Using Bio-Functionalized Magnetic Nanoparticles and Dynamic Nuclear Magnetic Resonance to Characterize the Time-Dependent Spin-Spin Relaxation Time for Sensitive Bio-Detection
Abstract
: In this work, we report the use of bio-functionalized magnetic nanoparticles (BMNs) and dynamic magnetic resonance (DMR) to characterize the time-dependent spin-spin relaxation time for sensitive bio-detection. The biomarkers are the human C-reactive protein (CRP) while the BMNs are the anti-CRP bound onto dextran-coated Fe3O4 particles labeled as Fe3O4-antiCRP. It was found the time-dependent spin-spin relaxation time, T2, of protons decreases as time evolves. Additionally, the ΔT2 of of protons in BMNs increases as the concentration of CRP increases. We attribute these to the formation of the magnetic clusters that deteriorate the field homogeneity of nearby protons. A sensitivity better than 0.1 μg/mL for assaying CRP is achieved, which is much higher than that required by the clinical criteria (0.5 mg/dL). The present MR-detection platform shows promise for further use in detecting tumors, viruses, and proteins.1. Introduction
A number of diagnostic platforms have been developed to measure the abundance of biomolecules with high sensitivity. Those platforms enable us to diagnose diseases at early stages and gain valuable insights into their biology at the detection level of the respective systems [1–3]. Some examples include SQUID-based magnetic detection [4–8], magnetic resonance (MR) [9,10], nanowires [11,12], nanoparticles [13], surface plasmon resonance [14], mass spectrometry [15]. Among these, assaying bio-molecules based on magnetic detection has received considerable attention. Magnetic detection can be implemented via the measurements of magnetic relaxation [4,16], remanent magnetization [5], alternating current (ac) susceptibility immunomagnetic reduction (IMR) assay [17], saturation magnetization [18], spin-spin relaxation [19]. The sensors to such a system would include SQUID sensors [20], magnetoresistive sensors [21], micro-MR sensors [22], and magnetic nanotag sensors [23]. However, some of the detection methods are time consuming. It is in our interest to develop a detection platform that is easy to operate and sensitive, enabling us to diagnose diseases at early stages and perform early treatment.
In this work, we report a sensitive diagnostic MR for assaying biomarkers (CRP in this study). In the detection platform we amplify the dynamic MR signals to study the molecular interaction via the measurements of the time-dependent spin-spin relaxation time, T2, of protons during the association of Fe3O4-antiCRP with CRPs. It was found that T2 decreases as the concentration of the CRP increases. Additionally, the ΔT2 of protons in BMNs decreases as the concentration of CRP decreases, where ΔT2 = |T2(t = 120 min) − T2(t = 0)|. We attribute this to the stray fields from the magnetization of magnetic clusters that will deteriorate the field homogeneity seen by protons nearby. A detection sensitivity better than 0.1 μg/mL CRP is achieved via the dynamic measurements of T2-relaxation. This MR-detection platform shows promise for further use in a broad number of biomedical applications, such as detecting viruses, and proteins, via relaxation measurements.
2. Experiments
The dextran-coated magnetic nano-particles were synthesized by chemical co-precipitation [24]. The XRD pattern of the synthesized nanopartices present the high purity for the synthesized Fe3O4 magnetic particles without other phase such as Fe(OH)3 or Fe2O3. Then the antigoat C-reactive protein was bound onto the dextran on Fe3O4 particles. Polyclonal goat anti-CRP (Sigma-Aldrich, St. Louis, MO, USA) was covalently bound onto the dextran coated Fe3O4 particles labeled as Fe3O4-antiCRP (MagQu Co, New Taipei City, Taiwan) [18]. The BMNs, consisted of anti-C-reactive protein, were dispersed in phosphate buffered saline solution with a pH value of 7.4. Measured with a vibration sample magnetometer, the saturated magnetization of magnetic reagents was 0.3 emu/g measured by vibration sample magnetometer (Model 4500, EG&G, San Francisco, CA, USA), which corresponds to a concentration of 8.5 mg-Fe/mL. A laser scattering analysis (LSA) system (Nanotrac-150, Microtrac, Montgomeryville, PA, USA) was used to obtain the dispersion and average hydrodynamic diameter of biofunctionalized anti-CRP magnetic particles. The hydrodynamic diameter of biofunctionalized anti-CRP magnetic particles is 46.1 nm with a standard deviation of 9.3 nm. The details of the the preparation process and the characterization of BMNs are addressed in references [18,24]. The biotargets of biofunctionalized anti-CRP magnetic particles are human C-reactive protein (CRP).
The schematic of the MR detection is shown in Figure 1. The permanent magnet supplies a magnetic field strength of 0.35 Tesla, which corresponds to a resonant frequency of protons at 15.41 MHz. In the spin-echo MR signal detection, a 90° pulse was first applied to the sample via the pulse coil and the spin-echo signals were detected via the receiving coil by applying 180°-pulses. Typical time duration was 25 μs for the 90° pulse and 50 μs for the 180° pulse. The time delay between the 90°-pulse and 180°-pulse was 150 μs, and the time delay between the 180°-pulses (TE time) is 300 μs. The spin-echo signal was amplified and detected via a mixer. The T2 relaxation time was measured every five minutes for a total time of 120 min to study the dynamic T2 of protons as Fe3O4-antiCRP becomes associated with CRP.
3. Results and Discussion
Figure 2 shows a spin-echo MR signal of protons for a 120 μL reagent consisted of Fe3O4-anti CRP with a saturated magnetization of 0.025 emu/g. The strength of the spin-echo MR signal of protons in BMNs satisfies the relation:
The term T2 = 992 μs is derived by fitting the data of spin-echo MR signal to Equation (1). Figure 3 shows the relaxation rate T2−1 of protons in reagents consisted of Fe3O4-antiCRP as a function of saturated magnetization. In the examination there is no CRP added in to the magnetic reagent. The T2−1 was 500 s−1 as the saturated magnetization of reagents was 0.05 emu/g and the T2-relaxation rate increases to 1700 s−1 as the saturated magnetization of reagent increases to 0.1 emu/g. Therefore, the T2-relaxation rate of protons increases monotonically as its concentration increases. The reagent with magnetic susceptibility of 0.1 emu/g was used to study molecular interactions.
Figure 4 shows the spin-echo MR signals of protons when a reagent of Fe3O4-antiCRP with saturated magnetization = 0.025 emu/g associated with CRP at t = 0 before the association and t = 120 min after the association. The T2 is 545 μs at t = 0 and T2 decreases to 290 μs after at t = 120 min. The decrease of T2 at t = 120 min in comparison to t = 0 is due to the molecular interaction between BMNs with CRP, which causes the conjugation of the magnetic clusters. The stray fields generated from the magnetization of magnetic clusters deteriorate the field homogeneity seen by nearby protons. Therefore the T2 relaxation time at t = 120 min is shorter than T2 at t = 0.
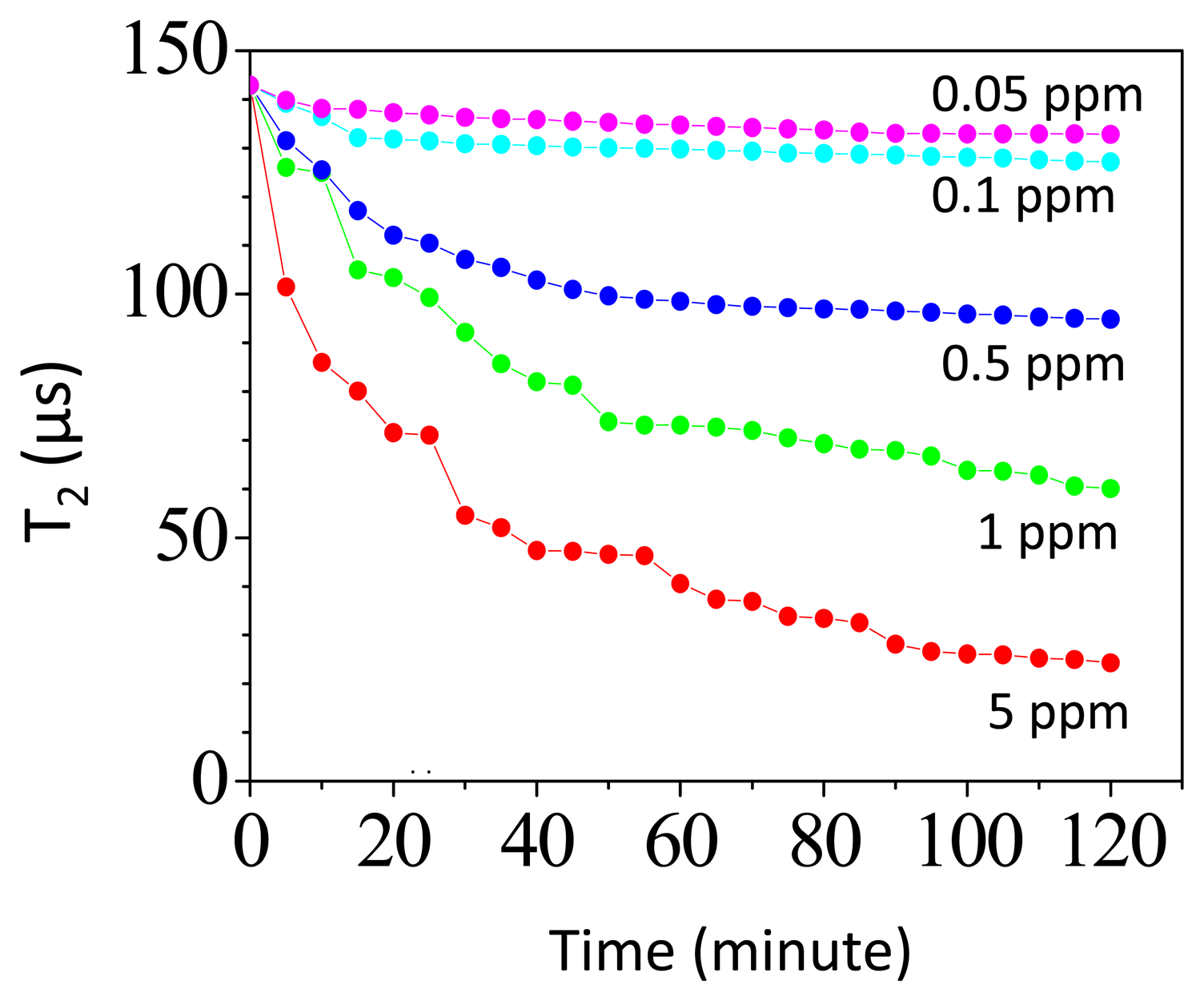
Figure 5 shows the time-dependent T2 in assaying different amount of CRPs. The volume of the reagent is 60 μL and has a saturated magnetization of 0.1 emu/g. In assaying 0.5 ppm CRP, the T2 of protons at t = 0 is 142 μs and T2 decreases to 100 μs at t = 120 min. In assaying 0.05 ppm CRP, the T2 of protons at t = 0 is 142 μs and T2 decreases to 136 μs at t = 120 min. As we assay the CRP, we find that there is a reduction in T2-relaxation time as time evolves. This is due to the formation of the magnetic clusters during the association of Fe3O4-antiCRP with CRPs. The magnetization from magnetic clusters will deteriorate the field homogeneity seen by protons nearby. Therefore the T2-relaxation time of protons decreases as time evolves [19,25].
Figure 6 shows the ΔT2 of protons as a function of CRP concentrations, where ΔT2 = |T2(t = 120 min) − T2(t = 0)|. The ΔT2 = 135 μs as the concentration of CRP is 10 ppm and the ΔT2 decreases to 10 μs as the concentration of the CRP decreases to 0.05 ppm. The ΔT2 increases as the concentration of CRP increases. We attribute this to the enhanced molecular interaction that will increase the strength of magnetization in magnetic clusters as the concentration of CRP increases. The enhanced stray fields from the magnetization will increasingly deteriorate the field homogeneity seen by protons nearby. The solid curve is the fitting curve to a logistic function:
The diagnostic applications using magnetic nanoparticles and microMR were reported [19,22]. That paper addressed the use of MNPs for in vitro detection of cellular biomarkers based on the effects of molecular interaction on T2-relaxation of protons. That platform showed parallel and rapid measurements from small sample volumes, and a wide range of targets, including whole cells, proteins, DNA/mRNA, metabolites, drugs, viruses and bacteria. The present study reports the time-dependent detection of T2-relaxation with the sensing coil wound around the reagent, Fe3O4-antiCRP. The sensing coil is simple to make and shows high detection sensitivity. The time-dependent T2-relaxation of protons is characterized with a reagent of 120 μL within a couple of minutes. To reduce assaying time, we can first mix the reagents with CRPs and let them stand there to complete the association, and then characterize the change of T2 using MR, which can be achieved in minutes.
CRP levels in human blood are a key indicator of infectious/noninfectious diseases or acute tissue. The normal concentration in healthy human serum is lower than 10 μg/mL, slightly increasing with aging. Higher levels are found in late pregnant women, mild inflammation and viral infection (10–40 μg/mL), and bacterial infection (40–200 μg/mL) [26]. Examining CRP levels in blood is helpful in the diagnosis of diseases or acute tissue. At present, there are several reported quantitative methods used for assaying CRP, which include enzyme-linked immunosorbent assay [27], radioimmunoassay [28], and immunonephelometry [29], and immunomagnetic reduction assay [17,30]. The present MR detection sensitivity shows high detection sensitivity better than 0.1 μg/mL, which is much higher than that required by the clinical criteria (0.5 mg/dL). The present dynamic MR detection method is robust, easy-to-use and shows potential in the application of detecting a wide variety of biomarkers.
4. Conclusions
In this work, we report the time-dependent T2-relaxation of protons as the Fe3O4-AntiCRP is associated with CRP. There is a reduction in the T2-relaxation time of protons as time evolves. Additionally, the ΔT2 increases as the concentration of CRP increases. We attribute these to the molecular interaction that increases the magnetization due to the presence of magnetic clusters. The local fields created from the magnetization of magnetic clusters deteriorate the field homogeneity seen by protons nearby. A detection sensitivity better than 0.1 μg/mL (0.1 ppm) CRP is verified via the measurements of dynamic T2-relaxation. The present MR-detection platform is easy to use and shows promise for detecting tumors, viruses, and proteins.
Acknowledgments
This work is supported by the National Science Council of Taiwan under grant number: NSC 102-2112-M-003-008-MY2, NSC 103-2420-H-003-006-, NSC 102-2923-M-003-001-, NSC 103-2923-M-003-002 , NSC 103-2112-M-003-010, NSC 102-2120-M-168-001-, NSC 102-2112-M-003-017—and by “Aim for the Top University Plan” of the National Taiwan Normal University and the Ministry of Education, Taiwan, R.O.C under grant number 103J1A27.
Author Contributions
S.H. Liao: Experimental design, experimental validation, data analysis, article writing.
K.L. Chen: Experimental design, experimental validation.
C.M. Wang: Experimental validation, data analysis.
J.J. Chieh: Experimental design, data analysis.
H. E. Horng: Experimental design, data analysis.
L.M. Wang: Experimental design, data analysis, article writing.
C.H. Wu: Experimental design.
H.C. Yang: Experimental design, article writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, M.M.-C.; Cuda, G.; Bunimovich, Y.L.; Gaspari, M.; Heath, J.R.; Hill, H.D.; Mirkin, C.A.; Nijdam, A.J.; Terracciano, R.; Thundat, T.; et al. Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 2006, 10, 11–19. [Google Scholar]
- Wulfkuhle, J.D.; Liotta, L.A.; Petricoin, E.F. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer 2003, 3, 267–275. [Google Scholar]
- Hood, L.; Heath, J.R.; Phelps, M.E.; Lin, B. Systems biology and new technologies enable predictive and preventative medicine. Science 2004, 306, 640–643. [Google Scholar]
- Kötitz, R.; Weitschies, W.; Trahms, L.; Brewer, W.; Semmler, W. Determination of the biding reaction between avidin and biotin by relaxation measurements of magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 194, 62. [Google Scholar]
- Enpuku, K.; Minotani, T.; Gima, T.; Kuroki, Y.; Itoh, Y.; Yamashita, M.; Katakura, Y.; Kuhara, S. Detection of Magnetic Nanoparticles with Superconducting Quantum Interference Device (SQUID) Magnetometer and Application to Immunoassays. Jpn. J. Appl. Phys. 1999, 38, L1102. [Google Scholar]
- Chiu, M.J.; Yang, S.Y.; Chen, T.F.; Chieh, J.J.; Huang, T.Z.; Yip, P.K.; Yang, H.C.; Cheng, T.W.; Chen, Y.F.; Hua, M.S.; et al. New assay for old markers-Plama beta amyloid of mild cognitive impairment and Alzheimer's disease. Curr. Alzheimer Res. 2012, 9, 1142–1148. [Google Scholar]
- Chieh, J.J.; Yang, S.-Y.; Horng, H.E.; Yu, C.Y.; Lee, C.L.; Wu, H.L.; Hong, C.-Y.; Yang, H.-C. Immnuomagnetic reduction assay using high-Tc superconducting quantum-interference device based magnetosusceptometry. J. Appl. Phys. 2010, 107, 074903. [Google Scholar]
- Yang, S.Y.; Chieh, J.J.; Yang, C.C.; Liao, S.H.; Chen, H.H.; Horng, H.E.; Yang, H.C.; Hong, C.Y.; Chiu, M.J.; Chen, T.F.; et al. Clinic Applications in Assaying Ultra-Low-Concentration Bio-Markers Using HTS SQUID-Based AC Magnetosusceptometer. IEEE Trans. Appl. Supercon. 2013, 23, 1600604. [Google Scholar]
- Schroder, L.; Lowery, T.; Hilty, C.; Wemmer, D.; Pines, A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science 2006, 314, 446–449. [Google Scholar]
- Haun, J.B.; Yoon, T.-J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.; Lieber, C. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar]
- Nam, J.M.; Thaxton, C.; Mirkin, C. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Plasmon light scattering in biology and medicine: New sensing approaches, visions and perspectives. Curr. Opin. Chem. Biol. 2005, 9, 538–544. [Google Scholar]
- Aebersold, R.; Mann, M. Mass spectrometry–based proteomics. Nature 2003, 422, 198–207. [Google Scholar]
- Lee, S.K.; Myers, W.R.; Grossman, H.L.; Cho, H.-M.; Chemla, Y.R.; Clarke, J. Magnetic gradiometer based on a high-transition temperature superconducting quantum interference device for improved sensitivity of a biosensor. Appl. Phys. Lett. 2002, 81, 3094. [Google Scholar]
- Hong, C.Y.; Wu, C.C.; Chiu, Y.C.; Yang, S.Y.; Horng, H.E.; Yang, H.C. Magnetic susceptibility reduction method for magnetically labeled immunoassay. Appl. Phys. Lett. 2006, 88, 212512. [Google Scholar]
- Horng, H.E.; Yang, S.Y.; Hong, C.-Y.; Liu, C.M.; Tsai, P.S.; Yang, H.C.; Wu, C.C. Biofunctionalized magnetic nanoparticles for high-sensitivity immunomagnetic detection of human C-reactive protein. Appl. Phys. Lett. 2006, 88, 252506. [Google Scholar]
- Lee, H.; Sun, E.; Ham, D.; Weissleder, R. Chip–NMR biosensor for detection and molecular analysis of cells. Nat. Med. 2008, 14, 869. [Google Scholar]
- Chemla, Y.R.; Grossman, H.L.; Poon, Y.; McDermott, R.; Stevens, R.; Alper, M.D.; Clarke, J. Ultrasensitive magnetic biosensor for homogeneous immunoassay. Proc. Natl. Acad. Sci. USA 2000, 97, 14268. [Google Scholar]
- Baselt, D.R.; G.U. Lee, M.; Natesan, S.W.; Metzger, P.E.; Sheehan, R.J. Colton. Biosens. Bioelectron. 1998, 13, 731.
- Shao, H.; Min, C.; Issadore, D.; Liong, M.; Yoon, T.-J.; Weissleder, R.; Lee, H. Magnetic Nanoparticles and microNMR for Diagnostic Applications. Theranostics 2012, 2, 55–65. [Google Scholar]
- Osterfeld, S.; Yu, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.J.; Hall, D.A.; Wilson, R.J.; Sun, S.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 2008, 105, 20637–20640. [Google Scholar]
- Jiang, W.; Yang, H.C.; Yang, S.Y.; Horng, H.E.; Hung, J.C.; Chen, Y.C.; Hong, C.Y. Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J. Magn. Magn. Mater. 2004, 283, 210–214. [Google Scholar]
- Shao, H.; Yoon, T.J.; Liong, M.; Weissleder, R.; Lee, H. Magnetic nanoparticles for biomedical NMR-based diagnostics. Beilstein J. Nanotechnol. 2010, 1, 142–154. [Google Scholar]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emergy Med. 1999, 17, 1019–1025. [Google Scholar]
- MacBeath, G.; Schreiber, S.L. Printing proteins as microarrays for high-throughput function determination. Science 2000, 289, 1760–1763. [Google Scholar]
- Yalow, R.S.; Berson, S.A. Assay of plasma insulin in human subjects by immunological methods. Nature 1959, 184, 1648–1649. [Google Scholar]
- Yang, S.Y.; Wu, R.M.; Chien, C.F.; Horng, H.E.; Hong, C.-Y.; Yang, H.C. One-sample measurement in laser nephelometric immunoassay using magnetic nanoparticles. Appl. Phys. Lett. 2006, 89, 244106. [Google Scholar]
- Chang, C.H.; Lai, Z.X.; Lin, H.L.; Yang, C.C.; Chen, H.H.; Yang, S.Y.; Horng, H.E.; Hong, C.Y.; Yang, H.C.; Lin, H.C. Use of immunomagnetic reduction for C-reactive protein assay in clinical samples. Int. J. Nanomedicine 2012, 7, 4335–4340. [Google Scholar]





© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liao, S.-H.; Chen, K.-L.; Wang, C.-M.; Chieh, J.-J.; Horng, H.-E.; Wang, L.-M.; Wu, C.H.; Yang, H.-C. Using Bio-Functionalized Magnetic Nanoparticles and Dynamic Nuclear Magnetic Resonance to Characterize the Time-Dependent Spin-Spin Relaxation Time for Sensitive Bio-Detection. Sensors 2014, 14, 21409-21417. https://doi.org/10.3390/s141121409
Liao S-H, Chen K-L, Wang C-M, Chieh J-J, Horng H-E, Wang L-M, Wu CH, Yang H-C. Using Bio-Functionalized Magnetic Nanoparticles and Dynamic Nuclear Magnetic Resonance to Characterize the Time-Dependent Spin-Spin Relaxation Time for Sensitive Bio-Detection. Sensors. 2014; 14(11):21409-21417. https://doi.org/10.3390/s141121409
Chicago/Turabian StyleLiao, Shu-Hsien, Kuen-Lin Chen, Chun-Ming Wang, Jen-Jie Chieh, Herng-Er Horng, Li-Min Wang, C. H. Wu, and Hong-Chang Yang. 2014. "Using Bio-Functionalized Magnetic Nanoparticles and Dynamic Nuclear Magnetic Resonance to Characterize the Time-Dependent Spin-Spin Relaxation Time for Sensitive Bio-Detection" Sensors 14, no. 11: 21409-21417. https://doi.org/10.3390/s141121409
APA StyleLiao, S.-H., Chen, K.-L., Wang, C.-M., Chieh, J.-J., Horng, H.-E., Wang, L.-M., Wu, C. H., & Yang, H.-C. (2014). Using Bio-Functionalized Magnetic Nanoparticles and Dynamic Nuclear Magnetic Resonance to Characterize the Time-Dependent Spin-Spin Relaxation Time for Sensitive Bio-Detection. Sensors, 14(11), 21409-21417. https://doi.org/10.3390/s141121409





