Development of a Portable Taste Sensor with a Lipid/Polymer Membrane
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
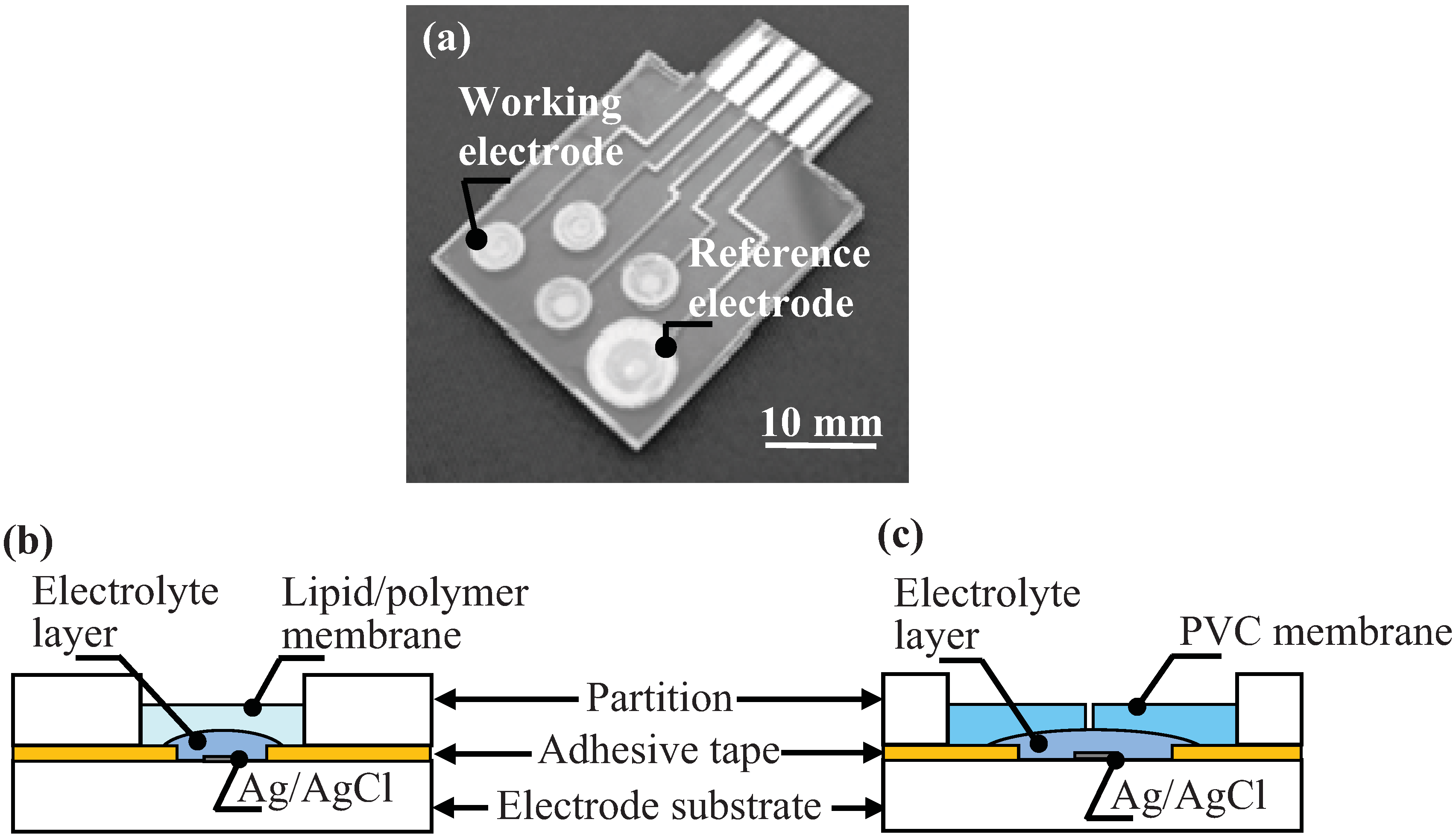
2.2. Taste Sensor Chip
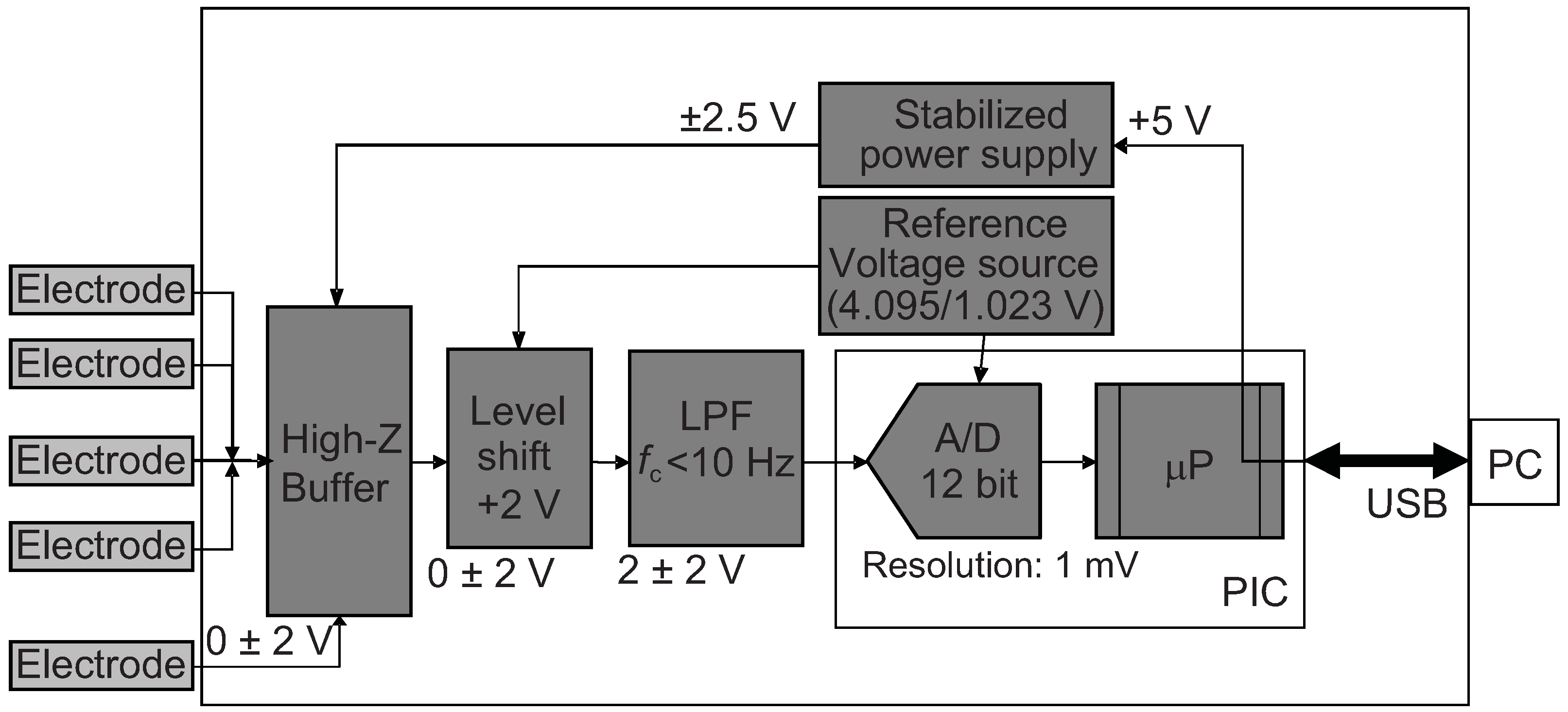
2.3. Portable Taste Sensor Device and the Electronics
2.4. The Performance of the Fabricated Reference Electrode
2.5. Performance of the Portable Taste Sensor
3. Results and Discussion
3.1. Characteristics of the Reference Electrode
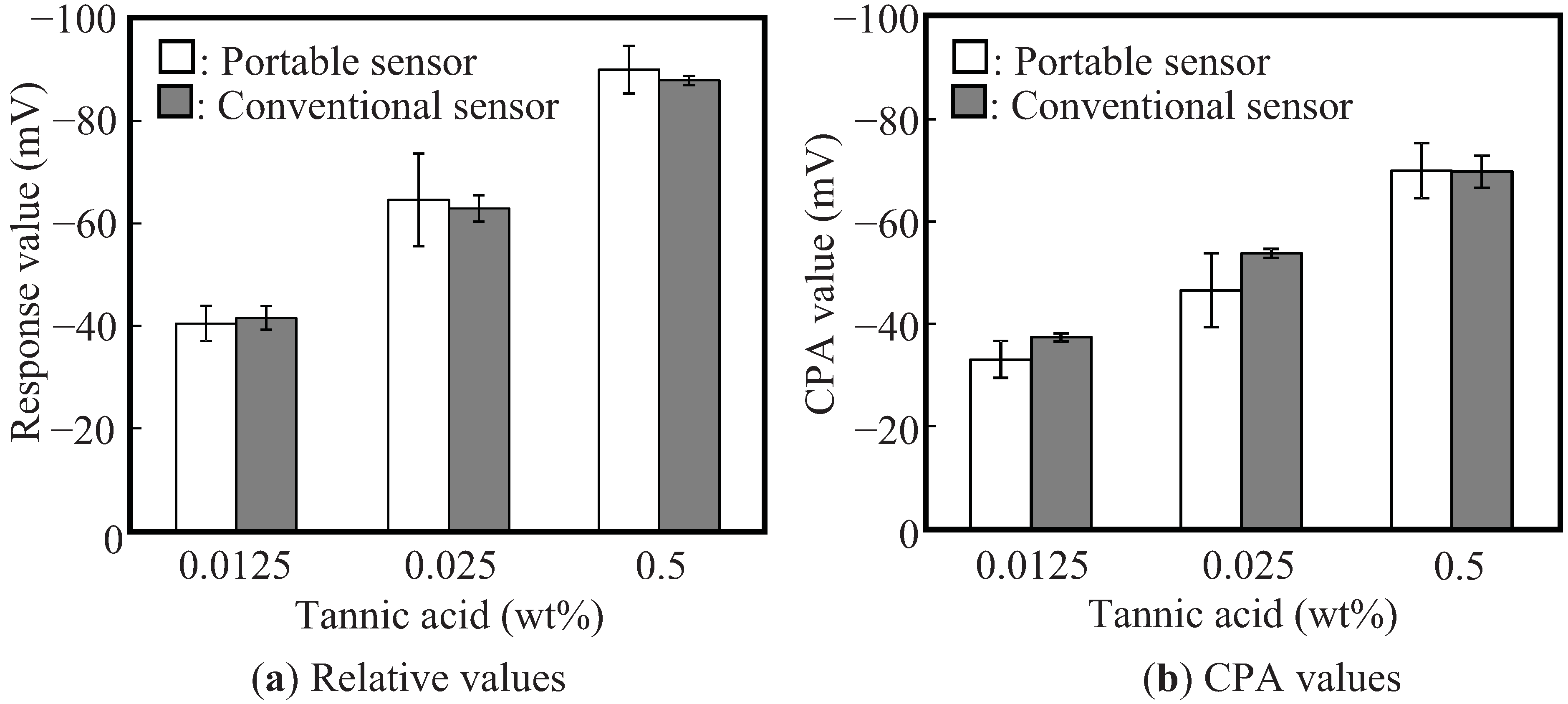
3.2. Performance of the Portable Taste Sensor
4. Conclusions
Acknowledgments
References
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar]
- Anand, V.; Kataria, M.; Kukkar, V.; Saharan, V.; Choudhury, P.K. The latest trends in the taste assessment of pharmaceuticals. Drug Discov. Today 2007, 12, 257–265. [Google Scholar]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Natale, C.D.; D'Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids. Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar]
- Iiyama, S.; Yahiro, M.; Toko, K. Measurements of soy sauce using taste sensor. Sens. Actuators B 2000, 66, 205–206. [Google Scholar]
- Hayashi, N.; Chen, R.; Ikezaki, H.; Ujihara, T. Evaluation of the umami taste intensity of green tea by a taste sensor. J. Agric. Food Chem. 2008, 56, 7384–7387. [Google Scholar]
- Toko, K. Taste Sensors, Biomimetic Sensor Technology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Toko, K. Taste sensor. Sens. Actuators B 2000, 64, 205–215. [Google Scholar]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar]
- Kawamura, Y.; Funakoshi, M.; Kasahara, Y.; Yamamoto, T. A neurophysiological study on astringent taste. Jpn. J. Physiol. 1969, 19, 851–865. [Google Scholar]
- Schiffman, S.S.; Suggs, M.S.; Sostman, A.L.; Simon, S.A. Chorda tympani and lingual nerve responses to astringent compounds in rodents. Physiol. Behav. 1992, 51, 55–63. [Google Scholar]
- Bajec, M.R.; Pickering, G.J. Astringency: mechanisms and perception. Crit. Rev. Food Sci. Nutr. 2008, 48, 858–875. [Google Scholar]
- Twomey, K.; de Eulate, E.A.; Alderman, J.; Arrigan, D.W.M. Fabrication and characterization of a miniaturized planar voltammetric sensor array for use in an electronic tongue. Sens. Actuators B 2009, 140, 532–541. [Google Scholar]
- Winquist, F.; Krantz-Rulcker, C.; Lundstrom, I. A miniaturized voltammetric electronic tongue. Anal. Lett. 2008, 41, 917–924. [Google Scholar]
- Twomey, K.; Truemper, A.; Murphy, K. A Portable sensing system for electronic tongue operations. Sensors 2006, 6, 1679–1696. [Google Scholar]
- Etoh, S.; Feng, L.; Nakashi, K.; Hayashi, K.; Ishii, A.; Toko, K. Taste sensor chip for portable taste sensor system. Sens. Mater. 2008, 20, 151–160. [Google Scholar]
- Etoh, S.; Iwakura, M.; Nakashi, K.; Hattori, R.; Hayashi, R.; Toko, K. Fabrication of taste sensor chip and portable taste sensor system. Proceedings of the International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 9–12 May 2006; pp. 180–183.
- Tahara, Y.; Ikeda, A.; Maehara, Y.; Habara, M.; Toko, K. Development and evaluation of a miniaturized taste sensor chip. Sensors 2011, 11, 9878–9886. [Google Scholar]

| Taste | Composition |
|---|---|
| Reference solution | 30 mM KCl, 0.3 mM tartaric acid |
| Saltiness | 300 mM KCl, 0.3 mM tartaric acid |
| Sourness | 30 mM KCl, 3mM tartaric acid |
| Umami | 10 mM monosodium glutamate |
| Bitterness (+) | 0.1 mM Quinine-HCl |
| Bitterness (–) | 0.01 vol% iso-alpha acid |
| Astringency | 0.05 wt% tannic acid |
| Sweetness | 1 M Sucrose |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tahara, Y.; Nakashi, K.; Ji, K.; Ikeda, A.; Toko, K. Development of a Portable Taste Sensor with a Lipid/Polymer Membrane. Sensors 2013, 13, 1076-1084. https://doi.org/10.3390/s130101076
Tahara Y, Nakashi K, Ji K, Ikeda A, Toko K. Development of a Portable Taste Sensor with a Lipid/Polymer Membrane. Sensors. 2013; 13(1):1076-1084. https://doi.org/10.3390/s130101076
Chicago/Turabian StyleTahara, Yusuke, Kenichi Nakashi, Ke Ji, Akihiro Ikeda, and Kiyoshi Toko. 2013. "Development of a Portable Taste Sensor with a Lipid/Polymer Membrane" Sensors 13, no. 1: 1076-1084. https://doi.org/10.3390/s130101076
APA StyleTahara, Y., Nakashi, K., Ji, K., Ikeda, A., & Toko, K. (2013). Development of a Portable Taste Sensor with a Lipid/Polymer Membrane. Sensors, 13(1), 1076-1084. https://doi.org/10.3390/s130101076





