Tunable Microfluidic Devices for Hydrodynamic Fractionation of Cells and Beads: A Review
Abstract
:1. Introduction
2. Mechanical Tunability
2.1. Elastomeric Substrate
2.2. Microstructure Design
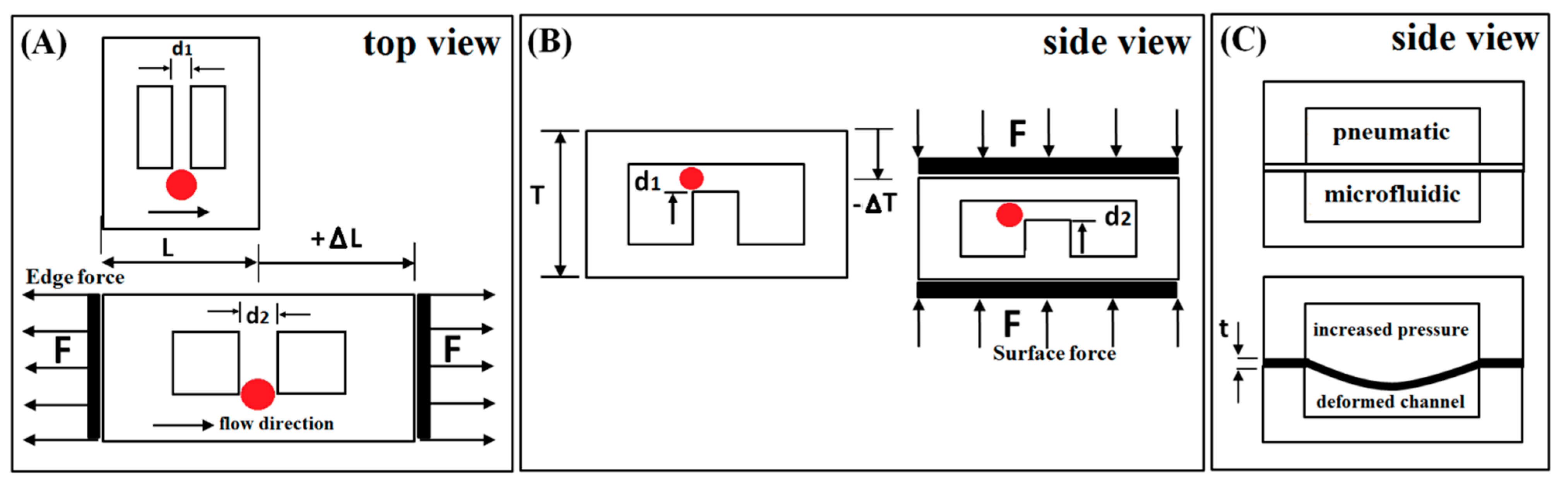
2.3. Device Tuning Methods
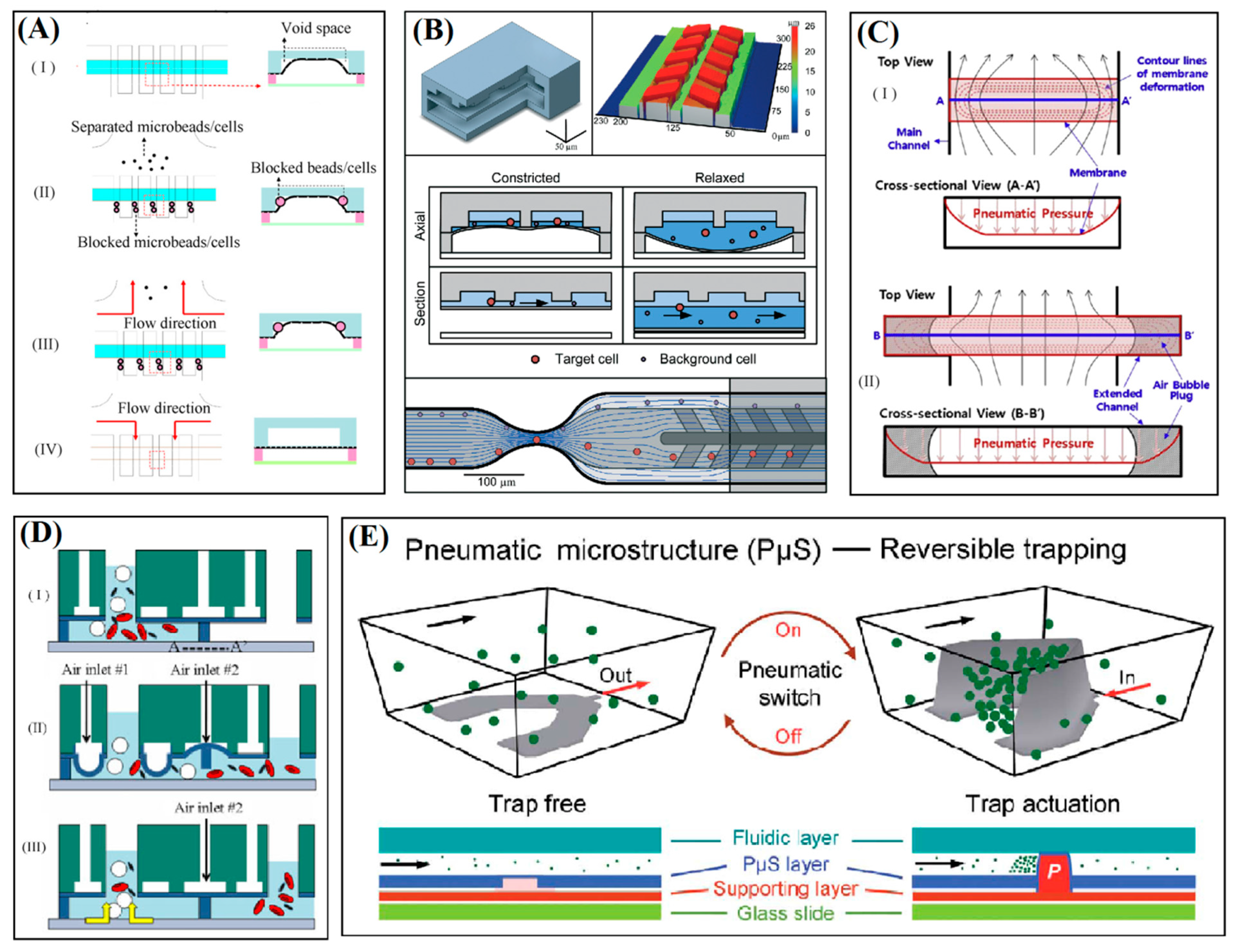
3. Deformation of Microstructure Layer
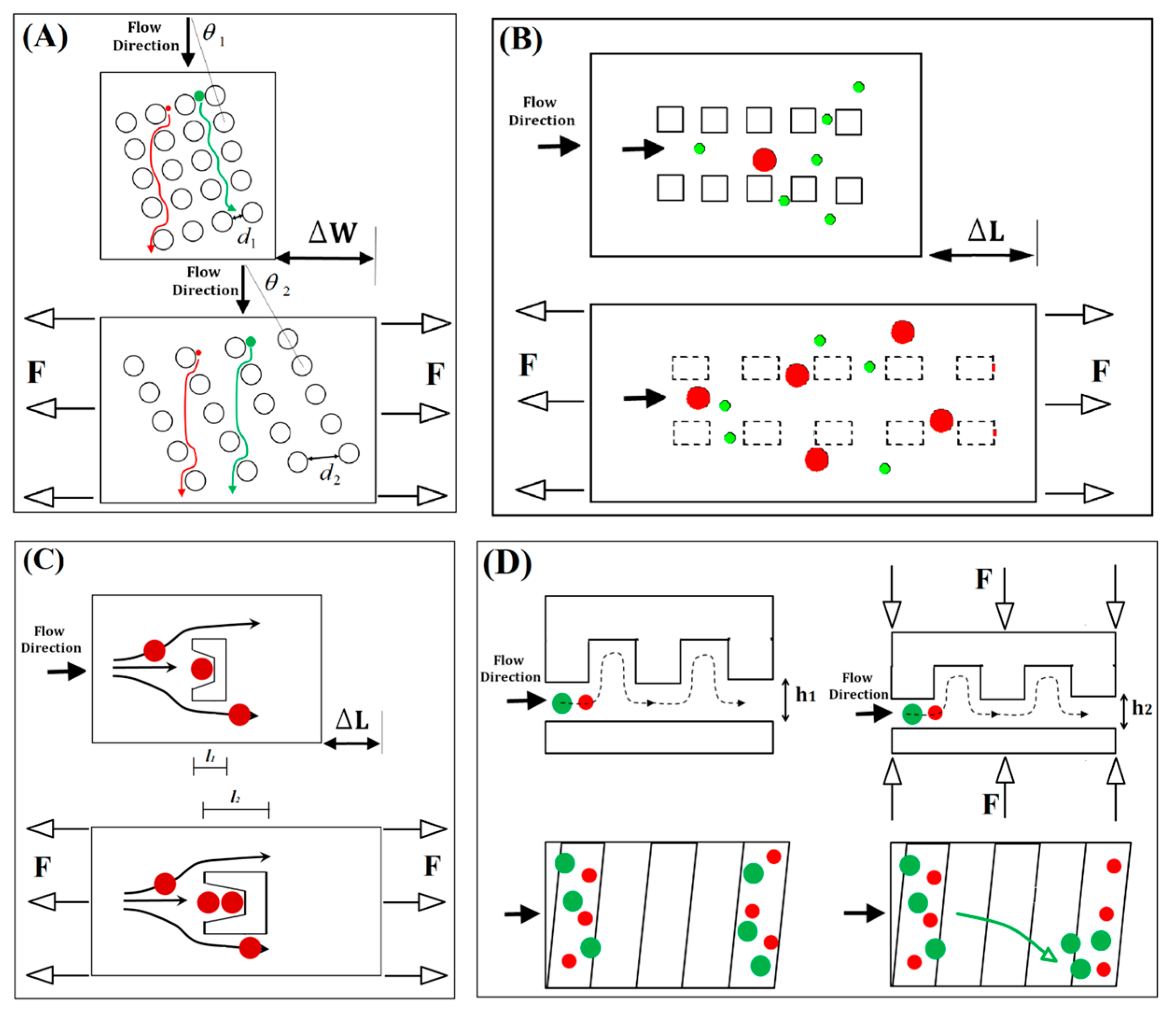
3.1. Separation by Arrays of Micropillars
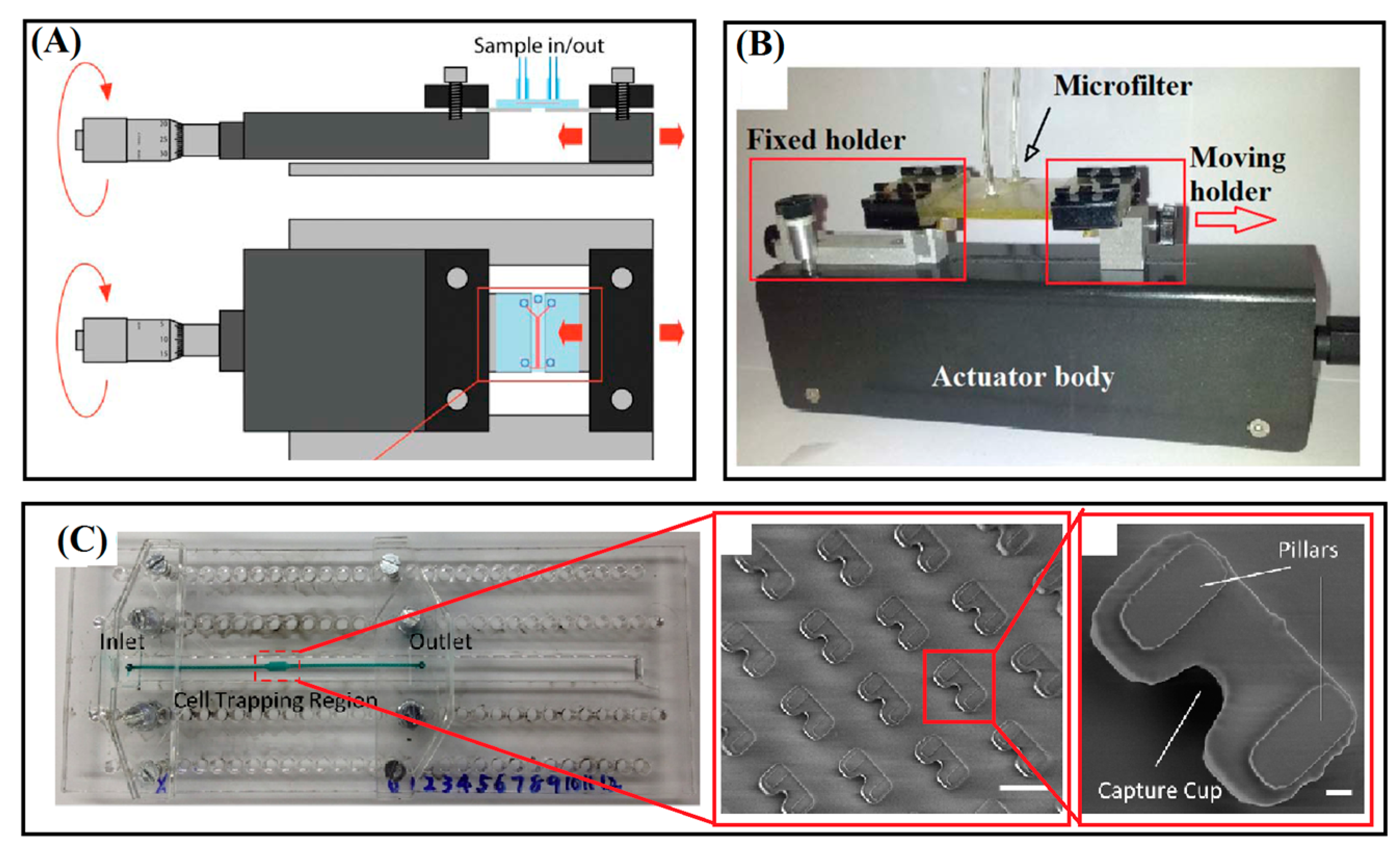
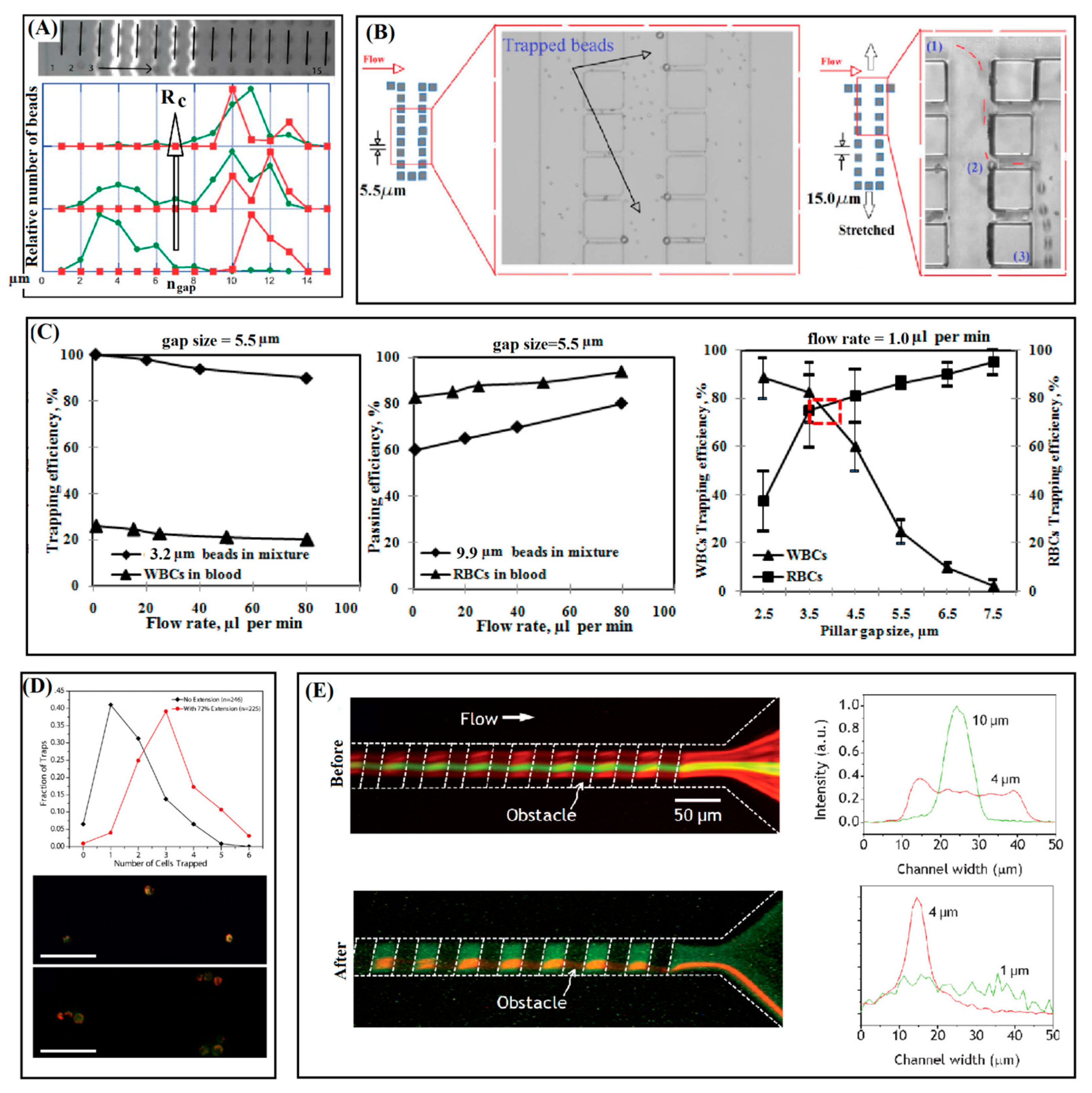
3.2. Cup-Shaped Elements for Trapping
3.3. Tuning of Hydrophoretic Effect
4. Elastomeric Membrane Deformation
4.1. Blockage of Microchannel Cross-Section
4.2. Actuation of Floating Microstructure
4.3. Dynamic Cup-Shaped Elements for Trapping
5. Conclusions
| Method | Tunable Geometry and Actuation | Application and Flow Conditions | Development Challenges |
|---|---|---|---|
| Stretchable DLD [40] | variable inter-pillar spacing and discrimination resolution of 10 nm | continuous fractionation, beads (5 and 8 µm), flow rate of 500 µm/s | stretcher integration to complex systems, non-uniformity of strains, stick-slip behavior |
| Stretchable pillar-based [15] | inter-pillar spacing of 5.5–15 µm and actuator resolution of 0.165 µm | Microfiltration beads (9.9 and 3.2 µm, 50/µL and 50,000/µL) and blood cells, flow rate of 1.0 µL/min | stretcher integration to complex systems |
| Stretchable pillar-based [14] | inter-pillar spacing of 2.5–7.5 µm and actuator resolution of 0.165 µm | microfiltration optimization, beads (9.9 and 3.2 µm, 50/µL and 50,000/µL) and blood cells, flow rates of 1.0–80 µL/min | stretcher integration to complex systems |
| Tunable hydrophoretic [43,66] | obstacle gap for hydrophoretic criterion adjusted on 7.0–2.5 µm | continuous focusing, beads (mixtures of 10,4, and 1 µm, 20, 7.3, and 1.8 × 102/µL), flow rates of 0.4 and 1.0 µL/min | nonuniform deformation of microchannel cross-section under mechanical press |
| Stretchable cup-shaped structures [42] | depth of elastomeric structures stretched to 79% of initial value | device modulation for number of trapped cells cancer cells (MCF-7, 1000/µL), flow rate of 10 µL/min | nonlinear deformation, stretcher integration to complex systems |
| Dynamic cup-shape structures [58] | controllable dynamic array of U-shape structures, pneumatic pressures of 0–20 psi | trap and release for patterning and manipulation of human cells, (A549, HepG2, MCF-7, 5000/µL), flow rates of 0–200 µL/min | typical in microfluidics |
| Channel cross-section corners [46] | corner voids of channel blocked by membrane to 5 µm, pulsing pneumatic pressures of 3–17 psi at 1–16 Hz | filtration and recovery, beads (5–20 µm, 2500/µL) and cells (chondrocytes), flow rates of 3.3–14.9 µL/min | typical in microfluidics |
| Floating block [45] | floating block forms narrow gap size of 1–13 µm, pneumatic pressures of 0–7.2 psi and pulsation of 1–11 Hz | separation, beads (1.0, 4.8, 10 µm and concentration of 16.63, 4.25, and 0.26 × 103/µL) and blood cells | typical in microfluidics |
| Resettable trap [16] | diaphragm deflection for size discrimination of <1 µm, pneumatic pressures of 0–5.8 psi | filtration and recovery by size and deformability, beads (6.4, 7.3, 9.5, 10.1 µm) and rare cancer cells from blood (UM-UC13, 1/1000 leukocytes), flow rate of 4–6 mm/s and 15,000 cells/min | typical in microfluidics |
Acknowledgments
Conflicts of Interest
References
- Yi, C.; Li, C.-W.; Ji, S.; Yang, M. Microfluidics technology for manipulation and analysis of biological cells. Anal. Chim. Acta 2006, 560, 1–23. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, K.; Lee, W.G. Cell manipulation in microfluidics. Biofabrication 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.; Bow, H.; Hou, H.; Tan, S.; Han, J.; Lim, C. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Aran, K.; Morales, M.; Sasso, L.A.; Lo, J.; Zheng, M.; Johnston, I.; Kamdar, N.; Ündar, A.; Zahn, J.D. Microfiltration device for continuous, label-free bacteria separation from whole blood for sepsis treatment. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011.
- Xuan, X.; Zhu, J.; Church, C. Particle focusing in microfluidic devices. Microfluid. Nanofluid. 2010, 9, 1–16. [Google Scholar] [CrossRef]
- Alvankarian, J.; Bahadorimehr, A.; Majlis, B.Y. A pillar-based microfilter for isolation of white blood cells on elastomeric substrate. Biomicrofluidics 2013, 7, 014102–014116. [Google Scholar] [CrossRef] [PubMed]
- Doh, I.; Kim, Y.; Cho, Y.-H. A particle trapping chip using the wide and uniform slit formed by a deformable membrane with air bubble plugs. Curr. Appl. Phys. 2013, 13, 902–906. [Google Scholar] [CrossRef]
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 2005, 26. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Evander, M.; Hammarström, B.; Laurell, T. Review of cell and particle trapping in microfluidic systems. Anal. Chim. Acta 2009, 649, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Bow, H.; Pivkin, I.V.; Diez-Silva, M.; Goldfless, S.J.; Dao, M.; Niles, J.C.; Suresh, S.; Han, J. A microfabricated deformability-based flow cytometer with application to malaria. Lab Chip 2011, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Bhagat, A.A.; Peng, W.K.; Lee, W.C.; Ong, C.J.; Chen, P.C.; Han, J. Size-independent deformability cytometry with active feedback control of microfluidic channels. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011.
- Wang, G.; Turbyfield, C.; Crawford, K.; Alexeev, A.; Sulchek, T. Cellular enrichment through microfluidic fractionation based on cell biomechanical properties. Microfluid. Nanofluid. 2015, 19, 987–993. [Google Scholar] [CrossRef]
- Alvankarian, J.; Majlis, B.Y. A technique of optimization of microfiltration using a tunable platform. J. Micromech. Microeng. 2015, 25. [Google Scholar] [CrossRef]
- Alvankarian, J.; Jaddi, N.S.; Vakilian, M.; Barantalab, F.; Jamal, R.; Majlis, B.Y. Consideration of nonuniformity in elongation of microstructures in a mechanically tunable microfluidic device for size-based isolation of microparticles. J. Microelectromech. Syst. 2015, 24, 309–318. [Google Scholar] [CrossRef]
- Beattie, W.; Qin, X.; Wang, L.; Ma, H. Clog-free cell filtration using resettable cell traps. Lab Chip 2014, 14, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F.; Åberg, L.; Swärd-Nilsson, A.-M.; Laurell, T. Free flow acoustophoresis: Microfluidic-based mode of particle and cell separation. Anal. Chem. 2007, 79, 5117–5123. [Google Scholar] [CrossRef] [PubMed]
- Applegate, R., Jr.; Squier, J.; Vestad, T.; Oakey, J.; Marr, D. Optical trapping, manipulation, and sorting of cells and colloids in microfluidic systems with diode laser bars. Opt. Express 2004, 12, 4390–4398. [Google Scholar] [CrossRef]
- Ozkan, M.; Wang, M.; Ozkan, C.; Flynn, R.; Esener, S. Optical manipulation of objects and biological cells in microfluidic devices. Biomed. Microdevices 2003, 5, 61–67. [Google Scholar] [CrossRef]
- Kang, Y.; Li, D. Electrokinetic motion of particles and cells in microchannels. Microfluid. Nanofluid. 2009, 6, 431–460. [Google Scholar] [CrossRef]
- Liu, C.; Stakenborg, T.; Peeters, S.; Lagae, L. Cell manipulation with magnetic particles toward microfluidic cytometry. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Gijs, M.A.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 2009, 110, 1518–1563. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Ho, C.-M. Cell separation by non-inertial force fields in microfluidic systems. Mech. Res. Commun. 2009, 36, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mirowski, E.; Moreland, J.; Zhang, A.; Russek, S.E.; Donahue, M.J. Manipulation and sorting of magnetic particles by a magnetic force microscope on a microfluidic magnetic trap platform. Appl. Phys. Lett. 2005, 86. [Google Scholar] [CrossRef]
- Hur, S.C.; Henderson-MacLennan, N.K.; McCabe, E.R.; Di Carlo, D. Deformability-based cell classification and enrichment using inertial microfluidics. Lab Chip 2011, 11, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.S.; Zhao, Y.; Schiro, P.G.; Ng, L.; Lim, D.S.W.; Shelby, J.P.; Chiu, D.T. Deformability considerations in filtration of biological cells. Lab Chip 2010, 10, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Yazdi, S.; Ardekani, A. Hydrodynamic mechanisms of cell and particle trapping in microfluidics. Biomicrofluidics 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Inertial microfluidics for continuous particle filtration and extraction. Microfluid. Nanofluid. 2009, 7, 217–226. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, S.H.; Suh, K.Y. Cell research with physically modified microfluidic channels: A review. Lab Chip 2008, 8, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Peoples, M.C.; Karnes, H.T. Microfluidic immunoaffinity separations for bioanalysis. J. Chromatogr. B 2008, 866, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Seki, M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Scherer, A.; Psaltis, D. Mechanically tunable optofluidic distributed feedback dye laser. Opt. Express 2006, 14, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- So, J.H.; Thelen, J.; Qusba, A.; Hayes, G.J.; Lazzi, G.; Dickey, M.D. Reversibly deformable and mechanically tunable fluidic antennas. Adv. Funct. Mater. 2009, 19, 3632–3637. [Google Scholar] [CrossRef]
- Chronis, N.; Liu, G.; Jeong, K.-H.; Lee, L. Tunable liquid-filled microlens array integrated with microfluidic network. Opt. Express 2003, 11, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Khare, K.; Zhou, J.; Yang, S. Tunable open-channel microfluidics on soft poly (dimethylsiloxane) (pdms) substrates with sinusoidal grooves. Langmuir 2009, 25, 12794–12799. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Yang, S. Mechanically switchable wetting on wrinkled elastomers with dual-scale roughness. SoftMatter 2009, 5, 1011–1018. [Google Scholar] [CrossRef]
- Johnston, I.; McCluskey, D.; Tan, C.; Tracey, M. Mechanical characterization of bulk sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24. [Google Scholar] [CrossRef]
- Beech, J.P.; Tegenfeldt, J.O. Tuneable separation in elastomeric microfluidics devices. Lab Chip 2008, 8, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Vasdekis, A.E.; Psaltis, D. Elastomer based tunable optofluidic devices. Lab Chip 2012, 12, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shang, J.; Olsen, T.; Liu, K.; Brenner, D.; Lin, Q. A mechanically tunable microfluidic cell-trapping device. Sens. Actuators A Phys. 2014, 215, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, J.-K. Tuneable hydrophoretic separation using elastic deformation of poly (dimethylsiloxane). Lab Chip 2009, 9, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, T.; Chen, Z.; Fei, P.; Yu, Z.; Pang, Y.; Huang, Y. Squeeze-chip: A finger-controlled microfluidic flow network device and its application to biochemical assays. Lab Chip 2012, 12, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Huang, C.-J.; Lee, G.-B. A tunable microfluidic-based filter modulated by pneumatic pressure for separation of blood cells. Microfluid. Nanofluid. 2012, 12, 85–94. [Google Scholar] [CrossRef]
- Huang, S.-B.; Wu, M.-H.; Lee, G.-B. A tunable micro filter modulated by pneumatic pressure for cell separation. Sens. Actuators B Chem. 2009, 142, 389–399. [Google Scholar] [CrossRef]
- Hopcroft, M.A.; Nix, W.D.; Kenny, T.W. What is the young’s modulus of silicon? J. Microelectromech. Syst. 2010, 19, 229–238. [Google Scholar] [CrossRef]
- Milman, Y.V.; Gridneva, I.; Golubenko, A. Construction of stess-strain curves for brittle materials by indentation in a wide temperature range. Sci. Sinter. 2007, 39, 67–75. [Google Scholar] [CrossRef]
- Huff, M.A.; Nikolich, A.D.; Schmidt, M.A. Design of sealed cavity microstructures formed by silicon wafer bonding. J. Microelectromech. Syst. 1993, 2, 74–81. [Google Scholar] [CrossRef]
- Schneider, F.; Fellner, T.; Wilde, J.; Wallrabe, U. Mechanical properties of silicones for MEMS. J. Micromech. Microeng. 2008, 18. [Google Scholar] [CrossRef]
- Greaves, G.N.; Greer, A.; Lakes, R.; Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 2011, 10, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Moy, P.; Gunnarsson, C.A.; Weerasooriya, T.; Chen, W. Stress-strain response of pmma as a function of strain-rate and temperature. In Dynamic Behavior of Materials; Springer: New York, NY, USA, 2011; Volume 1, pp. 125–133. [Google Scholar]
- Kuo, J.S.; Ng, L.; Yen, G.S.; Lorenz, R.M.; Schiro, P.G.; Edgar, J.S.; Zhao, Y.; Lim, D.S.; Allen, P.B.; Jeffries, G.D. A new usp class vi-compliant substrate for manufacturing disposable microfluidic devices. Lab Chip 2009, 9, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Alvankarian, J.; Majlis, B.Y. A new uv-curing elastomeric substrate for rapid prototyping of microfluidic devices. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Alvankarian, J.; Majlis, B.Y. Exploiting the oxygen inhibitory effect on uv curing in microfabrication: A modified lithography technique. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Alvankarian, J.; Majlis, B.Y. Low cost prototyping of microfluidic structure. In Proceedings of the IEEE International Conference on Semiconductor Electronics, Melaka, Malaysia, 28–30 June 2010; pp. 317–320.
- Liu, W.; Li, L.; Wang, J.-C.; Tu, Q.; Ren, L.; Wang, Y.; Wang, J. Dynamic trapping and high-throughput patterning of cells using pneumatic microstructures in an integrated microfluidic device. Lab Chip 2012, 12, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Grover, W.H.; Ivester, R.H.; Jensen, E.C.; Mathies, R.A. Development and multiplexed control of latching pneumatic valves using microfluidic logical structures. Lab Chip 2006, 6, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Takeuchi, S. A dynamic microarray with pneumatic valves for selective trapping and releasing of microbeads. In Proceedings of the IEEE 22nd International Conference on Micro Electro Mechanical Systems, Sorrento, Italy, 25–29 January 2009; pp. 371–374.
- Wu, Z.; Hjort, K. Microfluidic hydrodynamic cell separation: A review. Micro Nanosyst. 2009, 1, 181–192. [Google Scholar] [CrossRef]
- Tanyeri, M.; Ranka, M.; Sittipolkul, N.; Schroeder, C.M. A microfluidic-based hydrodynamic trap: Design and implementation. Lab Chip 2011, 11, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.; Kim, J. Hydrodynamic trap-and-release of single particles using dual-function elastomeric valves: Design, fabrication, and characterization. Microfluid. Nanofluid. 2012, 13, 835–844. [Google Scholar] [CrossRef]
- Huang, L.R.; Cox, E.C.; Austin, R.H.; Sturm, J.C. Continuous particle separation through deterministic lateral displacement. Science 2004, 304, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.; Aghdam, N.; Lee, L.P. Single-cell enzyme concentrations, kinetics, and inhibition analysis using high-density hydrodynamic cell isolation arrays. Anal. Chem. 2006, 78, 4925–4930. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Song, S.; Choi, C.; Park, J.-K. Continuous blood cell separation by hydrophoretic filtration. Lab Chip 2007, 7, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Irimia, D.; Toner, M. Cell handling using microstructured membranes. Lab Chip 2006, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, T.; Woo, S.; Ma, H. Chromatographic behaviour of single cells in a microchannel with dynamic geometry. Lab Chip 2011, 11, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvankarian, J.; Majlis, B.Y. Tunable Microfluidic Devices for Hydrodynamic Fractionation of Cells and Beads: A Review. Sensors 2015, 15, 29685-29701. https://doi.org/10.3390/s151129685
Alvankarian J, Majlis BY. Tunable Microfluidic Devices for Hydrodynamic Fractionation of Cells and Beads: A Review. Sensors. 2015; 15(11):29685-29701. https://doi.org/10.3390/s151129685
Chicago/Turabian StyleAlvankarian, Jafar, and Burhanuddin Yeop Majlis. 2015. "Tunable Microfluidic Devices for Hydrodynamic Fractionation of Cells and Beads: A Review" Sensors 15, no. 11: 29685-29701. https://doi.org/10.3390/s151129685
APA StyleAlvankarian, J., & Majlis, B. Y. (2015). Tunable Microfluidic Devices for Hydrodynamic Fractionation of Cells and Beads: A Review. Sensors, 15(11), 29685-29701. https://doi.org/10.3390/s151129685





