Microfabricated Reference Electrodes and their Biosensing Applications
Abstract
:1. Introduction
- The reference electrodes must have high exchange current densities and thus be non-polarizeable (hard to change the electrode’s potential).
- The potentials of all of the electrodes must be reproducible.
- The potential drift of the electrodes, from factors attributed to filling solution effusion or degradation of electrode coating, must be minimized over the duration of device operation.
- Other operational requirements needed for the working electrodes might be imposed based on the nature of the experiment. For example, invasive recording and stimulating electrodes should not introduce foreign toxic ions into humans, which necessitate the use of highly polarizeable electrodes.
2. Basic Theory
2.1. Electrode Potential
2.2. Current-Voltage Relationship
- j is the current density,
- η=V−Veq is the “overpotential”, defined as the difference between the applied potential and the built-in potential Veq of the electrode,
- α is the symmetry factor, which is a linearization coefficient for the effect of the overpotential on the energetics of the reaction,
- k is Boltzmann’s constant,
- q is the elementary charge, and
- j0 is the exchange current density.
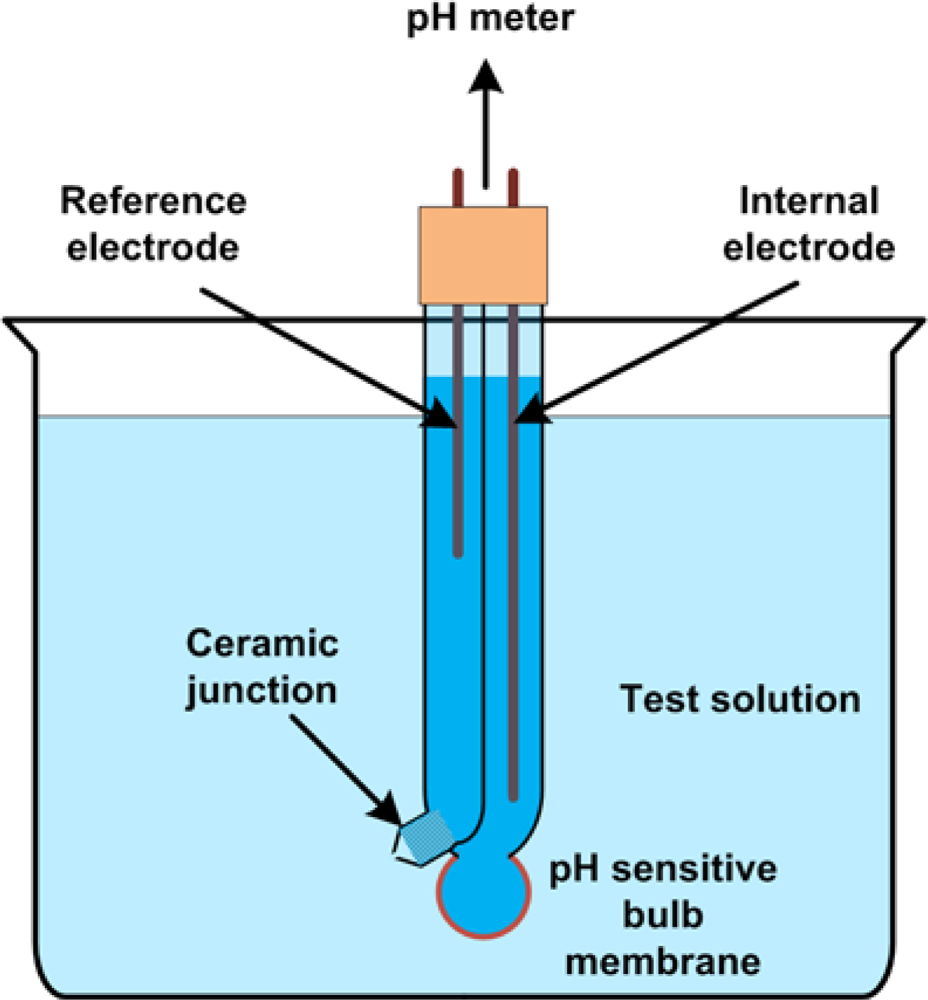
2.3. Reference Electrodes
- The reference electrode must have a high exchange current density, must be reversible and non-polarizeable. These properties will allow exchange of charge between the electrode-electrolyte interface due to electrochemical reactions and other environmental factors without significantly changing the electrode potential. It will also guard against fluctuations caused by random charge injection into the interface. Figure 2 shows typical current-voltage profiles, indicating the range of good reference electrodes.
- The electrode reaction should not consume the electrode or change its surface area in any way, so as not to change the reaction area and hence the current.
- The solution in contact with the reference electrode should be saturated. There are many reasons for having a saturated inner filling solution and separating it from the external test solution. First, evaporation of the solvent will not affect the concentration of the solution and hence the electrode potential. Second, a high concentration of reactants allows for higher and stable exchange current density, which allows the reference electrode to maintain its potential when current is flowing through it. Third, any minute fluctuation in the concentration of a saturated solution will not change the electrochemical potential (and hence, the electrode potential) greatly because of the logarithmic dependence of the chemical potential on the concentration. Finally, the larger portion of the electrode potential occurs due to the large net dipole at the metal-electrolyte interface. Since the composition of the test solution is application-dependent, it would be desirable to have the reference electrode potential as independent as possible from the test solution. Liquid junction potentials are very small and will not change much by changing the test solution. However, if the test solution were in direct contact with the electrode, then the surface potential could vary greatly.
- The interface between the test liquid and the saturated solution must be designed to minimize any form of rapid convective mixing. Such rapid mixing contaminates the reference electrode’s environment and the test solution, possibly affecting the chemical and biological reactions of interest in the test solution and definitely changing the reference potential.
- The liquid junction potential should be the minimum possible, and should be constant with time. This is generally done by ensuring that the solvent is the same to avoid net dipole moments at this surface. Detailed liquid junction analysis has been cited extensively in literature [16,17]. Generally, electrochemical equilibrium is reached rapidly while the effects of convective mixing are hindered by the porous membrane [6]. However, if other species are allowed to interact and pass through this membrane, they can take place in the equilibrium process and the final potential can be disturbed. Equilibrium might never be reached and this process can assist in rapid leakage of the reference electrode solution.
- The reaction should be simple enough that the exchange current density law (Equation 2) is valid. Many electrode reactions are not modeled by the Butler-Volmer equation and require many different steps for the reaction to occur. For the electrode reactions in which intermediate processes do not occur, there might be a need for the adsorption of reactant ions onto the electrode’s surface, and the energetics of the reaction become complicated. In such reactions, the equivalent exchange current density becomes quite small, possibly needing a very long time to reach equilibrium and hence are not reversible.The junction should be free of other possible contaminating species that can affect the reference electrode potential by changing the chemical potential of the reactants, or worse, by participating in the electrochemical reaction and making it impossible to attain equilibrium.
- The characteristics of the reference electrode must be reproducible. Many different electrodes might have excellent stability and low drift. However, due to the limited control over the fabrication procedure, they might end up having different potentials.
3. Common Reference Electrode Types
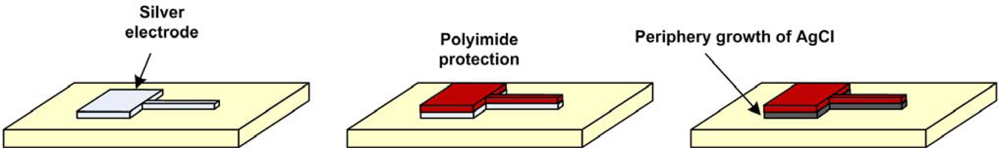
3.1. Ag/AgCl Electrode
3.2. Calomel Electrode
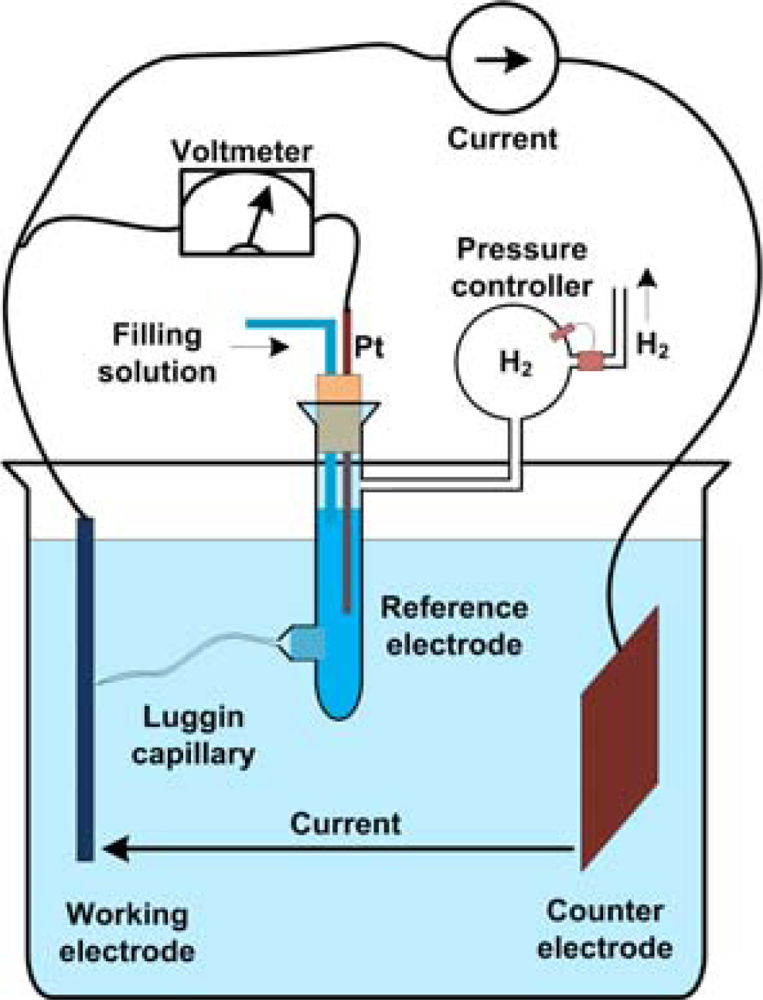
3.3. Hydrogen Electrode
3.4. Other Pseudo-Reference Electrodes
4. Miniaturization and Fabrication Techniques
- This film deposition: Evaporation, sputter deposition or chemical vapor deposition (CVD) of the electrode material (e.g., Ag) onto a lithographically patterned metallic lead.
- Electroplating: The substrate is electrochemically treated in a bath containing cations of the required metal. Electroplating allows for higher thicknesses of the electrode material [34].
- Screen printing: This involves pressing the electrode metal “ink” onto a surface using a blocking meshed stencil. This technique can produce the highest thickness of electrode material [35], but is normally done as a manual step and not integrated with automated processing techniques.
- Direct mixture deposition: As in the case of screen printing Ag/AgCl, a compound paste is deposited directly onto the metallic surface. However, the adhesive properties of this layer to the supporting electrode might not be very good, and reliability of the electrode will depend on the homogeneity of the paste used.
- Ion exchange reaction: A spontaneous chemical reaction can form the needed film. In the case of Ag/AgCl, bathing the electrode in a solution of FeCl3 is a known method to form the silver chloride layer.
- Electrochemical coating: An electrochemically induced reaction allows controlled surface chemistry and a reportedly more Nernstian behavior [34], albeit slower than ion-exchange reactions. The AgCl coatings can be electrochemically made by anodization of silver in a HCl bath. Platinization of platinum electrodes is also done using an electrochemical coating reaction.
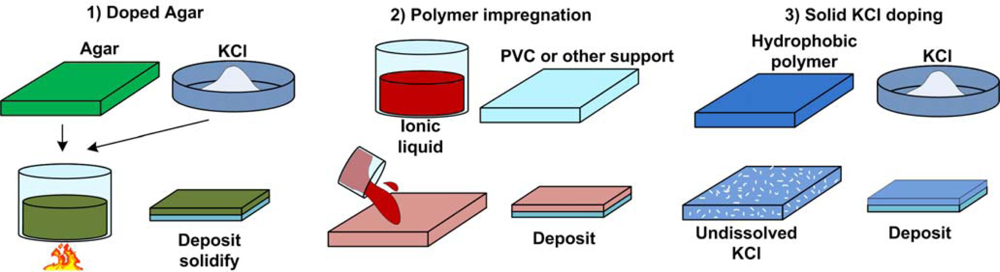
- Porous materials: Introduction of naturally porous materials, such as agarose gel, between the reference and test solutions.
- Gels: Use of synthetic gels, such as polyacrylamide gel.
- Porous glass: This is most used in conventional reference electrodes.
- Heterogeneous designs: Specially engineered multilayer structures can provide different reaction chemistries at the two different solution interfaces, providing selective passage of potential-determining ions.
- Nano-porous membranes: Nano-channels are made on polymer membranes using excimer laser ablation. The size of the nanopores can be adjusted to achieve the required junction resistance. Such membranes were used for conventional reference electrodes [36] and are seen as good candidates for the microfabrication of true reference electrodes.
4.1. Micro Ag/AgCl Electrodes in Capillaries
4.2. Microfabricated Solid-State Ag/AgCl
4.3. Microfabricated Ag/AgCl with Filling Solution
4.4. Microfabricated Ag/AgCl without Filling Solution
4.5. Miniaturized Mercury and Calomel Electrodes
4.6. Miniaturized Hydrogen Electrodes
4.7. IrOx
- First, current-voltage relationships (Tafel plots) of the electrodes are required to deduce the exchange current densiy, as its value differs with device parameters. A poorly designed Ag/AgCl reference electrode might not have its nominal exchange current density, and this is critical when discussing the dynamics of the electrode.
- Second, the internal filling solution is very often disregarded and the fabricated electrode is used as a quasi-electrode. This makes the reference potential very sensitive to the conducting ion activity, which can vary in the test solution. Unless the test conditions are extremely well controlled, the reference electrode’s potential will vary.
- Third, only a few publications document the effect of adding different species on the electrode’s potential. Most of the literature focus primarily on pH dependence. Potential contaminant species for the sensor in question must be identified and their effects on the reference electrode potential studied.
- Fourth, the choices for the test solutions and filling solutions vary, making the comparison of the reference electrodes’ performances complicated. A standard reference solution should be incorporated for each electrode type. This solution might be chosen to be that of highest relevance to the application using the electrode of interest.
5. Applications of Reference Electrodes in Chemical and Biomedical Sensing
5.1. pH Sensing
5.2. Glucose Sensors
5.3. Gas Sensors
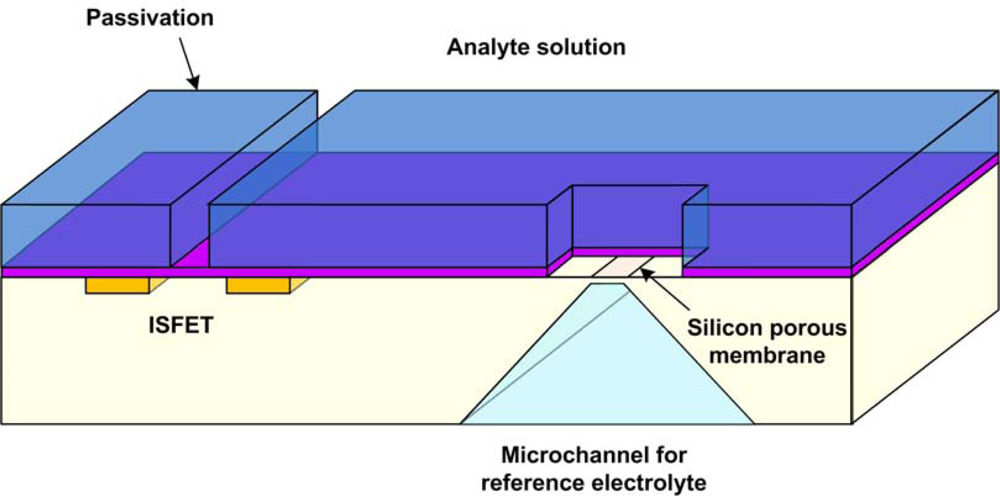
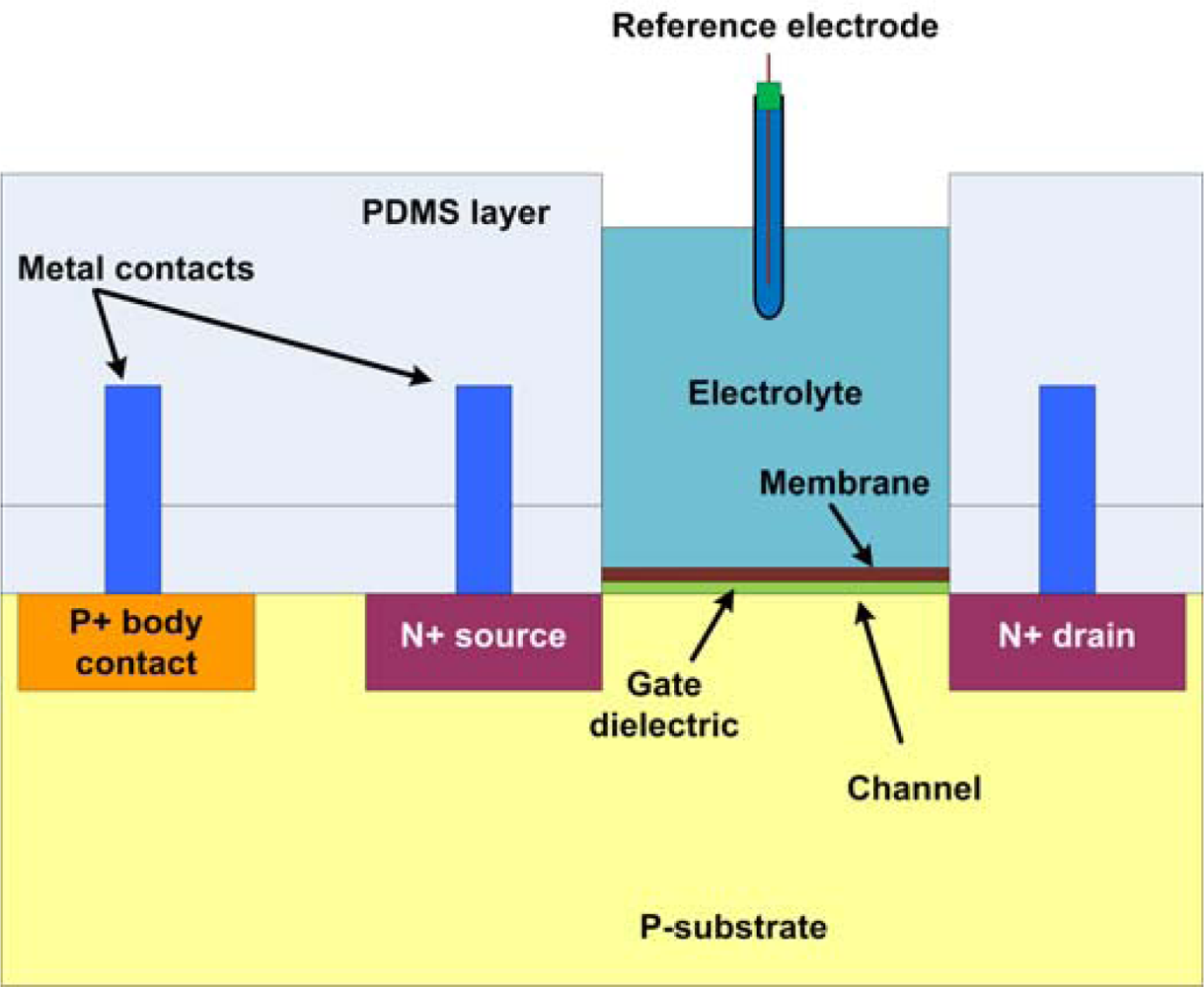
5.4. FET Based Sensors
5.5. Other Types of Sensors
6. Conclusions and Future Perspectives
Acknowledgments
References and Notes
- Suzuki, H. Advances in microfabrication of electrochemical sensors and systems. Electroanalysis 2000, 12, 703–715. [Google Scholar]
- Ravindra, N.; Prodan, M.C.; Fnu, S.; Padron, I.; Sikha, S.K. Advances in the manufacturing, types, and applications of biosensors. JOM 2007, 59, 37–43. [Google Scholar]
- Suzuki, H. Microfabrication of chemical sensors and biosensors for environmental monitoring. Mater. Sci. Eng. C 2000, 12, 55–61. [Google Scholar]
- Koudelka-Hep, M.; van der Wal, P. Microelectrode sensors for biomedical and environmental applications. Electrochim. Acta 2000, 45, 2437–2441. [Google Scholar]
- Brezinski, D.P. Kinetic, static and stirring errors of liquid junction reference electrodes. The Analyst 1983, 108, 425–442. [Google Scholar]
- Sharma, M. Electrodes and Electrolytes. Lecture notes, The University of Sydney, Available online: http://www.physics.usyd.edu.au/super/life_sciences/electricity.html/ (Accessed on September 8, 2009).
- Sevilla, F., III; Kullick, T.; Scheper, T. A bio-FET sensor for lactose based on co-immobilized β-galactosidase/glucose dehydrogenase. Biosens. Bioelectron 1994, 9, 275–281. [Google Scholar]
- Shinwari, M.W.; Deen, M.J.; Landheer, D. Study of the electrolyte-insulator-semiconductor field-effect transistor (EISFET) with applications in biosensoe design. Microelectron. Reliab 2007, 47, 2025–2057. [Google Scholar]
- Benjamin, H.; Bhansali, S.; Hoath, S.B.; Pickens, W.L.; Smallwood, R. A planar micro-sensor for bio-impedance measurements. Sens. Actuat. B 2005, 111–112, 430–435. [Google Scholar]
- Duan, Y.Y.; Millard, R.E.; Tykocinski, M.; Lui, X.; Clark, G.M.; Cowan, R. Potential applications of a small and high surface area platinum electrode as an implanted impedance bio-sensor or recording electrode. Proc. SPIE 2001, 4235, 145–152. [Google Scholar]
- Milazzo, G.; Caroli, S.; Sharma, V.K. Tables of Standard Electrode Potentials; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Bott, A.W. Mass Transport. Curr. Sep 1996, 14, 104–109. [Google Scholar]
- Bockris, J.; Reddy, A.; Gamboa-Aldeco, M. Modern Electrochemistry 2A: Fundamentals of Electrodics; Kluwer Academic: New York, NY, USA, 2000. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Jayalakshmi, V.; Ramaswamy, R. Influence of working electrodes in Belousov-Zhabotinsky oscillatory system. Can. J. Chem 1997, 75, 547–558. [Google Scholar]
- Dorta-Rodríguez, R.; Barrera-Niebla, M.; González, S.; Hernández-Luis, F. Calculation of liquid junction potentials. J. Electroanal. Chem 1997, 436, 173–188. [Google Scholar]
- Bass, L. Potential of liquid junctions. Trans. Faraday Soc 1964, 60, 1914–1919. [Google Scholar]
- Elzanowska, H.; Birss, V. I. Reversible ageing of iridium oxide electrodes in acidic solutions. J. Appl. Electrochem 1993, 23, 646–654. [Google Scholar]
- Yu, P.; Dong, S. The fabrication and performance of an Ag/AgCl reference electrode in human serum. Anal. Chim. Acta 1996, 330, 167–174. [Google Scholar]
- Shibata, M.; Siegfried, B.; Huston, J.P. Miniature calomel electrode for recording DC potential changes accompanying spreading depression in the freely moving rat. Physiol. Behav 1977, 18, 1171–1174. [Google Scholar]
- Bockris, J.O’M.; Devanathan, M.A.V.; Reddy, A.K.N. The anodic formation of calomel films on mercury electrodes-an ellipsometric-galvanostatic study. Proc. R. Soc. Lond. A 1964, 279, 327–345. [Google Scholar]
- Lill, K.A.; Hassel, A.W. A combined μ-mercury reference electrode/Au counter-electrode system for microelectrochemical applications. J. Solid State Electrochem 2006, 10, 941–946. [Google Scholar]
- Dohner, R.E.; Wegmann, D.; Morf, W.E.; Simon, W. Reference electrode with free-flowing free-diffusion liquid junction. Anal. Chem 1986, 58, 2585–2589. [Google Scholar]
- Reiss, H.; Heller, A. The absolute potential of the standard hydrogen electrode: a new estimate. J. Phys. Chem 1985, 89, 4207–4213. [Google Scholar]
- Kita, H. Periodic variation of exchange current density of hydrogen electrode reaction with atomic number and reaction mechanism. J. Electrochem. Soc 1966, 113, 1095–1111. [Google Scholar]
- Kita, H. Periodic variation of exchange current density of hydrogen electrode reaction with atomic number and reaction mechanism. Trans. Symp. Elec. Proc. 1966, 113, 79–90. [Google Scholar]
- Appelby, A.J.; Chemla, M.; Kita, H.; Bronoel, G. Encyclopedia of the Electrochemistry of Elements; Marcel Dekker: New York, NY, USA, 1982; Volume IX, p. 383. [Google Scholar]
- Backholm, J. Electrochromic properties of iridium oxide based thin films; Doctoral Thesis; Uppsala University: Uppsala, Sweden, April 2008. [Google Scholar]
- Franklin, R.K.; Johnson, M.D.; Scottt, K.A.; Shim, J.H.; Nam, H.; Kipket, D.R.; Brown, R.B. Iridium oxide reference electrodes for neurochemical sensing with MEMS microelectrode arrays. IEEE Sensors 2005, 1400–1403. [Google Scholar] [CrossRef]
- Yang, H.; Kang, S.K.; Choi, C.A.; Kim, H.; Shin, D.H.; Kim, Y.S.; Kim, Y.T. An iridium oxide reference electrode for use in microfabricated biosensors and biochips. Lab Chip 2004, 4, 42–46. [Google Scholar]
- Wang, M.; Yao, S.; Madou, M. A long-term stable iridium oxide pH electrode. Sens. Actuat. B 2002, 81, 313–315. [Google Scholar]
- Goffe, R.A.; Tseung, A.C. Internally charged palladium hydride reference electrode-Part 1 : The effect of charging current density on long-term stability. Med. & Biol. Eng. & Comput 1978, 16, 670–676. [Google Scholar]
- Imokawa, T.; Williams, K-J.; Denuault, G. Fabrication and Characterization of Nanostructured Pd Hydride pH Microelectrodes. Anal. Chem 2006, 78, 265–271. [Google Scholar]
- Polk, B.J.; Stelzenmuller, A.; Mijares, G.; MacCrehan, W.; Gaitan, M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sens. Actuat. B 2006, 114, 239–247. [Google Scholar]
- Simonis, A.; Dawgul, M.; Lüth, H.; Schöning, M.J. Miniaturised reference electrodes for field-effect sensors compatible to silicon chip technology. Electrochim. Acta 2005, 51, 930–937. [Google Scholar]
- Broadley, S.T.; Ragsdale, S.R.; Silverman, H.P. Reference electrode having a microfluidic flowing liquid junction. US Patent 6616821. 2003. [Google Scholar]
- Boodts, J.C.F.; Trasatti, S. Hydrogen evolution on iridium oxide cathodes. J. Appl. Electrochem 1989, 19, 255–262. [Google Scholar]
- McGill, I.R.; McEnanay, B. A novel reference electrode arrangement for high temperature polarisation studies. Corros. Sci 1978, 18, 257–259. [Google Scholar]
- Garbett, K.; Torrance, K. Capillary tip reference electrode based on a glass syringe [for metal surface corrosion measurement]. Lab. Pract. 1975, 24, 248. [Google Scholar]
- Savinell, R.F.; Liu, C.C.; Kowalsky, T.E.; Puschett, J.B. Miniature Glass pH Electrode with Nonaqueous Internal Reference Solution. Anal. Chem 1981, 53, 552–554. [Google Scholar]
- Kitade, T.; KITAMURA, K.; Takegami, S.; Miyata, Y.; Nagatomo, M.; Sakaguchi, T.; Furukawa, M. Needle-type ultra micro silver/silver chloride reference electrode for use in micro-electrochemistry. Anal. Sci 2005, 21, 907–912. [Google Scholar]
- Zhang, X.; Ogorevc, B.; Tavčar, G.; Švegl, I.G. Over-oxidized polypyrrole-modified carbon fibre ultramicroelectrode with an integrated silver/silver chloride reference electrode for the selective voltammetric measurement of dopamine in extremely small sample volumes. Analyst 1996, 121, 1817–1822. [Google Scholar]
- Pedrotti, J.J.; Angnes, L.; Gutz, I.G.R. Miniaturized reference electrodes with microporous polymer junctions. Electroanalysis 1996, 8, 673–675. [Google Scholar]
- Czaban, J.D.; Rechnitz, G.A. Glass Microelectrode Probes for Routine pH Measurements. Anal. Chem 1976, 48, 277–281. [Google Scholar]
- Jin, X.; Lu, J.; Liu, P.; Tong, H. The electrochemical formation and reduction of a thick AgCl deposition layer on a silver substrate. J. Electroanal. Chem 2003, 542, 85–96. [Google Scholar]
- Jović, B.M.; Jović, V.D.; Dražić, D.M. Kinetics of chloride ion adsorption and the mechanism of AgCl layer formation on the (111), (100) and (110) faces of silver. J. Electroanal. Chem 1995, 399, 197–206. [Google Scholar]
- Eine, K.; Kjelstrup, S.; Nagy, K.; Syverud, K. Towards a solid state reference electrode. Sens. Actuat. B 1997, 44, 381–388. [Google Scholar]
- Huang, I-Y; Huang, R-S; Lo, L-H. Fabrication and characterization of a new planar solid-state reference electrode for ISFET sensors. Thin Solid Films 2002, 406, 255–261. [Google Scholar]
- Liao, W-Y; Chou, T-C. Fabrication of a planar-form screen printed solid electrolyte modified Ag/AgCl reference electrode for application in a potentiometric biosensor. Anal. Chem 2006, 78, 4219–4223. [Google Scholar]
- Mamińska, R.; Dybko, A.; Wróblewski, W. All-solid-state miniaturised planar reference electrodes based on ionic liquids. Sens. Actuat. B 2006, 115, 552–557. [Google Scholar]
- Zielińska, R.; Mulik, E.; Michalska, A.; Achmatowicz, S.; Maj-Żurawska, M. All-solid-state planar miniature ion-selective chloride electrode. Anal. Chim. Acta 2002, 451, 243–249. [Google Scholar]
- Yun, K-S; Kim, H-J; Joo, S.; Kwak, J.; Yoon, E. Analysis of heavy-metal ions using mercury microelectrodes and a solid-state reference electrode on a Si wafer. Jpn. J. Appl. Phys 2000, 39, 7159–7163. [Google Scholar]
- Valdés-Ramírez, G.; Álvarez-Romero, G.; Galán-Vidal, C.A.; Hernández-Rodríguez, P.R.; Ramírez-Silva, M. Composites: a novel alternative to construct solid-state Ag/AgCl reference electrodes. Sens. Actuat. B 2005, 110, 264–270. [Google Scholar]
- Nagy, K.; Eine, K.; Syverud, K.; Aune, O. Promising new solid-state reference electrode. J. Electrochem. Soc 1997, 144, L1–L2. [Google Scholar]
- Nolan, M.A.; Tan, S.H.; Kounaves, S.P. Fabrication and characterization of a solid state reference electrode for electroanalysis of natural waters with ultramicroelectrodes. Anal. Chem 1997, 69, 1244–1247. [Google Scholar]
- Moussy, F.; Harrison, D.J. Prevention of the rapid degradation of subcutaneously implanted Ag/AgCl reference electrodes using polymer coatings. Anal. Chem 1999, 66, 674–679. [Google Scholar]
- Kwon, N-H; Lee, K-S; Won, M-S; Shim, Y-B. An all-solid-state reference electrode based on the layer-by-layer polyumer coating. Analyst 2007, 132, 906–912. [Google Scholar]
- Tymecki, L.; Zwierkowska, E.; Koncki, R. Screen-printed reference electrodes for potentiometric measurements. Anal. Chim. Acta 2004, 526, 3–11. [Google Scholar]
- Smith, R.L; Scott, D.C. A solid state miniature reference electrode. Proc. IEEE/VSF Symp. Biosens, Los Angeles, CA; 1984; 15–17, pp. 61–62. [Google Scholar]
- Smith, R.L; Scott, D.C. Miniature liquid junction reference electrode and an integrated solid state electrochemical sensor including the same. US patent 4592824. 1986. [Google Scholar]
- Yee, S.; Jin, H.; Lam, L.K.C. Miniature liquid junction reference electrode with micromachined silicon cavity. Sens. Actuat 1988, 15, 337–345. [Google Scholar]
- van den Berg, A.; Grisel, A.; van den Vlekkert, H.H.; de Rooij, N.F. A micro-volume open liquid-junction reference electrode for pH-ISFETs. Sens. Actuat. B 1990, 1, 425–432. [Google Scholar]
- Sinsabaugh, S.L.; Fu, C.W.; Fung, C.D. A batch-processed reference micro electrode integrated on a silicon substrate. Proc. Electrochem. Soc 1986, 86, 66–73. [Google Scholar]
- Suzuki, H.; Hiratsuka, A.; Sasaki, S.; Karube, I. Problems associated with the thin-film Ag/AgCl reference electrode and a novel structure with improved durability. Sens. Actuat. B 1998, 46, 104–113. [Google Scholar]
- Suzuki, H.; Hirakawa, T.; Sasaki, S.; Karube, I. Micromachined liquid-junction Ag/AgCl reference electrode. Sens. Actuat. B 1998, 46, 146–154. [Google Scholar]
- Suzuki, H.; Shiroishi, H.; Sasaki, S.; Karube, I. Microfabricated liquid junction Ag/AgCl reference electrode and its application to a one-chip potentiometric sensor. Anal. Chem 1999, 71, 5069–5075. [Google Scholar]
- Sun, X.; Wang, M. Fabrication and characterization of planar reference electrode for on-chip electroanalysis. Electrochim. Acta 2006, 52, 427–433. [Google Scholar]
- Yalcinkaya, F.; Powner, E.T. Ag/AgCl/Cl− coated silver stripe reference electrode. Med. Eng. Phys 1997, 19, 299–301. [Google Scholar]
- Kim, S.K.; Lim, H.; Chung, T.D.; Kim, H.C. A miniaturized electrochemical system with a novel polyelectrolyte reference electrode and its application to thin layer electroanalysis. Sens. Actuat. B 2006, 115, 212–219. [Google Scholar]
- Tymecki, Ł.; Zwierkowska, E.; Koncki, R. Screen-printed reference electrodes for potentiometric measurements. Anal. Chim. Acta 2004, 526, 3–11. [Google Scholar]
- Simonis, A.; Lüth, H.; Wang, J.; Schöning, M.J. New concepts of miniaturised reference electrodes in silicon technology for potentiometric sensor systems. Sens. Actuat. B 2004, 103, 429–435. [Google Scholar]
- Ito, S.; Kobayashi, F.; Baba, K.; Asano, Y.; Wada, H. Development of long-term stable reference electrode with fluoric resin liquid junction. Talanta 1996, 43, 135–142. [Google Scholar]
- Cunnane, V.J.; Schiffrin, D.J.; Williams, D.E. Micro-cavity electrode: a new-type of liquid-liquid microelectrode. Electrochim. Acta 1995, 40, 2943–2946. [Google Scholar]
- Rivas, I.; Puente, D.; Ayerdi, I.; Castaño, E. Ag/AgI quasi-reference microelectrodes. Proceedings of 2005 Spanish Conference on Electron Devices, Tarragona, Spain, 2–4 February 2005; pp. 465–468.
- Ciobanu, M.; Wilburn, J.P.; Lowy, D.A. Miniaturized reference electrodes. II. use in corrosive, biological, and organic media. Electroanalysis 2004, 16, 1351–1358. [Google Scholar]
- Thomas, R.C.; Cohen, C.J. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes. Pflügers Archiv Eur. J. Physiol 1981, 390, 96–98. [Google Scholar]
- Saheb, A.; Janata, J.; Josowicz, M. Reference electrode for ionic liquids. Electroanalysis 2006, 18, 405–409. [Google Scholar]
- Matsumoto, T.; Ohashi, A.; Ito, N. Development of a micro-planar Ag/AgCl quasi-reference electrode with long-term stability for an amperometric glucose sensor. Anal. Chim. Acta 2002, 462, 253–259. [Google Scholar]
- Ferreira, H.E.A.; Daniel, D.; Bertotti, M.; Richter, E.M. A novel disposable electrochemical microcell: construction and characterization. J. Braz. Chem. Soc 2008, 19, 1538–1545. [Google Scholar]
- Silva, R.A.B.; Almeida, E.G.N.; Rabelo, A.C.; Silva, A.T.C.; Ferreira, L.F.; Richter, E.M. Three electrode electrochemical microfluidic cell: construction and characterization. J. Braz. Chem. Soc 2009, 20, 1235–1241. [Google Scholar]
- Yosypchuk, B.; Novotny, L. Reference electrodes based on solid amalgams. Electroanalysis 2004, 16, 238–241. [Google Scholar]
- Kunimatsu, M.; Qiao, H.; Okada, T. Microtubular hydrogen electrode, a reference electrode for electrochemical analyses. J. Electrochem. Soc 2005, 152, E161–E166. [Google Scholar]
- Will, F.G. A self-contained, miniature hydrogen reference electrode. J. Electrochem. Soc 1985, 133, 454–455. [Google Scholar]
- Gong, S.; Lu, J.; Yan, H. Developing the self-contained hydrogen reference electrode. J. Electoanal. Chem 1997, 436, 291–293. [Google Scholar]
- Nann, T.; Urban, G.A. A new dynamic hydrogen reference electrode for applications in thin-film sensor systems. Sens. Actuat. B 2000, 70, 188–195. [Google Scholar]
- Giner, J. A practical reference electrode. J. Electrochem. Soc 1964, 111, 376–377. [Google Scholar]
- Keller, O.C.; Buffle, J. Voltammetric and reference microelectrodes with integrated microchannels for flow through microvoltammetry. 1. The microcell. Anal. Chem 2000, 72, 936–942. [Google Scholar]
- Wipf, D.O.; Ge, F.; Spaine, T.W.; Baur, J.E. Microscopic measurement of pH with iridium oxide microelectrode. Anal. Chem 2000, 72, 4921–4927. [Google Scholar]
- Yang, H.; Kang, S.K.; Shin, D-H.; Kim, H.; Kim, Y.T. Microfabricated iridium oxide reference electrode for continuous glucose monitoring sensor. In Proceedings of the 12th International Conference on Solid State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; pp. 103–106.
- Park, S-I.; Jun, S.B.; Park, S.; Kim, H.C.; Kim, S.J. Application of a new Cl-plasma-treated Ag/AgCl reference electrode to micromachined glucose sensor. IEEE Sens. J 2003, 3, 267–272. [Google Scholar]
- Simonis, A.; Krings, T.; Lüth, H.; Wang, J.; Schöning, M.J. A “hybrid” thin-film pH sensor with integrated thick-film reference. Sensors 2001 2001, 1, 183–192. [Google Scholar]
- Kim, H.R.; Kim, Y.D.; Kim, K.I.; Shim, J.H.; Nam, H.; Kang, B.K. Enhancement of physical and chemical properties of thin film Ag/AgCl reference electrode using a Ni buffer layer. Sens. Actuat. B 2004, 97, 348–354. [Google Scholar]
- Bergstorm, P.L.; Patel, S.V.; Schwank, J.W.; Wise, K.D. A micromachined surface work-function gas sensor for low-pressure oxygen detection. Sens. Actuat. B 1997, 42, 195–204. [Google Scholar]
- Gergintschew, Z; Kornetzky, P.; Schipanski, D. The capacitively controlled field effect transistor (CCFET). Sens. Actuat. B 1996, 35–36, 285–289. [Google Scholar]
- Fedirko, N.; Svichar, N.; Chesler, M. Fabrication and use of high-speed, concentric H+ - and Ca2+ -selective microelectrodes suitable for in vitro extracellular recording. J. Neurophysiology 2006, 96, 919–924. [Google Scholar]
- Kurkdijan, A.C.; Barbier-Brygoo, H. A hydrogen ion-selective liquid-membrane microelectrode for measurement of the vacuolar pH of plant cells in suspension culture. Anal. Biochem 1983, 132, 96–104. [Google Scholar]
- Zine, N.; Bausells, J.; Ivorra, A.; Aguilo, J.; Zabala, M.; Teixidor, F.; Masalles, C.; Vinas, C.; Errachid, A. Hydrogen-selective microelectrodes based on silicon needles. Sens. Actuat. B 2003, 91, 76–82. [Google Scholar]
- Chao, P.; Ammann, D.; Oesch, U.; Simon, W.; Lang, F. Extra- and intracellular hydrogen ion-selective microelectrode based on neutral carriers with extended pH response range in acid media. Pflügers Arch. European J. Physiol 1988, 411, 216–219. [Google Scholar]
- Koryta, J. Ion-selective electrodes. Ann. Rev. Mat. Sci 1986, 16, 13–27. [Google Scholar]
- Covington, A.K.; Bates, R.G.; Durst, R.A. Definition of pH scales, standard reference values, measurement of pH and related terminology. Pure Appl. Chem 1985, 57, 531–542. [Google Scholar]
- Lengyel, B.; Blum, E. The behaviour of the glass electrode in connection with its chemical composition. Trans. Faraday Soc 1934, 30, 461–471. [Google Scholar]
- Kerridge, P.T. The use of the glass electrode in biochemistry. Biochem. J 1925, 19, 611–617. [Google Scholar]
- Vanýsek, P. The glass pH electrode. Interface-The Electrochem. Soc. Pub 2004, 13, 19–20. [Google Scholar]
- Gooding, J.; Hibbert, D.; Yang, W. Electrochemical metal ion sensors. Exploiting amino acids and peptides as recognition elements. Sensors 2001, 1, 75–90. [Google Scholar]
- Eisenman, G. Cation selective glass electrodes and their mode of operation. Emerging Techniques in Biophysics 1962, 2, 259–323. [Google Scholar]
- Ikeda, T.; Hamada, H.; Miki, K.; Senda, M. Glucose oxidase-immobilized benzoquinone- carbon paste electrode as glucose sensor. Agric. Biol. Chem 1985, 49, 541–543. [Google Scholar]
- Wang, J.; Walcarius, A. Zeolite-modified carbon paste electrode for selective monitoring of dopamine. J. Electroanal. Chem 1996, 407, 183–187. [Google Scholar]
- Guan, W-J; Li, Y.; Chen, Y-Q; Zhang, X-B; Hu, G-Q. Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes. Biosens. Bioelectron 2005, 21, 508–512. [Google Scholar]
- Zhang, X.; Wang, J.; Ogorevc, B.; Spichiger, U.E. Glucose nanosensor based on prussian-blue modified carbon-fiber cone nanoelectrode and an integrated reference electrode. Electroanalysis 1999, 11, 945–949. [Google Scholar]
- Hiratsuka, A.; Kojima, K-I.; Suzuki, H.; Muguruma, H.; Ikebukuro, K.; Karube, I. Integration of microfabricated needle-type glucose sensor devices with a novel thin-film Ag/AgCl electrode and plasma-polymerized thin film: mass production techniques. The Analyst 2001, 126, 658–663. [Google Scholar]
- Patolsky, F.; Zayats, M.; Katz, E.; Willner, I. Precipitation of an insoluble product on enzyme monolayer electrodes for biosensor applications: characterization by faradaic impedance spectroscopy, cyclic voltammetry, and microgravimetric quartz crystal microbalance analyses. Anal. Chem 1999, 71, 3171–3180. [Google Scholar]
- Cui, G.; Lee, J.S.; Kim, S.J.; Nam, H.; Cha, G.S.; Kim, H.D. Potentiometric pCO2 sensor using polyaniline-coated pH-sensitive electrodes. Analyst 1998, 123, 1855–1859. [Google Scholar]
- Suzuki, H.; Kojima, N.; Sugama, A.; Fujita, S. Micromachined clark oxygen electrode. Proceedings of 1991 Int. Conf. Solid-State Sens. Actuators, Stockholm, Sweden; 1991; pp. 339–342. [Google Scholar]
- Suzuki, H.; Hirakawa, T.; Sasaki, S.; Karube, I. An integrated module for sensing pO2, pCO2, and pH. Anal. Chim. Acta 2000, 405, 57–65. [Google Scholar]
- Souteyrand, E; Cloarec, J.P.; Martin, J.R.; Wilson, C.; Lawrence, I.; Mikkelsen, S.; Lawrence, M.F. Direct detection of the hybridization of synthetic homo-oligomer DNA sequences by the field-effect. J. Phys. Chem. B 1997, 101, 2980–2985. [Google Scholar]
- Ingebrandt, S.; Offenhäusser, A. Label-free detection of DNA using field-effect transistors. Phys. Status Solidi A 2006, 203, 3399–3411. [Google Scholar]
- Kim, D.-S.; Park, H.-J.; Jung, H.M.; Shin, J.K.; Choi, P.; Lee, J.H.; Lim, G. Field effect transistor-based bimolecular sensor employing a Pt reference electrode for the detection of deoxyribonucleic acid sequence. Jpn. J. Appl. Phys 2004, 43, 3855–3859. [Google Scholar]
- Park, K-M; Lee, S-K; Sohn, Y-S; Choi, S-Y. BioFET sensor for detection of albumin in urine. Electron. Lett 2008, 44, 185–186. [Google Scholar]
- Minot, E.D.; Janssens, A.M.; Heller, I.; Heering, H.A.; Dekker, C.; Lemay, S.G. Carbon nanotube biosensors: the critical role of the reference electrode. Appl. Phys. Lett 2007, 91, 093507. [Google Scholar]
- Jamasb, S.; Collins, S.; Smith, R.L. A physical model for drift in pH ISFETs. Sens. Actuat. B 1998, 49, 146–155. [Google Scholar]
- Jamasb, S. An analytical technique for counteracting drift in ion-selective field effect transistors. IEEE Sens. J 2004, 4, 795–801. [Google Scholar]
- Jamasb, S; Collins, S.D.; Smith, R.L. Correction of instability in ion-selective field-effect transistors (ISFET’s) for accurate continuous monitoring of pH. Proceedings of 19th Int. Conf. IEEE /EMBS, Chicago, IL, USA; 1997; pp. 2337–2340. [Google Scholar]
- Mascini, M.; Palchetti, I.; Marrazza, G. DNA electrochemical biosensors. Fresenius J. Anal. Chem 2001, 369, 15–22. [Google Scholar]
- Brett, C.M.A.; Inzelt, G.; Kertesz, V. Poly(methylene blue) modified electrode sensor for haemoglobin. Anal. Chim. Acta 1999, 385, 119–123. [Google Scholar]
- Schwarz, M.A.; Galliker, B.; Fluri, K.; Kappes, T.; Hauser, P.C. A two-electrode configuration for simplified amperometric detection in a microfabricated electrophoretic separation device. Analyst 2001, 126, 147–151. [Google Scholar]
- Safari-Mohsenabad, Salman; Selvaganapathy, P.R.; Deen, M.J. Surface micromachined PDMS microfluidic devices fabricated using a sacrificial photoresist. Proceedings of Electrochemical Society Spring Symposium, Vancouver, BC, Canada, 25–30 April 2010.
- Safari-Mohsenabad, Salman; Selvaganapathy, P.R.; Deen, M.J. Microfluidic Reference Electrode with Flowing Liquid Junction, 2010; Unpublished.
- Safari-Mohsenabad, Salman; Selvaganapathy, P.R.; Derardja, A.; Deen, M.J. Nanosheet formation by modified electrodeposition method and its application to miniaturized reference electrodes. 2010; (Unpublished). [Google Scholar]
- Shinwari, M.W; Deen, M.J.; Selvaganapathy, P.R. Analytic Modeling of Biotransistors. IET Circuits, Devices & Systems 2008, 2, 158–165. [Google Scholar]
- Deen, M.J.; Shinwari, M.W.; Ranuárez, J.C.; Landheer, D. Noise Considerations in Field-Effect Biosensors. J. Appl. Phys 2006, 100, 074703–074703-8. [Google Scholar]
- Landheer, D.; Aers, G.; McKinnon, W.R.; Deen, M.J.; Ranuarez, J.C. Model for the Field-Effect from Layers of Biological Macromolecules on the Gates of Metal-Oxide-Semiconductor Transistors. J. Appl. Phys 2005, 98, 044701–044701-15. [Google Scholar]
- Bustillo, J.M.; Howe, R.T.; Muller, R.S. Surface micromachining for microelectromechanical systems. Proc. IEEE 1998, 86, 1552–1574. [Google Scholar]
- Subramani, B.G.; Selvaganapathy, P.R. Surface micromachined PDMS microfluidic devices fabricated using a sacrificial photoresist. J. Micromech. Microeng 2009, 19, 015013. [Google Scholar]








| Electrode type | Dimensions | Fabrication | Filling solution | Test solution | Electrode potential (V) | Stability | Year | [Ref.] |
|---|---|---|---|---|---|---|---|---|
| Ag/AgCl | 13mm × 1.5mm × 0.4mm | Photolithography, Sputtering, Electrochemical oxidation | None (quasi-RE) | 0.1 M KCl in 20mM NaOH/KH2PO4 buffer, pH 7 | 90mV vs. commercial Ag/AgCl | < 1mV in 24h, 1mV in 8h with saturated AgCl and KCl in solution | 1998 | [64] |
| Ag/AgCl | 13mm × 1.5mm × 0.9mm | Photolithography, Electrochemical oxidation, Bulk micromachining | Saturated KCl, AgCl. Pin hole liquid junction | 0.1 M KCl in 20mM NaOH/KH2PO4 buffer, pH 7 | 7mV vs. commercial Ag/AgCl | <1mV in 3h. | 1998 | [65] |
| Ag/AgCl | 13mm × 1.5mm × 0.9mm | Photolithography, Electrochemical oxidation, Screen printing | Saturated KCl, AgCl. Hydrophillic Polymer liquid junction | 0.1 M KCl in 50mM NaOH/KH2PO4 buffer, pH 7 | 8mV vs. commercial Ag/AgCl | <2mV in 100h. | 1999 | [66] |
| Ag/AgCl | N/A (macroscopic Ag wire used) | Photo-polymerization for junction polymer | 1M KCl. pDADMAC plug junction | PBS at pH 7.4 with 0.15M NaCl | 19.3mV vs. commercial Ag/AgCl in 3M KCl | <12mV in 30h. | 2006 | [69] |
| Ag/AgCl | N/A | Photolithography, Lift-off, Sputtering, Electrochemical oxidation | Saturated KCl in Agarose supporting gel. | 10mM KCl, pH range 4–10 | 0.45mV vs. commercial Ag/AgCl | <1.5mV in 42h. | 2002 | [34] |
| Ag/AgCl | 2cm×0.1 mm | Electroless plating on glass, Electroplating | None (quasi-RE) | 3M KCl | 13.5mV vs. commercial Ag/AgCl | <30mV in 14 days | 2006 | [67] |
| Ag/AgCl | 106 μm2 | Photolithography, Chemical oxidation | None (quasi-RE) | 1mM KCl | 32mV vs. commercial Ag/AgCl at 1mM KCl, with identical electrode variation of 10mV | <2mV in 5000s | 2006 | [48] |
| Ag/AgCl | 1μm diameter capillary | Pre-made capillary and silver wire. Capillary action for salt bridge and filling solution | 3.3M KCl + AgCl | Distilled water | (−4–0) mV vs. commercial Ag/AgCl | <2 mV in 2400s | 2005 | [1] |
| Ag/AgCl | 1.2mm2 | Photolithography, Lift-off, Plasma chlorination | None (quasi-RE) | PBS (0.1M Na2HPO4, 0.15M NaCl, 0.1 g/l NaN3 at pH 7.4) | 70mV vs. commercial Ag/AgCl electrode in 3M KCl | <13mV in 5h | 2003 | [90] |
| Ag/AgCl | 10mm×20mm | Screen-printing thick film | None (quasi-RE) | Technical buffer with 0.05mM Cl− | ≈230mV vs. commercial Ag/AgCl | <70mV in 12h | 2001 | [91] |
| Ag/AgCl | 2mm×1.8mm (exposed area only) | Sputtering, Ni layer added, Photolithography, Chemical chloridizing | None (quasi-RE) | 50mM Tris buffer (pH 7.4) with 3.5M KCl | 0mV vs. commercial Ag/AgCl | <1mV in 2h | 2004 | [92] |
| Graphite/AgCl | N/A (macroscopic) | None (macroscopic PVC encasing used) | None (quasi-RE) | KCl 0.1M | 40.8mV vs. commercial AgCl in saturated KCl | <0.2mV in 1h | 2005 | [53] |
| Hydrogen | 4mm×7mm | Evaporation, Lift-off, Galvanic platenization of Pt | None. One variant uses pHEMA membrane | KCl 1M | −850mV vs. fabricated pseudo-Ag/AgCl (after 120–1100 s of initialization drifts) | <1.5mV/h | 2000 | [85] |
| Ag/AgI | 1mm diameter | Sputtering, Lift-off, PECVD, RIE, Electrodeposition | None (quasi-RE) | PBS, pH 7.0 | −94mV vs. SCE | <1mV in 20h | 2005 | [73] |
| IrOx | 0.1mm×1mm | Photolithography, Sputtering, CVD, Electrodeposition | None (quasi-RE) | PBS | 195mV vs. Ag/AgCl | <20mV in 9 days, after initial 120 mV/day drift. 4mV std. dev. | 2003 | [30] |
| IrOx | 1500μm2 | Sputtering, Anodic growth (Electrochemical deposition) | None (quasi-RE) | 0.1M PBS | 40mV vs. Ag/AgCl | <100mV in 15 days | 2005 | [29] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated Reference Electrodes and their Biosensing Applications. Sensors 2010, 10, 1679-1715. https://doi.org/10.3390/s100301679
Shinwari MW, Zhitomirsky D, Deen IA, Selvaganapathy PR, Deen MJ, Landheer D. Microfabricated Reference Electrodes and their Biosensing Applications. Sensors. 2010; 10(3):1679-1715. https://doi.org/10.3390/s100301679
Chicago/Turabian StyleShinwari, M. Waleed, David Zhitomirsky, Imran A. Deen, P. R. Selvaganapathy, M. Jamal Deen, and D. Landheer. 2010. "Microfabricated Reference Electrodes and their Biosensing Applications" Sensors 10, no. 3: 1679-1715. https://doi.org/10.3390/s100301679
APA StyleShinwari, M. W., Zhitomirsky, D., Deen, I. A., Selvaganapathy, P. R., Deen, M. J., & Landheer, D. (2010). Microfabricated Reference Electrodes and their Biosensing Applications. Sensors, 10(3), 1679-1715. https://doi.org/10.3390/s100301679