2000; pp. 2189–2203. [Google Scholar]
- Menil, F.; Coillard, V.; Lucat, C. Critical review of nitrogen monoxide sensors for exhaust gases of lean burn engines. Sens. Actuators B 2000, 67, 1–23. [Google Scholar] [CrossRef]
- Vilar, M.R.; El-Beghdadi, J.; Debontridder, F.; Naaman, R.; Arbel, A.; Ferraria, A.M.; do Rego, A.M. Development of nitric oxide sensor for asthma attack prevention. Mater. Sci. Eng. C 2006, 26, 253–259. [Google Scholar] [CrossRef]
- Bisgaard, H.; Loland, L.; AnhØj, J. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am. J. Respir. Crit. Care Med. 1999, 160, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Taylor, D.R. Is exhaled nitric oxide measurement a useful clinical test in asthma. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Pijnenburg, M.W.; Smith, A.D.; Jongste, J.C.D. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax 2006, 61, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Ellis, C.G.; Freeman, D.J. Optimization of nitric oxide chemiluminescence operating conditions for measurement of plasma nitrite and nitrate. Clin. Chem. 2002, 48, 570–573. [Google Scholar] [PubMed]
- Mondal, S.P.; Dutta, P.K.; Hunter, G.W.; Ward, B.J.; Laskowski, D.; Dweik, R.A. Development of high sensitivity potentiometric NOx sensor and its application to breath analysis. Sens. Actuators B 2011, 158, 292–298. [Google Scholar] [CrossRef]
- Sun, C.; Maduraiveeran, G.; Dutta, P. Nitric oxide sensors using combination of p- and n-type semiconducting oxides and its application for detecting NO in human breath. Sens. Actuators B 2013, 186, 117–125. [Google Scholar] [CrossRef]
- Zhang, P.S.; Zhang, W.; Gerlach, F.; Ahlborn, K.; Guth, U. Electrochemical investigations on multi-metallic electrodes for amperometric NO gas sensors. Sens. Actuators B 2005, 108, 797–802. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. Improved Response Characteristics of SnO2 Film Based NO2 Gas Sensor with Nanoscaled Metal Oxide Catalysts. In Proceedings of the 14th International Meeting on Chemical Sensors, Nuremberg, Germany, 20–23 May 2012; pp. 702–705.
- Kukkola, J.; Maklin, J.; Halonen, N.; Kyllonen, T.; Toth, G.; Szabo, M.; Shchukarev, A.; Mikkola, J.P.; Jantunen, H.; Kordas, K. Gas sensors based on anodic tungsten oxide. Sens. Actuators B 2011, 153, 293–300. [Google Scholar] [CrossRef]
- Wang, S.H.; Kuo, S.H.; Shen, C.Y. A nitric oxide gas sensor based on Rayleigh surface acoustic wave resonator for room temperature operation. Sens. Actuators B 2011, 156, 668–672. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C—Catalysts, mechanism, kinetics. Catal. Sci. Technol. 2014, 4, 14–25. [Google Scholar] [CrossRef]
- Fox, C.G.; John Alder, F. Surface acoustic wave sensors for atmospheric gas monitoring. A review. Analyst 1989, 114, 997–1004. [Google Scholar] [CrossRef]
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw. Theory Tech. 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Thompson, M.; Stone, D.C. Surface-Launched Acoustic Wave Sensors: Chemical Sensing and Thin Film Characterization; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Shen, C.Y.; Hsu, C.L.; Su, M.Y.; Tsai, R.T.; Wang, S.H. Propagation characteristics of surface acoustic wave and properties of gas sensors on quartz. In IUTAM Symposium on Recent Advances of Acoustic Waves in Solids; Springer: London, UK, 2010; Volume 26, pp. 293–302. [Google Scholar]
- Wang, S.H.; Chou, T.C.; Liu, C.C. Nano-crystalline tungsten oxide NO2 sensor. Sens. Actuators B 2003, 94, 343–351. [Google Scholar] [CrossRef]
- Shen, C.Y.; Huang, H.M.; Wang, S.H.; Chiu, Y.C. Room temperature detection properties of a surface acoustic wave gas sensor with Cu2+/PANI/SnO2 nanocomposite thin film to nitric oxide. Appl. Mech. Mater. 2013, 312, 732–735. [Google Scholar] [CrossRef]
- Schulz, M.B.; Matsinger, B.J.; Holland, M.G. Temperature dependence of surface acoustic wave velocity on α quartz. J. Appl. Phy. 1970, 7, 2755–2765. [Google Scholar] [CrossRef]
- Sinha, B.K.; Tiersten, H.F. On the temperature dependence of the velocity of surface waves in quartz. J. Appl. Phys. 1980, 51, 4659–4665. [Google Scholar] [CrossRef]
- Henry-Briot, E.; Ballandras, S.; Marianneau, G.; Martin, G. Influence of metal thickness on phase velocity and thermal sensitivity of SAW devices. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W.; Stephen, J.M.; Richard, M.W. Acoustic wave microsensors. Anal. Chem 1993, 65, 940A–948A. [Google Scholar] [CrossRef]
- Montress, G.K.; Parker, T.E.; Andrea, D. Review of SAW Oscillator Performance. In Proceedings of the IEEE Ultrasonics Symposium, Cannes, France, 31 October–3 November 1994; pp. 43–54.
- Ricco, A.J. SAW chemical sensors, an expanding role with global impact. Electrochem. Soc. Interface 1994, 3, 38–45. [Google Scholar]
- Wohltjen, H. Mechanism of operation and design consideration for surface acoustic wave device vapor sensor. Sens. Actuators 1984, 5, 307–325. [Google Scholar] [CrossRef]
- Ray, S.; Gupta, P.S.; Singh, G. Electrical and optical properties of sol-gel prepared Pd-doped SnO2 thin films: Effect of multiple layers and its use as room temperature methane gas sensor. J. Ovonic Res. 2010, 6, 23–34. [Google Scholar]
- Geng, L.; Zhao, Y.; Huang, X.; Wang, S.; Zhang, S.; Wu, S. Characterization and gas sensitivity study of polyaniline/SnO2 hybrid material prepared by hydrothermal route. Sens. Actuators B 2007, 120, 568–572. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Shen, C.-Y.; Su, J.-M.; Chang, S.-W. A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite. Sensors 2015, 15, 7084-7095. https://doi.org/10.3390/s150407084
Wang S-H, Shen C-Y, Su J-M, Chang S-W. A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite. Sensors. 2015; 15(4):7084-7095. https://doi.org/10.3390/s150407084
Chicago/Turabian StyleWang, Shih-Han, Chi-Yen Shen, Jian-Ming Su, and Shiang-Wen Chang. 2015. "A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite" Sensors 15, no. 4: 7084-7095. https://doi.org/10.3390/s150407084
APA StyleWang, S.-H., Shen, C.-Y., Su, J.-M., & Chang, S.-W. (2015). A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite. Sensors, 15(4), 7084-7095. https://doi.org/10.3390/s150407084




