Semiconductor Quantum Dots for Biomedicial Applications
Abstract
: Semiconductor quantum dots (QDs) are nanometre-scale crystals, which have unique photophysical properties, such as size-dependent optical properties, high fluorescence quantum yields, and excellent stability against photobleaching. These properties enable QDs as the promising optical labels for the biological applications, such as multiplexed analysis of immunocomplexes or DNA hybridization processes, cell sorting and tracing, in vivo imaging and diagnostics in biomedicine. Meanwhile, QDs can be used as labels for the electrochemical detection of DNA or proteins. This article reviews the synthesis and toxicity of QDs and their optical and electrochemical bioanalytical applications. Especially the application of QDs in biomedicine such as delivering, cell targeting and imaging for cancer research, and in vivo photodynamic therapy (PDT) of cancer are briefly discussed.1. Introduction
Quantum dots (QDs) as colloidal nanocrystalline semiconductors have unique photophysical properties due to quantum confinement effects. They emit different wavelengths over a broad range of the light spectrum from visible to infrared, depending on their sizes and chemical compositions. Compared with the traditional organic fluorophores (e.g., organic dyes and fluorescent proteins), QDs have unique optical and electronic properties, such as larger absorption coefficients, size-tunable light emission, superior signal brightness, resistance to photobleaching and simultaneous excitation of multiple fluorescence colors [1–6]. In addition, the large-surface area of QDs is beneficial to covalently link to biorecognition molecules, such as peptides, antibodies, nucleic acids or small-molecule ligands for further application as fluorescent probes (Figure 1).
These properties of QDs herald a revolution from electronic materials science to biological applications [7]. Current and projected applications of QDs include using fluorescent labels for cellular labeling [1,8,9], intracellular sensors [10,11], deep-tissue and tumor targeting and imaging agents [7,12–17], sensitizers for photodynamic therapy (PDT) [18–21], vectors for gene therapy [22–26], magnetic resonance imaging (MRI) contrast agents [27,28] and so on. This review mainly summarizes the development of synthesis, the surface modification and toxicity of QDs, and briefly focuses on the application developments of QDs in the biomedical field.
2. The Surface Chemistry and Toxicity of QDs
Early in the 1990s, Bawendi and coworkers first reported a synthesis protocol for QDs with highly monodisperse, regular core structure and tunable particle size [29,30]. Up to now, the most successful and well-developed method to prepare highly luminescent II–VI QDs is the TOP/TOPO synthetic approach [31]. However, these QDs are insoluble in water, which limits their biological applications. Therefore, a number of surface functionalization studies have been developed to make QDs water-soluble and biologically compatible [29–37].
In one common approach, the original hydrophobic coatings are replaced by water-soluble functional molecules (e.g., dithiothreitol [38–40], mercaptocarbonic acids [41–44], 2-aminoethanethiol [33,45], dihydrolipoic acid [34–36,46,47], oligomeric phosphines [37,48], peptides [49–57], and cross-linked dendrons [58–61]) through the ligand exchange reactions. Because the optical properties of the inorganic core are often very sensitive to the surface, the ligand exchange process may result in poorer performance, particularly in the case of quantum dots [62].
The second approach is to encapsulate QDs in an amphiphile whose hydrophobic ends interleave with, but do not replace, the organic coating on QDs. This improvement for QDs synthesis is significant: (1) protecting the core/shell structure and maintaining the original photophysics of QDs; (2) making QDs water-soluble; (3) providing a biological interface and multiple functions [7]. However these kinds of QDs are not stable in biological settings because of relativelyweak anchoring of the single and double hydrophobic tails to the particle. Additionally, the hydrophilic end groups of even biocompatible surfactants may not protect nanocrystals from nonspecific biomolecular interactions [31]. Scientists have used amphiphilic polymers instead of simple amphiphile because single polymer chains can contain multiple hydrophobic units, their interactions with the native organic coatings on QDs can be numerous, and thus the encapsulant can be bound more strongly than conventional surfactants. However, the range of amphiphilic polymers for creating stable and nonaggregating QDs in biological settings has been relatively limited. Up to now, most of the amphiphilic polymers used are commercial and their hydrophobic/hydrophilic ratios are fixed, hence the cost is high and it may be different to control the process of forming water-soluble QDs and to optimize the forming conditions [31].
Although QDs have great prospects, the toxicity of QDs cannot be overlooked. During the processing of biological applications (e.g., cancer imaging, targeting and PDT treatment), the degradation products of QDs will do harm to the cells which they contact with, or produce immune responses with the components in blood [17]. The toxic degradation production routes are: first, the oxidation of the nanoparticle core/shell material can cause the release of free cadmium or other heavy metals, which will interrupt the normal cell activities [18]; secondly, the photosensitized production of reactive oxygen intermediates (ROI) also plays an important role in mediating the cell damage [63]; thirdly, the toxicity of capping materials should also be considered, several groups in capping materials such as mercaptoacetic acid and tri-n-octylphosphine oxide (TOPO) could produce toxicity to cells [12].
To reduce the cytotoxicity of QDs, replacement of the cadmium by nontoxic or less-toxic metals such as indium (In), or encapsulation of the core with a biocompatibile shell should be considered. Though In-based semiconducting dots contain arsenic, another toxin, the cytotoxicity of these dots may be small enough to keep the toxicity low. Fisher and coworkers [64] found that QDs could remain within the body for very long periods. Kim [8] reported that larger QDs generally accumulated in the reticuloendothelial system, such as the liver, spleen and lymphatic system for several months, but the size less than 5 nm could be removed by the kidney quickly. So in order to minimize the toxicity of QDs, QDs can be designed as smaller as they can, which can help them more easily to clean them out from the body.
In spite of the fact many investigators have paid close attention to and observed the side-effect of QDs, the definite metabolism of QDs in vivo remains uncertain [65–68]. Thus, it is still a necessary issue to investigate the detailed biochemical and pharmacological mechanism for further application of QDs in the human body.
3. Delivering QDs into Cells
Effective delivery of QDs into the targeted-cell is the primary requirement for the bioapplications of QDs [9,15,17,20,32,54]. It is a major step because if QDs cannot reach their site of action in vivo, they is useless. Furthermore, efficient delivery can also allow a reduction in dosage level, avoid non-specific side effects and reduce toxicity risks [66,69,70]. The current methods for delivering QDs into cells mainly include passive delivery, facilitated delivery and active delivery.
The general passive delivery for QDs is endocytosis, which is simple, without further functionalization of the QDs surface with a targeting ligand for uptake [66]. By incubating with the cells at appropriate concentration and exposure time, QDs will enter into cells though the nonspecific cell endocytosis. However, the nonspecific ingestion of this mode caused ineffective endosomal escape, and would impede the delivery of QDs to the cytoplasm or other organelles. Furthermore, high intracellular concentration of QDs can enhance the cytotoxicity in some cases [69].
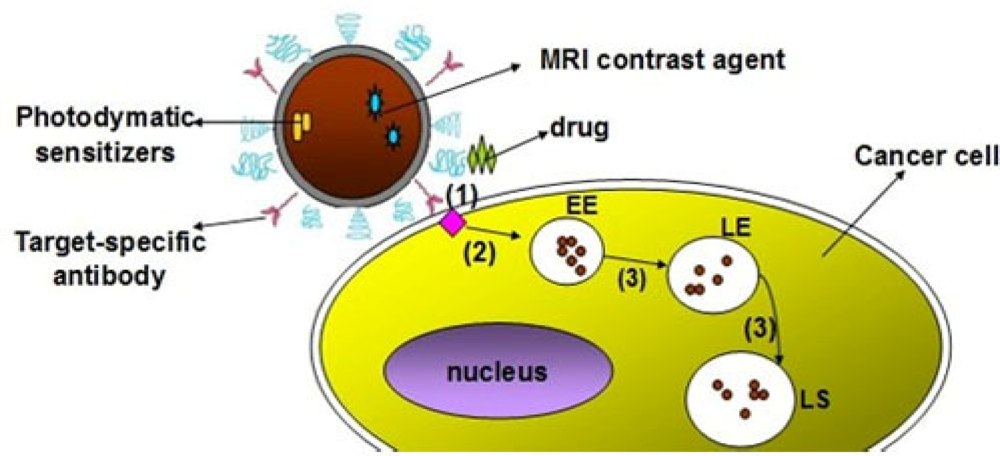
Facilitated delivery includes four ways: peptide-mediated uptake, protein-mediated delivery, polymer-mediated delivery and small molecule-mediated delivery [66]. Generally, these molecules are noncovalently assembled onto the surface of QDs for bioconjugation. Facilitated delivery could reduce the nonspecific absorption and side effects. However, QDs could also be uptaken by cell through endocytosis, leading to endosomal sequestration during the facilitated delivery strategies (Figure 2). As is well known, the high acidic of endosomes could degrade the QDs conjugates over time, thus free Polyethyleneimine (PEI) was used to encapsule the QDs conjugates to increase the stability [70]. Considering further application of cell imaging, more general endosomal escape strategies need to be developed in order to expand the application of facilitated delivery.
Active delivery is a direct physical manipulation of the cell by electroporation and microinjection. In comparison to facilitated delivery, QDs conjugates are delivered directly to the cytoplasm via electroporation by an endocytic pathway, without subsequent endosomal escape. However, the high cellular mortality rate and intracellar aggregation occurring during the delivery should be conquered [71]. Compared with electroporation, microinjection could deliver the QDs directly to the cytoplasm with lower cell death rate, and the rate of microinjection of QDs conjugates to cells depends on the physical constraints of cells, including morphology, membrane thickness, height, etc. [66]. Furthermore, this technology is very expensive. Therefore, considering the coexistence of advantages and drawbacks of the mentioned approaches, the appropriate way for delivering QDs into cell should be determined according to the specific experimental requirements. The relationship between the specific examples and the delivery strategies are listed in Table 1 [66].
4. QDs-Based Cancer Targeting and Imaging
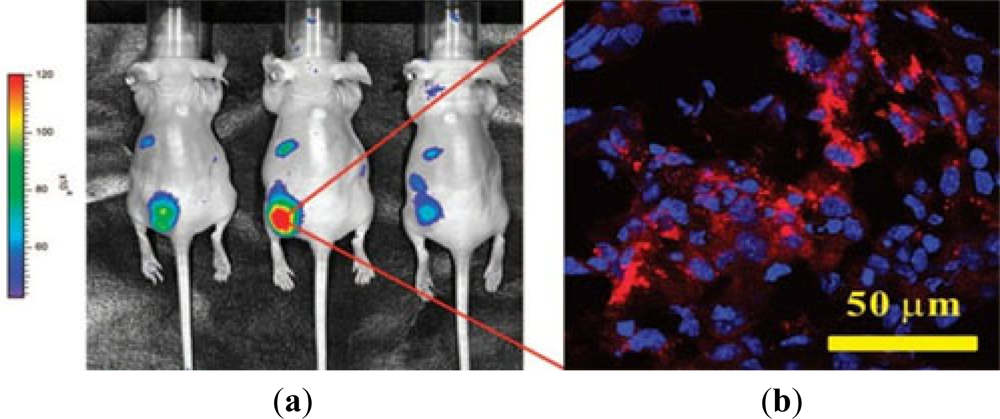
The photoluminescence (PL) of QDs is exceptionally bright and stable, making them potential candidates for biomedical imaging and therapeutic interventions. QDs conjugated with cancer specific ligands/antibodies/peptides were found to be effective for detecting and imaging human cancer cells. Gao and coworkers [67] firstly reported the QDs-antibody conjugates for in vivo targeting and imaging cancer, in which QDs-antibody conjugates were used as imaging probe for investigating and tracing QDs-PSMA antibody conjugates in mouse bearing subcutaneous human prostate cancer. It was found that the QDs-antibody conjugates were efficiently and uniformly distributed in prostate tumors due to the specific binding between PSMA antigen in prostate cancer cells and PSMA antibody on QDs. Cai and coworkers [105] conjugated NIR QDs with RED peptide, which could bind to the over-expressed αvβ3 integrin on the surface of U87MG glioblastoma cells and MDA-MB-435 human breast cancer cells to target cancer cell in vivo. By linking QDs to AFP (alpha-fetoprotein) antibody, an important marker for hepatocellular carcinoma cell lines, a specific immunofluorescent probes was obtained for further detection of AFP antibody in human serum. Yu et al. [106] demonstrated that the probe could target the specific hepatocellular carcinoma cells, and the expected results was obtained by investigating distribution of the probes in cancer cells by using a site-by-site measurement. Weng et al. [107] functionalized QDs with anti-HER2 scFv to synthesize the immunoliposome-based nanoparticles (QD-ILs). After incubating with HER2-overexpressing SK-BR-3 and MCF-7/HER2 cells, the QD-ILs exhibited efficient receptor-mediated endocytosis. In vivo fluorescence imaging showed that QD-ILs had localized prominently in tumors as well as in MPS organs (Figure 3). Liu et al. [68] reported a QDs-based wavelength-resolved spectral imaging for molecular mapping of tumor heterogeneity on human prostate cancer tissue specimens. By conjugating different QDs with specific protein biomarkers, such as E-cadherin, high-molecular-weight cytokeratin, p63, and α-methylacyl CoA racemase, structural distinct prostate glands and single cancer cells could be detected and characterized within the complex microenvironments of radical prostatectomy and needle biopsy tissue specimens using the wavelength-resolved spectral imaging.
The main advantage of QDs imaging is that it is non-ionizing and less hazardous [108]. In recent years, several groups have used QD probes for fluorescence immunostaining of fixed cells and tissue specimens [109–113]. QD-based immunohistochemistry (IHC) can improve both diagnostic sensitivity and specificity. In addition, because multiplexed QD staining can be carried out on intact cells and tissue specimens, it is expected to provide correlated molecular and morphological information, at the same time, this type of integrated biomarker and morphological data are not available from traditional analytical methods such as mass spectrometry, gene chips, protein microarrays, and polymerase chain reactions [109]. However, medical applications of QD-based IHC have achieved only limited success. A major bottleneck is the lack of robust protocols to define the key parameters and steps [109]. For example, there are no consensuses on methods for QD-antibody (QD-Ab) bioconjugation, tissue specimen preparation, multicolor QD staining, image processing and data quantification. So it is necessary to solve these problems, and let the QDs move further.
5. QDs Related Photodynamic Therapy for Cancer
Presently, the conventional types of cancer treatment (chemotherapy and radiation therapy), work by destroying fast-growing cells, but other types of fast-growing healthy cells (such as blood and hair cells) also can be damaged along with cancer cells, causing adverse reactions, or side effects. These side effects can range from fatigue and flu-like symptoms to hair loss and blood clotting problems. PDT developed in last century has become an FDA-approved therapy for different malignancies and with potential in other ailments such as coronary heart disease, AIDS and psoriasis [63].
Exploration of the use of light-activated drugs known as photosensitizers (PS) has been one of the most active areas of photomedical research in recent years [18–21,63,114,115]. PDT uses the combination of a photosensitizing drug and light in the presence of oxygen to cause selective damage to the targeting tissue. During PDT, reactive oxygen intermediates (ROI) is generated in the diseased cells by a simple and controllable light-activated process, which involves a photosensitizer that is capable of absorbing light appropriate wavelength and transfers energy or electron to oxygen or other molecules, and creates ROI such as singlet oxygen (1O2), hydroxyl radical (OH), super oxide anion (O2−) and hydrogen peroxide (H2O2). Then ROI will immediately react with vital biomolecules in cell organelles, leading to cell damage, mutation, death and photooxidation of cell constituents [19,20,63,114,115]. Singlet oxygen (1O2) is regarded as the main mediator of photo-induced cytotoxicity in PDT, which causes oxidation and degradation of cellular components, and ultimately cell apoptosis. [20,63,114,115] (Figure 4).
The standard PS drugs for PDT are porphyrin, phthalocyanines and chlorine derivatives. Porphyrin derivatives are the first generation photosensitizer. Despite the clinical success of porphyrin derivatives, some of their disadvantages like prolonged cutaneous photosensitivity, chemical impurity and weak absorption at therapeutic wavelengths have inspired the development of new PDT photosensitizers with improved optical and chemical properties. Phthalocyanines derivatives have favorable photophysical and chemical properties, which include strong absorbance at long wavelengths and chemical tunability through substituent addition on the periphery of the macrocycle or on the axial ligands. However, like most photosensitizing agents, these PS have poor solubility in water and tend to aggregate in aqueous solutions, which can result in loss of photochemical activity and affect their cell penetrating properties [63]. To resolve such issues nanoparticles are currently being explored as potential delivery systems for PDT photosensitizers or directly as PDT agents. The novel QDs-PS conjugates are used as a high ratio of PDT agents and anticancer targeting antibodies, where QDs can act as nanoscaffolds and solubilizers. They can also function as “energy-harvesting antenna” for PDT therapy due to their large one- or two-photon absorption cross-sections. Thus, QDs can be efficiently exited even deep within tissues and sensitized proximal PDT agents via energy transfer from QDs to PDT [21].
The novel QDs-PS conjugates showed many advantages over conventional PS drugs [17–21,63,114,115]: (1) they are species with well-defined size, shape, and composition, and can be synthesized by relatively simple and inexpensive methods; (2) they have been shown to be nontoxic in the absence of light but have the potential to be cytotoxic under irradiation; (3) they have photostability, and tunable and strong absorption, which can be tuned from the UV their composition and size; (4) the surface coating of QDs can be modified to enable them to become water soluble, biocompatible and target-specific.
However, researchers should be further investigated on the basis of predominances of the QDs-PS compared to the convention PS drugs. Despite many desirable properties of QDs for PDT, there still remain several important issues that need to be addressed to fully assess their applicability as PS in PDT. One major issue is the toxicity profile of the QDs inside the cells and their overall photostability once exposed to biological environments [63]. Another important matter that should be carefully investigated is how their surface composition affects the photosensitization process. Still, QDs-PS conjugates for cancer therapy are only suitable to superficial tumours is also need to be resolved [18].
6. Conclusions and Outlook
In the last decade, the unique photophysical properties and functions of QDs have been widely investigated, making them one of the most promising nanomaterials. Their outstanding performances such as high fluorescence yields, stability against photobleaching and the size-dependent luminescence features of QDs provide broad variety of applications for QDs in many fields. By acting as fluorescent, and photoelectrochemical as well as electrochemical probes, various QDs-based optical and electrochemical bioanalysis have already been successfully explored for sensing a wide range of molecules with high sensitivity and specificity. Furthermore, as a biomedical label, QDs can make a worthy contribution to the development of new diagnostic and delivery systems due to their unique optical properties. By combination of functional biomolecule-nanoparticle hybrid systems and the optical imaging and biophysics, QDs have been used as optical reporter units of biocatalytic transformations and can probe intracellular processes in vitro. QDs as a novel probe for in vivo analysis and clinic therapy such as PDT open an attractive new field with promising prospectives in biomedicine.
Acknowledgments
This review was financially supported by the National Basic Research Program of China (2010CB732400), the National Natural Science Foundation of China (21075055), the Program for Six Peak Talents of Jiangsu province (66) and the Leading Medical Talents Program from Department of Health of Jiangsu Province.
References
- Bruchez, M.J.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar]
- Chan, W.C.; Maxwell, D.J.; Gao, X.H.; Bailey, R.E.; Han, M.Y.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol 2002, 13, 40–46. [Google Scholar]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe) ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem 1997, 101, 9463–9475. [Google Scholar]
- Lim, Y.T.; Kim, S.; Nakayama, A.; Stott, N.E.; Bawendi, M.G.; Frangioni, J.V. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging 2003, 2, 50–64. [Google Scholar]
- Mattoussi, H.; Kuno, M.K.; Goldman, E.R.; George, P.; Mauro, J.M. Colloidal semiconductor quantum dot conjugates in biosensing. In Optical Biosensors: Present and Future; Ligler, F.S., Rowe Taitt, C.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Chapter 17; pp. 537–569. [Google Scholar]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar]
- Kim, S.; Lim, Y.T.; Soltesz, E.G.; Grand, A.M.; Lee, J.; Nakayama, A. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol 2004, 22, 93–97. [Google Scholar]
- Chen, F.Q.; Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett 2004, 4, 1827–1832. [Google Scholar]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. Nanocrystals as sensors. Green Chem 2007, 9, 403–410. [Google Scholar]
- Qian, J.; Yong, K.T.; Roy, I.; Ohulchanskyy, T.Y.; Bergey, E.J.; Lee, H.H.; Tramposch, K.M.; He, S.; Maitra, A.; Prasad, P.N. Imaging pancreatic cancer using surface-functionalized quantum dots. J. Phys. Chem 2007, 111, 6969–6972. [Google Scholar]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev 2008, 60, 1226–1240. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater 2005, 4, 435–446. [Google Scholar]
- Jaiswal, J.K.; Mattoussi, H.; Mauro, J.M.; Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol 2003, 21, 47–51. [Google Scholar]
- Bakalova, R.; Zhelev, Z.; Aoki, I.; Kanno, I. Designing quantum-dots probes. Nat. Photonics 2007, 1, 487–489. [Google Scholar]
- Baron, R.; Willner, B.; Willner, I. Biomolecule-nanoparticle hybrids as functional units for nanobiotechnology. Chem. Commun 2007, 18, 323–332. [Google Scholar]
- Biju, V.; Muraleedharan, D.; Nakayama, K.; Shinohara, Y.; Itoh, T.; Baba, Y.; Ishikawa, M. Quantum dot-insect neuropeptide conjugates for fluorescence imaging, transfection, and nucleus targeting of living cells. Langmuir 2007, 23, 10254–10261. [Google Scholar]
- Juzenas, P.; Chen, W.; Sun, Y.P.; Coelho, M.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapy of cancer. Adv. Drug Deliv. Rev 2008, 60, 1600–1614. [Google Scholar]
- Ochsner, M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B 1997, 39, 1–18. [Google Scholar]
- Oleinick, N.L.; Evans, H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res 1998, 150, 146–156. [Google Scholar]
- Tardivo, J.P.; Giglio, A.D.; Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; Turchiello, R.F.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther 2005, 2, 175–191. [Google Scholar]
- Jiang, Z.; Li, R.; Todd, N.W.; Stass, S.A.; Jiang, F. Detecting genomic aberrations by fluorescence in situ hybridization with quantum dots-labeled probes. J. Nanosci. Nanotechnol 2007, 7, 4254–4259. [Google Scholar]
- Burke, R.S.; Pun, S.H. Extracellular barriers to in vivo PEI and PEGylated PEI polyplex-mediated gene delivery to the liver. Bioconjug. Chem 2008, 19, 693–704. [Google Scholar]
- Delehanty, J.B.; Boeneman, K.; Bradburne, C.E.; Robertson, K.; Medintz, I.L. Quantum dots: A powerful tool for understanding the intricacies of nanoparticle-mediated drug delivery. Expert Opin. Drug Deliv 2009, 6, 1091–1112. [Google Scholar]
- Zhao, Y.; Zhao, L.; Zhou, L.; Zhi, Y.; Xu, J.; Wei, Z.; Zhang, K.X.; Ouellette, B.F.; Shen, H. Quantum dot conjugates for targeted silencing of bcr/abl gene by RNA interference in human myelogenous leukemia K562 cells. J. Nanosci. Nanotechnol 2010, 10, 5137–5143. [Google Scholar]
- Bolhassani, A.; Safaiyan, S.; Rafati, S. Improvement of different vaccine delivery systems for cancer therapy. Mol. Cancer 2011. [Google Scholar] [CrossRef]
- Nabiev, I.; Mitchell, S.; Davies, A.; Williams, Y.; Kelleher, D.; Moore, R.; Gun’ko, Y.K.; Byrne, S.; Rakovich, Y.P.; Donegan, J.F.; Sukhanova, A.; Conroy, J.; Cottell, D.; Gaponik, N.; Rogach, A.; Volkov, Y. General strategy for designing functionalized magnetic microspheres for different bioapplications. Nano Lett 2007, 7, 3452–3461. [Google Scholar]
- Lewin, M.; Carlesso, N.; Tung, C.H.; Tang, X.W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol 2000, 18, 410–414. [Google Scholar]
- Dabbousi, B.O.; Murray, C.B.; Rubner, M.F.; Bawendi, M.G. Langmuir-Blodgett manipulation of size-selected CdSe nanocrystallites. Chem. Mater 1994, 6, 216–219. [Google Scholar]
- Danek, M.; Jensen, K.F.; Murray, C.B.; Bawendi, M.G. Electrospray organometallic chemical vapor deposition-A novel technique for preparation of II–VI quantum dot composites. Appl. Phys. Lett 1994, 65, 2795–2797. [Google Scholar]
- Wu, Z.; Zhao, Y.; Qiu, F.; Li, Y.; Wang, S.; Yang, B.; Chen, L.; Sun, J.; Wang, J. Forming water-soluble CdSe/ZnS QDs using amphiphilic polymers, stearyl methacrylate/methylacrylate copolymers with different hydrophobic moiety ratios and their optical properties and stability. Colloids Surf. A Physicochem. Eng. Asp 2009, 350, 121–129. [Google Scholar]
- Ghasemi, Y.; Peymani, P.; Afifi, S. Quantum dot: Magic nanoparticle for imaging, detection and targeting. Acta Biomed 2009, 80, 156–165. [Google Scholar]
- Lee, C.M.; Jang, D.; Cheong, S.J.; Kim, E.M.; Jeong, M.H.; Kim, S.H.; Kim, D.W.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Surface engineering of quantum dots for in vivo imaging. Nanotechnology 2010. [Google Scholar] [CrossRef]
- Uyeda, H.T.; Medintz, I.L.; Jaiswal, J.K.; Simon, S.M.; Mattoussi, H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. J. Am. Chem. Soc 2005, 127, 3870–3878. [Google Scholar]
- Pons, T.; Uyeda, H.T.; Medintz, I.L.; Mattoussi, H. Hydrodynamic dimensions, electrophoretic mobility, and stability of hydrophilic quantum dots. J. Phys. Chem. B 2006, 110, 20308–20316. [Google Scholar]
- Smith, A.M.; Nie, S. Minimizing the hydrodynamic size of quantum dots with multifunctional multidentate polymer ligands. J. Am. Chem. Soc 2008, 130, 11278–11279. [Google Scholar]
- Kim, S.; Bawendi, M.G. Oligomeric ligands for luminescent and stable nanocrystal quantum dots. J. Am. Chem. Soc 2003, 125, 14652–14653. [Google Scholar]
- Stewart, M.H.; Susumu, K.; Mei, B.C.; Medintz, I.L.; Delehanty, J.B.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Multidentate poly(ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions. J. Am. Chem. Soc 2010, 132, 9804–9813. [Google Scholar]
- Kong, X.L.; Qi, H.; Zhou, H.X.; Ren, L.L.; Deng, C.Y.; Li, F.R. A novel sensitive immunoassay by nucleic acid barcode dot and its application in the detection of prostate-specific antigen. Clin. Chem. Lab. Med 2010, 48, 279–283. [Google Scholar]
- Thangadurai, P.; Balaji, S.; Manoharan, P.T. Surface modification of CdS quantum dots using thiols-structural and photophysical studies. Nanotechnology 2008, 19, 435708:1–435708:8. [Google Scholar]
- Yuan, Z.; Zhang, A.; Cao, Y.; Yang, J.; Zhu, Y.; Yang, P. Effect of mercaptocarboxylic acids on luminescent properties of CdTe quantum dots. J. Fluoresc 2011. [Google Scholar] [CrossRef]
- Petkar, K.C.; Chavhan, S.S.; Agatonovik-Kustrin, S.; Sawant, K.K. Nanostructured materials in drug and gene delivery: A review of the state of the art. Crit. Rev. Ther. Drug Carr. Syst 2011, 28, 101–164. [Google Scholar]
- Zhao, L.; Pang, X.; Adhikary, R.; Petrich, J.W.; Jeffries-El, M.; Lin, Z. Organic-inorganic nanocomposites by placing conjugated polymers in intimate contact with quantum rods. Adv. Mater 2011, 23, 2844–2849. [Google Scholar]
- Song, S.; Qin, Y.; He, Y.; Huang, Q.; Fan, C.; Chen, H.Y. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem. Soc. Rev 2010, 39, 4234–4243. [Google Scholar]
- Zorn, M.; Bae, W.K.; Kwak, J.; Lee, H.; Lee, C.; Zentel, R.; Char, K. Quantum dot-block copolymer hybrids with improved properties and their application to quantum dot light-emitting devices. ACS Nano 2009, 3, 1063–1068. [Google Scholar]
- Goldman, E.R.; Mattoussi, H.; Anderson, G.P.; Medintz, I.L.; Mauro, J.M. Fluoroimmunoassays using antibody-conjugated quantum dots. Methods Mol. Biol 2005, 303, 19–34. [Google Scholar]
- Liu, W.; Howarth, M.; Greytak, A.B.; Zheng, Y.; Nocera, D.G.; Ting, A.Y.; Bawendi, M.G. Compact biocompatible quantum dots functionalized for cellular imaging. J. Am. Chem. Soc 2008, 130, 1274–1284. [Google Scholar]
- Sheng, W.; Kim, S.; Lee, J.; Kim, S.W.; Jensen, K.; Bawendi, M.G. In situ encapsulation of quantum dots into polymer microspheres. Langmuir 2006, 22, 3782–3790. [Google Scholar]
- Bolhassani, A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim. Biophys. Acta 2011, 1816, 232–246. [Google Scholar]
- Wang, J.; Xia, J. Preferential binding of a novel polyhistidine peptide dendrimer ligand on quantum dots probed by capillary electrophoresis. Anal. Chem 2011, 83, 6323–6329. [Google Scholar]
- Li, Y.; Zhou, Y.; Wang, H.Y.; Perrett, S.; Zhao, Y.; Tang, Z.; Nie, G. Chirality of glutathione surface coating affects the cytotoxicity of quantum dots. Angew. Chem. Int. Ed. Engl 2011, 50, 5860–5864. [Google Scholar]
- Iyer, G.; Pinaud, F.; Xu, J.; Ebenstein, Y.; Li, J.; Chang, J.; Dahan, M.; Weiss, S. Aromatic aldehyde and hydrazine activated Peptide coated quantum dots for easy bioconjugation and live cell imaging. Bioconjug. Chem 2011, 22, 1006–1011. [Google Scholar]
- Ranjbarvaziri, S.; Kiani, S.; Akhlaghi, A.; Vosough, A.; Baharvand, H.; Aghdami, N. Quantum dot labeling using positive charged peptides in human hematopoetic and mesenchymal stem cells. Biomaterials 2011, 32, 5195–5205. [Google Scholar]
- Kuo, C.W.; Chueh, D.Y.; Singh, N.; Chien, F.C.; Chen, P. Targeted nuclear delivery using peptide-coated quantum dots. Bioconjug. Chem 2011, 22, 1073–1080. [Google Scholar]
- Sapsford, K.E.; Granek, J.; Deschamps, J.R.; Boeneman, K.; Blanco-Canosa, J.B.; Dawson, P.E.; Susumu, K.; Stewart, M.H.; Medintz, I.L. Monitoring botulinum neurotoxin a activity with peptide-functionalized quantum dot resonance energy transfer sensors. ACS Nano 2011, 5, 2687–2699. [Google Scholar]
- Liu, H.Y.; Gao, X. Engineering monovalent quantum dot-antibody bioconjugates with a hybrid gel system. Bioconjug. Chem 2011, 22, 510–517. [Google Scholar]
- Jin, T.; Tiwari, D.K.; Tanaka, S.; Inouye, Y.; Yoshizawa, K.; Watanabe, T.M. Antibody-protein A conjugated quantum dots for multiplexed imaging of surface receptors in living cells. Mol. Biosyst 2010, 6, 2325–2331. [Google Scholar]
- Das, A.; Sanjayan, G.J.; Kecskés, M.; Yoo, L.; Gao, Z.G.; Jacobson, K.A. Nucleoside conjugates of quantum dots for characterization of G protein-coupled receptors: Strategies for immobilizing A2A adenosine receptor agonists. J. Nanobiotechnol 2010. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Song, Y.; Jiang, W.; Wu, Z.; Wang, Y.A.; Sun, J.; Wang, J. Architecture of stable and water-soluble CdSe/ZnS core-shell dendron nanocrystals via ligand exchange. J. Colloid. Interface Sci 2009, 339, 336–343. [Google Scholar]
- Hwang, S.H.; Moorefield, C.N.; Wang, P.; Jeong, K.U.; Cheng, S.Z.; Kotta, K.K.; Newkome, G.R. Dendron-tethered and templated CdS quantum dots on single-walled carbon nanotubes. J. Am. Chem. Soc 2006, 128, 7505–7509. [Google Scholar]
- Advincula, R.C. Hybrid organic-inorganic nanomaterials based on polythiophene dendronized nanoparticles. Dalton Trans 2006, 23, 2778–2784. [Google Scholar]
- Rogach, A.L.; Nagesha, D.; Ostrander, J.W.; Giersig, M.; Kotov, N.A. “Raisin bun”-type composite spheres of silica and semiconductor nanocrystals. Chem. Mater 2000, 12, 2676–2685. [Google Scholar]
- Samia, A.C.; Dayal, S.; Burda, C. Quantum dot-based energy transfer: Perspectives and potential for applications in photodynamic therapy. Photochem. Photobiol 2006, 82, 617–625. [Google Scholar]
- Fischer, H.; Liu, L.; Pang, K.S.; Chan, W. Pharmacokinetics of nanoscale quantum dots: In vivo distribution, sequestration, and clearance in the rat. Adv. Funct. Mater 2006, 16, 1299–1305. [Google Scholar]
- Robert, F.S. Nanotechnology takes aim at cancer. Science 2005, 310, 1132–1134. [Google Scholar]
- Delehanty, J.B.; Mattoussi, H.; Medintz, I.L. Delivering quantum dots into cells: Strategies, progress and remaining issues. Anal. Bioanal. Chem 2009, 393, 1091–1105. [Google Scholar]
- Gao, X.H.; Cui, Y.Y.; Levenson, R.M.; Chung, L.W.; Nie, S.M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol 2004, 22, 969–976. [Google Scholar]
- Liu, J.; Stephen, K.L.; Vijay, A.V. Molecular mapping of tumor heterogeneity on clinical tissue specimens with multiplexed quantum dots. ACS Nano 2010, 4, 2755–2765. [Google Scholar]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicohemical and environmental factors. Environ. Health Perspect 2006, 114, 165–172. [Google Scholar]
- Ciofani, G.; Raffa, V.; Menciass, A.; Cuschieri, A. Cytocompatibility, interactions, and uptake of polyethyleneimine-coated boron nitride nanotubes by living cells: Confirmation of their potential for biomedical applications. Biotechnol. Bioeng 2008, 101, 850–858. [Google Scholar]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Intracellular delivery of quantum dots for live cell labeling of organelle tracking. Adv. Mater 2004, 16, 961–966. [Google Scholar]
- Chen, H.; Wang, L.; Yeh, J.; Wu, X.; Cao, Z.; Wang, Y.A.; Zhang, M.; Yang, L.; Mao, H. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PgammaMPS copolymer coating. Biomaterials 2010, 31, 5397–5407. [Google Scholar]
- Pellegrino, T.; Parak, W.J.; Boudreau, R.; le Gros, M.A.; Gerion, D.; Alivisatos, A.P.; Larabell, C.A. Quantum dot-based cell motility assay. Differentiation 2003, 71, 542–548. [Google Scholar]
- Parak, W.J.; Boudreau, R.; le Gros, M.; Gerion, D.; Zanchet, D.; Micheel, C.M.; Williams, S.C.; Alivisatos, A.P.; Larabell, C. Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Adv. Mater 2002, 14, 882–885. [Google Scholar]
- Thein, M.; Cheng, A.; Khanna, P.; Zhang, C.; Park, E.J.; Ahmed, D.; Goodrich, C.J.; Asphahani, F.; Wu, F.; Smith, N.B.; Dong, C.; Jiang, X.; Zhang, M.; Xu, J. Site-specific sonoporation of human melanoma cells at the cellular level using high lateral-resolution ultrasonic micro-transducer arrays. Biosens. Bioelectron 2011, 27, 25–33. [Google Scholar]
- Susumu, K.; Uyeda, H.T.; Medintz, I.L.; Pons, T.; Delehanty, J.B.; Mattoussi, H. Design of biotin-functionalized luminescent quantum dots. J. Am. Chem. Soc 2007, 129, 13987–13996. [Google Scholar]
- Delehanty, J.B.; Medintz, I.L.; Pons, T.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjug. Chem 2006, 17, 920–927. [Google Scholar]
- Rozenzhak, S.M.; Kadakia, M.P.; Caserta, T.M.; Westbrook, T.R.; Stone, M.O.; Naik, R.R. Cellular internalization and targeting of semiconductor quantum dots. Chem. Commun 2005, 17, 2217–2219. [Google Scholar]
- Lieleg, O.; Lopez-Garcia, M.; Semmrich, C.; Auernheimer, J.; Kessler, H.; Bausch, A.R. Specific integrin labeling in living cells using functionalized nanocrystals. Small 2007, 3, 1560–1565. [Google Scholar]
- Smith, B.R.; Cheng, Z.; De, A.; Koh, A.L.; Sinclair, R.; Gambhir, S.S. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett 2008, 8, 2599–2606. [Google Scholar]
- Pan, Y.L.; Cai, J.Y.; Qin, L.; Wang, H. Atomic force microscopy-based cell nanostructure for ligand-conjugated quantum dot endocytosis. Acta Biochim. Biophys. Sin 2006, 38, 646–652. [Google Scholar]
- Yong, K.T.; Qian, J.; Roy, I.; Lee, H.H.; Bergey, E.J.; Tramposch, K.M.; He, S.; Swihart, M.T.; Maitra, A.; Prasad, P.N. Quantum rod bioconjugates as targeted probes for confocal and two-photon fluorescence imaging of cancer cells. Nano Lett 2007, 7, 761–765. [Google Scholar]
- Zhang, H.; Sachdev, D.; Wang, C.; Hubel, A.; Gaillard-Kelly, M.; Yee, D. Detection and downregulation of type I IGF receptor expression by antibody-conjugated quantum dots in breast cancer cells. Breast Cancer Res. Treat 2009, 114, 277–285. [Google Scholar]
- Lidke, D.S.; Nagy, P.; Heintzmann, R.; Arndt-Jovin, D.J.; Post, J.N.; Grecco, H.E.; Jares-Erijman, E.A.; Jovin, T.M. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat. Biotechnol 2004, 22, 198–203. [Google Scholar]
- Liu, W.; Howarth, M.; Greytak, A.B.; Zheng, Y.; Nocera, D.G.; Ting, A.Y.; Bawendi, M.G. Compact biocompatible quantum dots functionalized for cellular imaging. J. Am. Chem. Soc 2008, 130, 1274–1284. [Google Scholar]
- Diagaradjane, P.; Orenstein-Cardona, J.M.; Colón-Casasnovas, N.E.; Deorukhkar, A.; Shentu, S.; Kuno, N.; Schwartz, D.L.; Gelovani, J.G.; Krishnan, S. Imaging epidermal growth factor receptor expression in vivo: Pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobe. Clin. Cancer Res 2008, 14, 731–741. [Google Scholar]
- Dudu, V.; Rotari, V.; Vazquez, M. Targeted extracellular nanoparticles enable intracellular detection of activated epidermal growth factor receptor in living brain cancer cells. Nanomedicine 2011, 7, 896–903. [Google Scholar]
- Chakraborty, S.K.; Fitzpatrick, J.A.; Phillippi, J.A.; Andreko, S.; Waggoner, A.S.; Bruchez, M.P.; Ballou, B. Cholera toxin B conjugated quantum dots for live cell labeling. Nano Lett 2007, 7, 2618–2626. [Google Scholar]
- Jaiswal, J.K.; Goldman, E.R.; Mattoussi, H.; Simon, S.M. Use of quantum dots for live cell imaging. Nat. Methods 2004, 1, 73–78. [Google Scholar]
- Rajan, S.S.; Liu, H.Y.; Vu, T.Q. Ligand-bound quantum dot probes for studying the molecular scale dynamics of receptor endocytic trafficking in live cells. ACS Nano 2008, 2, 1153–1166. [Google Scholar]
- Rajan, S.S.; Vu, T.Q. Quantum dots monitor TrkA receptor dynamics in the interior of neural PC12 cells. Nano Lett 2006, 6, 2049–2059. [Google Scholar]
- Schroeder, J.E.; Shweky, I.; Shmeeda, H.; Banin, U.; Gabizon, A. Folate-mediated tumor cell uptake of quantum dots entrapped in lipid nanoparticles. J. Control. Release 2007, 124, 28–34. [Google Scholar]
- Dudu, V.; Ramcharan, M.; Gilchrist, M.L.; Holland, E.C.; Vazquez, M. Liposome delivery of quantum dots to the cytosol of live cells. J. Nanosci. Nanotechnol 2008, 8, 2293–2300. [Google Scholar]
- Voura, E.B.; Jaiswal, J.K.; Mattoussi, H.; Simon, S.M. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat. Med 2004, 10, 993–998. [Google Scholar]
- Duan, H.; Nie, S. Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. J. Am. Chem. Soc 2007, 129, 3333–3338. [Google Scholar]
- Fuente, J.M.; Fandel, M.; Berry, C.C.; Riehle, M.; Cronin, L.; Aitchison, G.; Curtis, A.S. Quantum dots protected with tiopronin: A new fluorescence system for cell-biology studies. ChemBioChem 2005, 6, 989–991. [Google Scholar]
- Farias, PM.; Santos, B.S.; Menezes, F.D. Core-shell CdS/Cd(OH)2 quantum dots: Synthesis and bioconjugation to target red cells antigens. J. Microsc 2005, 219, 103–108. [Google Scholar]
- Coulon, J.; Thouvenin, I.; Aldeek, F.; Balan, L.; Schneider, R. Glycosylated quantum dots for the selective labelling of Kluyveromyces bulgaricus and Saccharomyces cerevisiae yeast strains. J. Fluoresc 2010, 20, 591–597. [Google Scholar]
- Bharali, D.J.; Lucey, D.W.; Jayakumar, H.; Pudavar, H.E.; Prasad, P.N. Folate-receptor-mediated delivery of InP quantum dots for bioimaging using confocal and two-photon microscopy. J. Am. Chem. Soc 2005, 127, 11364–11371. [Google Scholar]
- Clarke, S.; Nadeau, J.; Bahcheli, D.; Zhang, Z.; Hollmann, C. Quantum dots as phototoxic drugs and sensors of specific metabolic processes in living cells. Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society, Shanghai, China, 17–18 January 2005; Volume 1. pp. 504–507.
- Xue, X.; Pan, J.; Xie, H.; Wang, J.; Zhang, S. Fluorescence detection of total count of Escherichia coli and Staphylococcus aureus on water-soluble CdSe quantum dots coupled with bacteria. Talanta 2009, 77, 1808–1813. [Google Scholar]
- Clarke, S.J.; Hollmann, C.A.; Zhang, Z.J.; Suffern, D.; Bradforth, S.E.; Dimitrijevic, N.M.; Minarik, W.G.; Nadeau, J.L. Photophysics of dopamine-modified quantum dots and effects on biological systems. Nat. Mater 2006, 5, 409–417. [Google Scholar]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar]
- Medintz, I.L.; Pons, T.; Delehanty, J.B.; Susumu, K.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H. Intracellular delivery of quantum dot-protein cargos mediated by cell penetrating peptides. Bioconjug. Chem 2008, 19, 1785–1795. [Google Scholar]
- Cai, W.B.; Shin, D.W.; Chen, K.; Gheysens, O.; Cao, Q.Z.; Wang, S.X. Peptide-labeled nearinfrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett 2006, 6, 669–676. [Google Scholar]
- Yu, X.; Chen, L.; Li, K.; Li, Y.; Xiao, S.; Luo, X. Immunofluorescence detection with quantum dot bioconjugates for hepatoma in vivo. J. Biomed. Opt 2007, 12, 1–5. [Google Scholar]
- Weng, K.C.; Noble, C.O.; Papahadjopoulos-Sterberg, B.; Chen, F.F.; Drummond, D.C.; Kirpotin, D.B. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett 2008, 8, 2851–2857. [Google Scholar]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev 2010, 62, 1052–1063. [Google Scholar]
- Matsuno, A.; Mizutani, A.; Okinaga, H.; Takano, K.; Yamada, S.; Yamada, S.M.; Nakaguchi, H.; Hoya, K.; Murakami, M.; Takeuchi, M.; Sugaya, M.; Itoh, J.; Takekoshi, S.; Osamura, R.Y. Molecular morphology of pituitary cells, from conventional immunohistochemistry to fluorescein imaging. Molecules 2011, 16, 3618–3635. [Google Scholar]
- Byers, R.J.; Hitchman, E.R. Quantum dots brighten biological imaging. Prog. Histochem. Cytochem 2011, 45, 201–237. [Google Scholar]
- Isse, K.; Grama, K.; Abbott, I.M.; Lesniak, A.; Lunz, J.G.; Lee, W.M.; Specht, S.; Corbitt, N.; Mizuguchi, Y.; Roysam, B.; Demetris, A.J. Adding value to liver (and allograft) biopsy evaluation using a combination of multiplex quantum dot immunostaining, high-resolution whole-slide digital imaging, and automated image analysis. Clin. Liver Dis 2010, 14, 669–685. [Google Scholar]
- Tholouli, E.; Sweeney, E.; Barrow, E.; Clay, V.; Hoyland, J.A.; Byers, R.J. Quantum dots light up pathology. J. Pathol 2008, 216, 275–285. [Google Scholar]
- Smith, A.M.; Nie, S. Chemical analysis and cellular imaging with quantum dots. Analyst 2004, 129, 672–677. [Google Scholar]
- Yaghini, E.; Seifalian, A.M.; MacRobert, A.J. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine 2009, 4, 353–363. [Google Scholar]
- Rakovich, A.; Savateeva, D.; Rakovich, T.; Donegan, J.F.; Rakovich, Y.P.; Kelly, V.; Lesnyak, V.; Eychmüller, A. CdTe quantum dot/dye hybrid system as photosensitizer for photodynamic therapy. Nanoscale Res. Lett 2010, 5, 753–760. [Google Scholar]


| Strategy | Mechanism | Examples | Targeted Cells | References |
|---|---|---|---|---|
| Passive uptake | Electrostatic interactions | - | HeLa | [15] |
| Human macrophages | [27,72] | |||
| Breast cancer (MDA-MB-231) | [73,74] | |||
| Human melanoma cells (LU1205) | [75] | |||
| Facilitated delivery | Peptide-mediated | TAT | Human embryonic kidney | [76] |
| HeLa | [77] | |||
| Mesenchymal stem cells | [53] | |||
| Jurkat cells | [28] | |||
| Pep-1 (Chariot) | Osteoblast | [78] | ||
| Vascular endothelial cells | [78] | |||
| RGD motify | Fibroblast (NIH 3T3) | [79] | ||
| Epidermoid carcinoma | [80] | |||
| Protein-mediated | Neuropeptide | HeLa | [17] | |
| Transferrin | Human pancreatic cancer | [2,81,82] | ||
| Antibody | Breast cancer (MCF-7) | [11] | ||
| EGF | Mesenchymal stem cells | [83] | ||
| Chinese hamster ovary | [84–86] | |||
| Medulloblastoma tumors | [87] | |||
| Glioma tumors | [87] | |||
| Cholera toxin B | Fibroblast | [88,89] | ||
| NGF | PC12 neural cells | [90,91] | ||
| Polymer/lipid-mediated | Lipid polymers | Mouse lymphoma | [92] | |
| HeLa | [93] | |||
| A549 epithelial lung HeLa | [94] | |||
| Polyethyleneimine | HeLa | [95] | ||
| Drug-mediated | Tiopronin | Fibroblast | [96] | |
| Small molecule | Glucose/sugar | S. cerevisiae (Baker’s yeast) | [97,98] | |
| Folate | Epidermal carcinoma | [99] | ||
| Adenine/AMP | Bacteria (Bacillus subtilis, E. coli) | [100,101] | ||
| Dopamin | A9 mouse fibroblast with transfected dopamine receptor | [102] | ||
| Active Delivery | Electroporation | - | HeLa | [71] |
| Mouse neural stem progenitor cells | [9] | |||
| Microinjection | - | [71] | ||
| Xenopus embryo | [103] | |||
| HeLa | [104] | |||
| Human embryonic kidney | ||||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shao, L.; Gao, Y.; Yan, F. Semiconductor Quantum Dots for Biomedicial Applications. Sensors 2011, 11, 11736-11751. https://doi.org/10.3390/s111211736
Shao L, Gao Y, Yan F. Semiconductor Quantum Dots for Biomedicial Applications. Sensors. 2011; 11(12):11736-11751. https://doi.org/10.3390/s111211736
Chicago/Turabian StyleShao, Lijia, Yanfang Gao, and Feng Yan. 2011. "Semiconductor Quantum Dots for Biomedicial Applications" Sensors 11, no. 12: 11736-11751. https://doi.org/10.3390/s111211736
APA StyleShao, L., Gao, Y., & Yan, F. (2011). Semiconductor Quantum Dots for Biomedicial Applications. Sensors, 11(12), 11736-11751. https://doi.org/10.3390/s111211736




