Abstract
Caves provide critical roosting habitats for bats globally, but are increasingly disturbed or destroyed by human activities such as tourism and extractive industries. In addition to degrading the habitats of cave-roosting bats, such activities often promote contact between humans and bats, which may have potential impacts on human health. Cave-roosting bats are hosts to diverse viruses, some of which emerged in humans with severe consequences (e.g., severe acute respiratory syndrome coronavirus and Marburg virus). Characterizing patterns of viral richness and sharing among bat species are therefore important first steps for understanding bat-virus dynamics and mitigating future bat-human spillover. Here we compile a database of bat-virus associations and bat species ecological traits, and investigate the importance of roosting behavior as a determinant of viral richness and viral sharing among bat species. We show that cave-roosting species do not host greater viral richness, when accounting for publication bias, diet, body mass, and geographic range size. Our global analyses, however, show that cave-roosting bats do exhibit a greater likelihood of viral sharing, especially those documented in the literature as co-roosting in the same cave. We highlight the importance of caves as critical foci for bat conservation, as well as ideal sites for longitudinal surveillance of bat-virus dynamics.
Keywords:
Chiroptera; caves; roosting behavior; viruses; ecological traits; virus-host associations; zoonosis 1. Introduction
1.1. Caves as Critical Habitat for Bats Globally
Caves represent critical roosting sites for bats on all continents, except Antarctica [1]. Caves provide permanent roosts with stable microclimatic conditions optimal for rearing young and as winter hibernacula. In addition, caves provide shelter from inclement weather and predators [2]. As a result, caves house some of the largest aggregations of bats in the world [3]. For example, Monfort Cave in the Philippines houses a colony of frugivorous bats (Rousettus amplexicaudatus) estimated at 2 million individuals with a roosting density of 452.3 individuals/m2 [4], while Bracken Cave in the United States is occupied by roughly 20 million insectivorous bats (Tadarida braziliensis) [5]. Caves are often structurally complex (e.g., passageways, cracks and crevices, chambers), providing a range of roosting opportunities that can accommodate species-specific preferences and support aggregations of a high diversity of cave-roosting species [6]. Particularly in the tropics, caves have been reported to house bats of more than 10 species [3,7,8]. Consequently, in many countries, a significant proportion of native bats depend on caves as roost sites. For example, roughly 80% of all bat species in Puerto Rico roost in caves [9], while 101 of 131 (77%) bat species in China depend on caves as roosts [10].
1.2. Viral Diversity in Cave- and Non-Cave-Roosting Bats
All mammals harbor viruses, but viral richness is not uniform among species or mammalian orders. Bats, rodents, primates, and two ungulate orders are each host to an overall greater total number of viruses per species as compared to other mammalian orders, after controlling for research effort and other species-specific factors [11]. However, bats have the greatest proportion of zoonotic viruses (i.e., viruses that can infect animals and humans) per species as compared to other mammalian orders [11], potentially due to their unique life-history traits or innate immune system [12,13]. Within the order Chiroptera, several studies have examined the ecological and life-history traits associated with viral richness per species [11,14,15,16,17,18]. Ecological traits known to increase viral richness in bats include large geographic range size and high number of sympatric species. High levels of population genetic structure, large colony size, low conservation threat level, heavier host body mass, and dietary breadth are some of the chiropteran life-history traits that have been associated with high pathogen richness. Luis et al. [14] included propensity to roost in caves as an explanatory variable for viral sharing among a subset of 52 bat species, but roosting behavior otherwise has not been explicitly tested as a determinant of viral richness or viral sharing.
Cave-roosting bat species are specifically known to be reservoirs for several zoonotic viruses including severe acute respiratory syndrome (SARS) coronavirus and Marburg virus [19,20], as well as host to novel viruses closely related to Ebolavirus [21,22]. Specific clades of viruses are often primarily associated with closely related bat taxa; for example, the SARS coronaviruses are primarily found in the genus Rhinolophus, which includes a number of cave-roosting species [23,24]. Other viral groups appear to be shared more widely across bat taxa with varying ecological and life-history traits, particularly generalist viruses such as lyssaviruses and mosquito-borne flaviviruses [25].
1.3. Disease Ecology and Unique Traits of Cave-Roosting Bat Species
Several traits unique to cave-roosting species may help in maintaining a greater pool of viruses (greater viral richness) and/or help facilitate viral sharing among species. Close contact between conspecific individuals roosting in confined caves could facilitate sustained viral maintenance and potentially greater viral richness, though there are conflicting findings in the literature about the importance of gregariousness in bat-virus dynamics [14,16]. Dense maternity colonies in caves could enhance intraspecific transmission. Synchronous and seasonal breeding in high-density bat colonies introduces a pulse of susceptible individuals (i.e., offspring) that could increase rates of viral transmission and circulation, with a peak typically 3–6 months after birth, when maternal antibodies have waned [26,27]. Diverse bat assemblages found co-roosting in caves will also likely promote viral circulation and maintenance between bats of different species that typically would not interact while foraging, or otherwise, outside of the roost. Interspecific contact between individuals in caves could also facilitate host switching of viruses between bat species. Successful viral establishment in a novel (previously uninfected) species, however, depends in part on other behavioral factors and on the phylogenetic relatedness of species [11,28].
Just as caves provide stable microclimatic conditions and shelter to bats, they may provide optimal environments that promote viral persistence outside the host. Caves typically maintain consistent temperature and humidity profiles and lack ultraviolet radiation, all of which are important environmental factors influencing viral decay [29,30]. These abiotic factors, in part, contribute to the long-term persistence of the fungal pathogen Pseudogymnoascus destructans, the causative agent of white nose syndrome in bat colonies across North America, and potentially other human pathogenic bacteria in caves [31,32]. The role of cave environments on viral survival needs further investigation through both environmental sampling and experimental studies. Recent detection of Group C Betacoronavirus RNA in bat guano from cave floors in Thailand, however, demonstrates the potential for indirect viral transmission in caves [33].
1.4. Human-Cave Bat Interactions
Nearly half of all bat species that use caves as roosts are threatened or endangered [15]. This highlights the increasing pressure that cave ecosystems, and cave-roosting bats, are under from a multitude of human disturbances. Anthropogenic stressors could also lead to an increase in viral shedding in bat populations, thus making human-bat interactions more hazardous [15]. Increasing evidence supports the assumption that habitat quality and bat health are inherently linked. For example, in paleotropical forests with intense logging and fragmentation, bats with poor body conditions were more likely to shed astroviruses in feces [34]. Seltmann et al. [34] speculated that increased susceptibility of individuals to acquire and shed viruses may have resulted from stress-induced immunosuppression.
Human visitation to caves for tourism, religious ceremonies, bat hunting, quarrying of limestone and phosphate, and harvesting other biological resources in caves can potentiate interactions between humans and cave-roosting bats. Quarrying poses the greatest threat to cave-roosting bats, resulting in the direct and irreversible loss of permanent roost sites [35,36]. Species loss is magnified further by overexploitation through unregulated hunting for human consumption as well as purported traditional Chinese remedies (e.g., asthma) [37]. Frequent and regular visits by tourists in caves has been shown to increase carbon dioxide levels and alter temperature and humidity profiles in cave chambers, causing bats to abandon roosts [38]. Extraction of biological resources in caves, particularly the harvesting of bat guano for fertilizer, cave swiftlet nests for bird’s nest soup, and mineral formations for souvenirs (e.g., stalactites), often disturbs roosting bats [8,35,36]. These activities typically put local workers in frequent contact with bat excreta, providing opportunities for exposure to viruses that may be shed in feces, urine, or saliva. For example, Marburg virus spillover from cave-roosting bats has been linked to gold mining [39,40] and tourism [41] in caves from Democratic Republic of Congo and Uganda. In addition, destruction of foraging sites surrounding caves can serve as an additional stressor to cave-roosting bats that may promote susceptibility to infection. Urban and agricultural expansion, in addition to commercial logging, often results in extensive deterioration and destruction of forested habitats that serve as critical foraging sites for cave-roosting bats [4,36]. Collectively, such threats represent inescapable stressors to bats that may also increase the susceptibility of individual bats to viral infections, promote contact between cave-roosting bats and humans, and/or provide opportunities for viral spillover.
1.5. Study Objectives
Cave-roosting bat species comprise a significant proportion of global bat diversity, yet are under increasing anthropogenic pressure that could lead to future viral spillover events. Given these two critical issues, our primary study objective is to aggregate existing data and develop comparative analyses to better understand patterns of viral richness and viral sharing within cave-roosting bat species and between cave- and non-cave-roosting bat species. Specifically, we aim to determine the relative importance of roosting behavior on both viral richness within and viral sharing among bat species. We address the following three questions: (1) Is cave-roosting behavior a driver of viral richness when considered together with a suite of other ecological and life-history traits? (2) What factors are the most important determinants of viral sharing between bat species? (3) Is there greater viral sharing between co-roosting species than species without documented co-roosting behavior in caves?
2. Materials and Methods
2.1. Database of Viral Associations and Host Traits
We compiled a database of 595 virus-host associations for 114 viral species and 205 bat species. Virus names were synonymized to the International Committee on the Taxonomy of Viruses (ICTV) 2016 version 1 [42], and only viruses assigned a species name were included in our database. Bat species were synonymized according to the International Union for Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species version 2017–1 [43]. A majority of the associations (n = 509) were collected from a recently published mammalian virus database [11], with additional associations added from an updated literature search from this study (n = 49), as well as publicly available host-virus associations from the PREDICT global surveillance program (2009–2014, n = 37), for virus and host species that met our above taxonomic criteria (Supplementary Table S1 and Item S1) [44]. We also analyzed a subset of the data limited to viral detection obtained through PCR or virus isolation—a ‘stringent’ database excluding serological (antibody and antigen) detection—to control for potential biases from cross-reactivity of related viruses. The stringent database includes 153 bat species, 82 viruses, and 277 virus-host associations. Viral richness, defined as the number of ICTV—recognized viruses detected in each bat species, was calculated separately for the main and stringent databases. To determine roosting behavior for each bat species, we performed a literature search using keywords in Web of Science and Google Scholar. Literature searches included each species binomial plus recent taxonomic synonyms AND “roost”. Additional literature sources to categorize roosting behavior were found in the reference section of publications obtained from the initial literature search. Roosting behavior was categorized as obligate cave-roosting if a species was documented to exclusively use caves as roost sites, facultative cave-roosting when a species roosts in caves as well as other roost sites (e.g., hollow trees, abandoned buildings, termite nests), or non-cave-roosting if a species has not been recorded to roost in caves (Supplementary Item S2).
We collected additional host life-history and ecological traits that may be important for explaining observed viral richness. Host taxonomy, threat status, diet (i.e., frugivore, insectivore, or other), and spatial range information were extracted from IUCN [43]. From the IUCN spatial shapefiles, we calculated geographic range size (log10 km2) and sympatric viral host species (i.e., count of bat species in both main and stringent virus databases that have overlapping spatial ranges) with the rgeos package in R. Species body mass was extracted from PanTHERIA [45]. As more closely related species will have similar body mass due to shared evolutionary history, we controlled for this by calculating the residuals from a phylogenetic eigenvector regression (PVR) on body mass [46,47]. As described in Olival et al. [11], we calculated PVR for body mass using the R package PVR and a pruned mammalian supertree that only included bat species in our viral database [48,49]. Artibeus planirostris was manually inserted in the tree next to Artibeus jamaicensis per Simmons [50]. To plot viral richness from our main database and roosting behavior onto the phylogeny, the picante R package was used. To address research bias among bat species, we extracted the total publication count for each species (and known synonyms) from PubMed using the rentrez R package. The zoonotic status of viruses followed Olival et al. [11], but was updated through literature searches of viral binomial AND (“case” OR “human”) using Google Scholar and PubMed.
With 205 bat species in our main database, there are 20,910 unique species-pairs with the potential for viral sharing and 11,628 unique species-pairs for the 153 bat species in our stringent database. Here, we define viral sharing as the dual detection of a virus in two bat species. We created several shared traits per species-pair to test their relationship with viral sharing. During literature searches to categorize roosting behavior, if two or more species were documented as roosting in the same cave, we noted them as co-roosting species (see Supplementary Table S3). For the 201 species with IUCN range information, we calculated spatial and sympatric species overlap between each species-pair as proxies for potential interspecific contact. To do this, we calculated square kilometers of overlap between each species-pair from their IUCN spatial file using gIntersection function in the rgeos R package. Sympatric species overlap was calculated as the number of hosts in our database whose range intersects with both species of the pair. From our host traits, we determined a binary cave-roosting behavior trait: “Yes” if both species were obligate or facultative cave-roosting and “No” if only one or neither species were obligate or facultative cave-roosting. Similarly, a shared diet binary trait was determined: “Yes” if both species had the same dietary niche (e.g., both frugivores) or “No” if they had different diets (e.g., one a frugivore and one an insectivore). To account for research bias in dual virus detection in the species-pair, the minimum publication count from the two species was used as described in Gómez et al. [51].
2.2. Viral Richness and Sharing Analyses
General linear models (GLMs) were used to identify significant predictors of viral richness and viral sharing for species in both our main and stringent databases. Variables for the viral richness models included roosting behavior and diet as categorical predictors, and range size, phylogenetically-corrected mass, research effort (i.e., publication count), and the number of sympatric species as continuous covariates (Supplementary Table S2). As range size and sympatric species were highly correlated (p < 0.001, r = 0.60), these collinear variables were alternated in our model selection. Only eleven species (5%) were obligate cave-roosting, so the obligate and facultative categories were merged to create binary roosting behavior variable of “caving” or “noncaving”. Covariates were normalized to account for scaling differences, while research effort and range size were log10-transformed. For viral richness, we calculated count data of viruses per species and used a Poisson distribution. For best viral richness GLMs, we confirmed variable significance while controlling for phylogenetic signal by including host family as a random effect (Supplementary Table S4).
Variables included in our GLMs to assess viral sharing included roosting behavior (cave- or non-cave-roosting), documentation of co-roosting from the literature, and shared diet as binary predictors, and phylogenetic relatedness, research effort (i.e., minimum number of publications for each species pair), and spatial overlap (km2) as continuous covariates (Supplementary Table S3). To calculate phylogenetic relatedness between bat species, we used the cophenetic function in the R package ape to calculate branch distance between each species pair on a version of the mammalian supertree pruned to only include the species in our database [49]. Covariates were normalized to account for scaling differences. Numeric variables publication count, phylogenetic distance, and range overlap size were log10-transformed, as above. Viral sharing was calculated as a binomial distribution: detection of at least one virus in both species (1) or no virus detected in both species (0).
For both the viral richness and viral sharing models, we started with a model that included only roosting behavior (cave- vs. non-cave-roosting) and research effort. We then performed additional model runs based on a priori ecological and life-history hypotheses and used Akaike Information Criterion (AIC) values to select the best model (Supplementary Tables S4 and S5). All analyses were tested on the main database as well as our stringent database. For the viral sharing model, we also used a database limited to species with literature verified co-roosting, meaning they co-roost with at least one other species in our main database (n = 74 hosts, n = 2701 species-pairs). Coefficient plots were visualized using the arm package in R. Using the igraph package in R, we constructed a bipartite network to visualize host-virus associations among cave- and non-cave-roosting bat species. We additionally forced a unipartite host network and calculated network-level metrics (components and modularity) to describe viral sharing among bat hosts.
3. Results
3.1. Database Summary and Descriptive Statistics
Our database contains 205 bat species associated with at least one virus from 12 taxonomic families. Only 5 of the 12 bat families (38%) contain both cave- and non-cave-roosting species (Emballonuridae, Molossidae, Phyllostomidae, Pteropodidae, Vespertilionidae). The other 7 families are comprised of only cave-roosting species (Hipposideridae, Megadermatidae, Miniopteridae, Mormoopidae, Natalidae, Nycteridae, Rhinolophidae). Cave-roosting species make up the majority (71%, 146 of 205 species) of species in our viral host database. The global distribution of cave-roosting species with viral information was distinct from the non-cave-roosting species, with greater richness of cave-roosting species at northern latitudes and in Southeast Asia and China (Figure 1). Based on PubMed article counts, bats of cave-roosting species (mean = 26.7 articles) have been studied more extensively than non-cave-roosting species (mean = 9.2 articles; t = 3.3921, df = 191.79, p < 0.001). Twenty-six species had no search results for their scientific name in PubMed. Bats of three species have been more extensively studied (>300 articles for each) than bats of all other species: Eptesicus fuscus, Myotis lucifugus, and Desmodus rotundus.
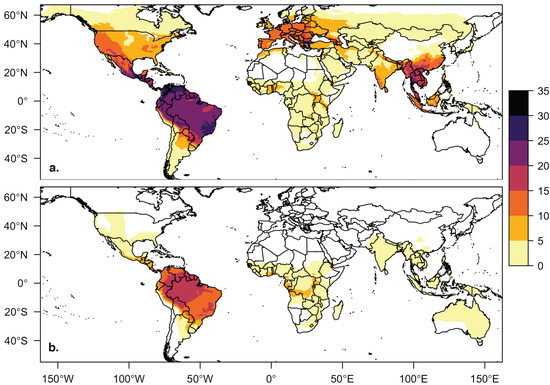
Figure 1.
Host distribution maps for bat species in the viral database for (a) cave-roosting bats (n = 142) and (b) non-cave-roosting bats (n = 59). Bat species richness, regardless of roosting behavior, is highest in South and Central America, but richness of cave-roosting species is also high in Southeast Asia, western North America, and southern Europe. Four bat species in our database without IUCN spatial files are not included in this figure or our geographic trait analyses.
The most represented bat genera in our database were Myotis (n = 25 species), Rhinolophus (n = 16, all cave-roosting), Pteropus (n = 12, all non-cave-roosting), and Hipposideros (n = 12, Figure 2). Species in the genus Pteropus are important natural reservoirs for Nipah [52] and Hendra [53] viruses, and the only genus listed above without represented species using caves as roost sites. Some important viral genera are exclusive to the bat genera Rhinolophus, Hipposideros, Miniopterus, Natalus, and Pipistrellus, which are largely comprised of cave-roosting species, while other viral genera are exclusive to bats of non-cave-roosting genera Pteropus and Cynomops or Artibeus, Eptesicus, Lasiurus, and Myotis, which include both cave- and non-cave-roosting species. Each bat species was observed to host on average 2.90 unique viruses (including serological detections) and 1.35 viruses (using stringent detections only). Fifty-three bat species have only had serological viral detection (35 cave-roosting and 18 non-cave-roosting species). Eidolon helvum and Artibeus lituratus had the highest observed viral richness (n = 18 viruses), while Rousettus aegyptiacus had the greatest number of viruses confirmed via PCR and viral isolation (n = 9). Observed viral richness per species did not significantly differ between cave-roosting bats and non-cave-roosting bats (t = 0.89, df = 120.57, p = 0.38), but this does not account for research effort or other explanatory variables (see GLM results below).
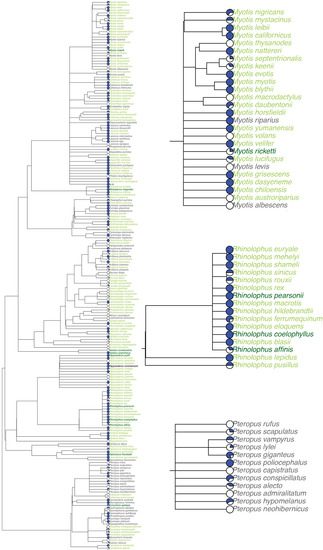
Figure 2.
Host phylogeny pruned from the mammalian supertree of (a) bat species included in our database (n = 205), and insets shown for the genera: (b) Myotis, (c) Rhinolophus, and (d) Pteropus. Facultative cave-roosting bat species in light green, obligate cave-roosting species in dark green, and non-cave-roosting species in grey. A pie chart showcasing the proportion of viruses detected in each species via serology (white) or nucleic acid (blue) is next to each species.
Nineteen viral families have been detected in bats, with the flaviviruses, rhabdoviruses, and coronaviruses exhibiting the greatest viral richness, with 26, 19, and 13 viral species respectively. On average, each virus has 5.22 hosts and 2.42 stringent hosts. Out of the 82 stringently detected viruses, 39 have been detected via PCR in only a single host species. Half of the bat-borne viruses (52%, 59 of 114 viruses) are zoonotic, detected in humans either serologically or through nucleic acid detection. Certain viruses have been exclusively detected (via serologic or nucleic acid methods) in cave-roosting bats, notably European bat lyssavirus 1 (n = 14 hosts), Issyk-Kul virus (n = 13), and SARS coronavirus (n = 8). Other well-known pathogens, however, have been detected in both cave- and non-cave-roosting species: rabies virus (24 non-cave- and 59 cave-roosting species), yellow fever virus (4 non-cave- and 10 cave-roosting species), and West Nile virus (5 non-cave and 9 cave-roosting species) (Figure 3). Zoonotic viruses have a greater number of bat hosts (mean = 7.85 species) than non-zoonotic viruses (mean = 2.4 species; t = −3.46, df = 63.92, p < 0.001).
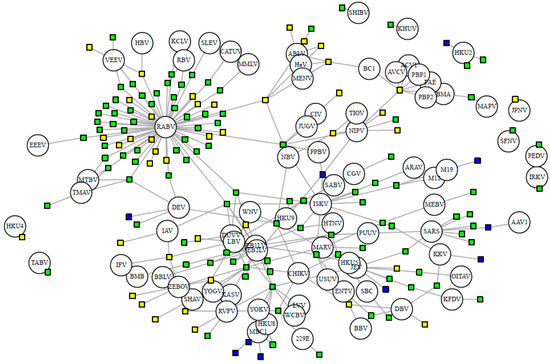
Figure 3.
Bipartite network of bat hosts (squares, n = 153 species) and PCR-detected viruses (circles, n = 82 species). Connected by 277 associations. Cave-roosting bats are shown in green (facultative, n = 102) and blue (obligate, n = 10), while non-cave-roosting species are shown in yellow. Viral abbreviations are listed with ICTV species name in Supplementary Table S1.
3.2. Viral Richness Analysis
The GLM for viral richness per bat species that best fit our main and stringent databases included per-species measures of research effort, phylogenetically-corrected mass, count of sympatric species, and roosting behavior (Supplementary Tables S2 and S4). In both sets of models, the research effort had the only significantly positive effect, while a non-frugivorous diet was the only variable with a significantly negative effect on viral richness. Phylogenetically-corrected mass, spatial variables (range size and count of sympatric viral host species), and roosting behavior did not have significant effects on the model. Threat status was not included in our analyses as only seven species included in our database (3%) were listed as vulnerable or endangered.
3.3. Viral Sharing Analysis
Every bat species in our database shares at least one virus with another species. On average, each bat species shares at least one virus with 31.38 other species in our main database or 22.16 other species when limiting to the stringently detected viruses (Figure 3). When we exclude rabies virus, viral sharing decreases to each bat species sharing at least one virus with 16.10 other species in our main database or 7.92 other species when limiting to the stringent database. This is exhibited by the connectivity of bat species in our forced unipartite network, as hosts are highly connected through viruses (Q = 0.27) and even more so for stringently detected viruses (Q = 0.21), and rabies as the most central (measured by eigenvector centrality) virus connected to the greatest number of hosts (n = 61; Figure 3). Out of the 19,900 species-pairs with spatial overlap information, 4563 (23%) species-pair exhibit spatial overlap. On average, each species has an overlapping geographic range with 46 ± 23 other bat species with known viral associations. Fifty-two percent of species share their dietary niche, 7% are both frugivores and 43% are both insectivores. Shared roosting behavior was exhibited by 59% of species-pairs, specifically 50% of species-pairs both roost in caves, while 9% are both non-cave-roosting species. Sixty-two percent of species-pairs are phylogenetically divergent, with branch distances ≥100 from the mammalian supertree. Roosting behavior was not a significant variable in the best-fit GLM for viral sharing among species in the main database. Our best-fit GLM for viral sharing among bat species in our stringent database included per-pair measures of research effort, phylogenetic distance, spatial overlap, shared diet, and roosting behavior (Figure 4a, Supplementary Table S5). For stringent bat species-pairs, each variable had a significantly positive effect except shared diet, which was not significant.
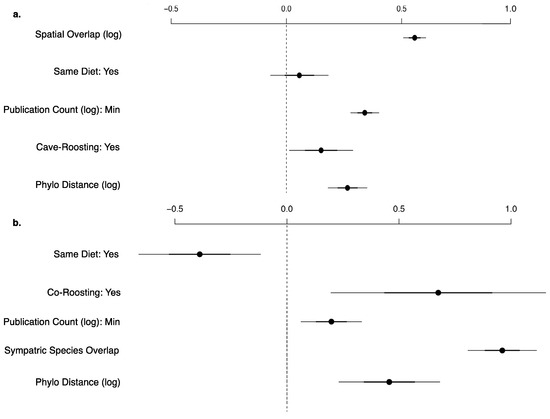
Figure 4.
Coefficient plot for the best viral sharing generalized linear models for shared stringent viruses (Y/N) between (a) all bat species-pairs (n = 7864) and (b) co-roosting bat species-pairs (n = 1378). Each model includes publication count, phylogenetic distance, shared roosting behavior, and shared dietary niche. Spatial overlap is the best predictor for all species, while number of sympatric viral hosts performed better in the co-roosting species subset. All variables are significant.
Seventy-four bat species in our database have literature-documented co-roosting behavior with other viral hosts in the literature, with 181 documented co-roosting pairs (7% of the species-pairs with co-roosting information). Based on our extensive literature review, on average, each species co-roosts with 4.89 other bat species, with a range of one to fifteen species. Natalus stramineus exhibited the greatest co-roosting behavior, with documented roost sharing in caves with fifteen other species. Our best-fit model for both the main and stringent databases for the subset of co-roosting species included reporting effort along with life-history and geographic traits. The best predictor for stringent viral sharing between two co-roosting species is number of bat species with an overlapping range, followed by documented co-roosting behavior, phylogenetic distance, publication count, and shared diet (Supplementary Table S5).
4. Discussion
4.1. Current State of Knowledge and Data Gaps
We compiled a comprehensive list of viruses that have been detected in bats globally, and used these data to specifically test the role that roosting behavior may play as a determinant of viral richness and viral sharing in bats. We found that most species in our database are cave-roosting, including obligate and facultative cave-roosting bat species. This partially reflects that many bat species exhibit a propensity to roost in caves, but also demonstrates important biases in our knowledge of roosting behavior to date. Many species that are foliage- and tree-roosting are very difficult to capture and observe (e.g., highly migratory hoary bats in North America) [54]. Often special trapping methods (e.g., canopy nets used in tall, vertical forest communities) are needed to adequately sample the full diversity of bats in a community [55]. Our database suggests a strong bias in viral sampling of bats at caves, where they are easier to capture and can be reliably located and sampled. Moreover, we only have documented viral associations (i.e., ICTV-named viruses) for 205 species globally, representing roughly 16% of the documented 1300 species in the order Chiroptera [56]. Additional data from bat species sampled across more diverse roost types may lead to a different picture on the role of roosting behavior and roosting substrate on viral richness and sharing.
4.2. Viral Richness Within Bat Species
When we control for research bias and other species-specific traits in our multivariate model, we found that roosting behavior (i.e., whether or not a species roosts in caves) was not a significant predictor of viral richness for the 205 bat species included in our analysis. This disagrees with the one other analysis of roosting behavior and viral richness [14]; however, this study was limited to 52 bat species with roosting behavior information. Our findings support previously published work, indicating that diet was an important life-history trait for viral richness [18,57]. Frugivorous bats host more viruses than other dietary guilds. Phylogenetically-corrected mass and spatial factors such as geographic range size and number of sympatric bat species, were surprisingly not significant predictors of viral richness in our study, as they had been previously identified as an important variable in other species-level analyses of viral richness [11,18,58].
Dietary niche and feeding behaviors may contribute to a bat’s exposure to and spread of viral pathogens. Frugivorous bats have been known to transmit henipaviruses via their urine, feces, and saliva in “drip zones” around fruiting trees [27]. While these infected foraging areas have been important for Nipah virus spillover into pigs and Hendra virus spillover into horses, the role of contaminated food in viral transmission among bats is less clear. The environmental and agricultural benefits of frugivorous bats, conversely, are well documented. Frugivorous cave-roosting bats are instrumental in the formation, regeneration, and maintenance of tropical forests, due to their seed dispersal capabilities [59]. For instance, lesser dawn bats (Eonycteris spelaea), an obligate cave-roosting species, are the primary pollinators of durian and petai [60,61], which generate revenues in southern Thailand that exceed $137 million annually [62]. These positive ecological and economic benefits must be considered in any discussion regarding the ecosystem services (i.e., pollination, insect control) or disservices (i.e., as disease reservoirs) that cave-roosting bats provide.
4.3. Viral Sharing Among Bat Species
Viral sharing among bats is common: half of the bat-associated viruses in our database (52%) have infected multiple bat species, demonstrating evidence for viral host shifts among species. The most shared virus is, unsurprisingly, rabies—a mammalian generalist virus hosted and detected in 61 of the 205 (30%) bat species in our database. Out of the 15 viruses in our database with the greatest number of bat host species, only two have not yet been detected in human populations: Lagos bat virus (n = 6 bat species) and Miniopterus bat coronavirus 1 (n = 5 bat species). For most known human viruses, there is a research effort bias that has led to the identification of a broader range of host species. Rabies in particular has received targeted surveillance due to its long history as a severe, and often fatal, viral infection in humans and dogs. Additionally, several of the viruses that would be categorized as specialist viruses in our database (only viral nucleic acid detection found in one bat species) are actually hosted by species in other vertebrate taxa, including Aves (birds; e.g., West Nile and Newcastle disease viruses) or Primates (e.g., Bocavirus 1 and 2). The inter-order host range of these viruses may be a key factor in their ability to infect, and be pathogenic, in humans, but further research is needed.
We found that sharing of viruses is more likely between cave-roosting (vs. non-cave roosting) species, though the greatest predictor of viral sharing is degree of spatial overlap between a species-pair. Co-roosting, however, consistently was the greatest predictor of viral sharing for both our main and our stringent databases. For models in which we included co-roosting behavior, phylogenetic distance between bat hosts had a significantly positive effect (only for stringent viral sharing), and shared diet was significantly negative. These findings differ from previous studies in bats [28], and also trends found more broadly across mammals [11]. This may be due to co-roosting bat species often being from divergent taxonomic groups or dietary niches. Thus, ecological opportunity and direct contact, rather than shared life-history traits like diet and phylogeny, are key to viral sharing between species.
The barriers to viral sharing among species are poorly understood. While some viruses have been detected in bats of phylogenetically distinct clades [63], others groups of viruses are thought to have closely evolved with a single species. New evolutionary analyses of herpesviruses, however, have recently demonstrated that these historic lineages may be comprised of more frequent host-switching events than previously thought [64]. Also, while some viruses are hosted by multiple species, specific strains of a viral species may be constrained to bats of a single species or phylogenetically related hosts [28]. Viral sharing among a diverse range of host taxa, as we have identified here among divergent cave-sharing bat species, is a known driver of zoonotic spillover [11,65], potentially due to adaption to multiple host cell receptors [66].
4.4. Caveats and Future Directions
Further work to determine and understand the virome of bats and expand the list of known host-virus associations will continue to shed light on the geographic, functional, and temporal dynamics of viral infection across multiple bat species [67], as well as identify spatial gaps in viral surveillance. Standardized field surveillance in targeted sites are beginning to fill these gaps [23]. Our calculated spatial variables rely on an assumption that viral distribution extends across a species’ entire geographic range [11,68], although pathogen distribution is likely more constrained to certain bat populations or habitat fragments. Host traits not assessed in this study, but may contribute to viral richness, include level of migration, gregariousness, and cave type [14]. Caves can vary greatly in size and microclimatic profiles, which may influence viral persistence and viral sharing among bat species communities within certain cave environments. For our analyses, we used a categorical host trait to describe roosting behavior, either cave-roosting (obligate or facultative) and non-cave-roosting. Finer scale criteria of the permanence, temperature, or accessibility of roost site may be important for pathogen persistence in the environment [69]. Exclusion of these additional factors was largely due to incomplete and scattered literature that would have greatly reduced the number of host species with complete data. As virus discovery becomes less time and cost intensive [70,71], these host-virus associations need to be continuously compiled into interpretable database in conjunction with adequate host ecological and life-history traits.
As the viral communities of cave-roosting bats have only been partially characterized, much remains unknown about the risk of viral spillover from cave ecosystems. However, what is known is that the risk of pathogen spillover to humans from any wildlife species is driven by anthropogenic disturbance and increased contact rates, along with host-specific factors like phylogenetic relatedness to humans [11,72]. Tragically, cave-roosting bats are under pressure from a multitude of human threats at roosting sites, and this contact not only stresses the bats, but also introduces opportunity for zoonotic spillover. While rare, virus spillover from cave-roosting bats to humans in caves have occurred. For example, Marburg virus fatally spilled over from cave-roosting bats into workers at the Kitaka Mine and tourists at the Python Cave in Uganda [26,73].
Our analysis shows that cave-roosting behavior is an important driver of viral sharing in bats. This finding alone suggests that more in-depth and longitudinal surveillance efforts aimed at cave-roosting bat species will be valuable to better understand the ecological mechanisms that promote viral sharing. Caves are ideal sites for viral surveillance as compared to other field sites, due to consistent bat populations, predictable exiting times, and ample bat guano for non-invasive sampling. As viral surveillance in cave-roosting species expands in the future, we must be conscious to utilized non-lethal sampling methods and minimize habitat disturbance to these vulnerable and ecologically important species, while simultaneously finding ways to assess and mitigate the risk of zoonotic spillover.
Supplementary Materials
The following are available online at www.mdpi.com/1424-2818/9/3/35/s1, Item S1: Virus References, Item S2: Roosting Behavior References, Table S1: Bat-Virus Associations, Table S2: Host Traits, Table S3: Species-Pair Traits, Table S4: Viral Richness GLM summaries, Table S5: Viral Sharing GLM summaries.
Acknowledgments
This work was made possible by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of the PREDICT Consortium and do not necessarily reflect the views of USAID or the United States Government. The authors thank the PREDICT in-country teams and global team for their contribution of bat-virus associations to this database. The authors thank C.N. Basaraba and B. Watson for assistance with spatial analyses.
Author Contributions
Anna R. Willoughby, Kevin J. Olival and PREDICT Consortium designed the study and statistical approach. Anna R. Willoughby and Kevin J. Olival wrote the code. Kendra L. Phelps, PREDICT Consortium and Anna R. Willoughby collected the data. Anna R. Willoughby generated figures. Anna R. Willoughby, Kendra L. Phelps and Kevin J. Olival wrote the manuscript. Detail information about PREDICT Consortium can be found by the website: www.consortium.predict.global.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: New York, NY, USA, 2009; ISBN 978-0-1992-1993-3. [Google Scholar]
- Kunz, T.H. Roosting ecology of bats. In Ecology of Bats; Kunz, T.H., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1982; pp. 1–55. ISBN 978-1-4613-3423-1. [Google Scholar]
- Arita, H.T. Conservation biology of the cave bats of Mexico. J. Mammal. 1993, 74, 693–702. [Google Scholar] [CrossRef]
- Hutson, A.M.; Mickleburgh, S.P. Microchiropteran Bats: Global Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 2001; Volume 56, ISBN 2-8317-0595-9. [Google Scholar]
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. A review of the global conservation status of bats. Oryx 2002, 36, 18–34. [Google Scholar] [CrossRef]
- Furey, N.M.; Racey, P.A. Conservation ecology of cave bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C., Kingston, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 463–500. ISBN 978-3-319-25220-9. [Google Scholar]
- Brunet, A.K.; Medellín, R.A. The species–area relationship in bat assemblages of tropical caves. J. Mammal. 2001, 82, 1114–1122. [Google Scholar] [CrossRef]
- Phelps, K.; Jose, R.; Labonite, M.; Kingston, T. Correlates of cave-roosting bat diversity as an effective tool to identify priority caves. Biol. Conserv. 2016, 201, 201–209. [Google Scholar] [CrossRef]
- Rodríguez-Durán, A. Bat assemblages in the West Indies: The role of caves. In Island Bats: Evolution, Ecology and Conservation; Fleming, T.H., Racey, P.A., Eds.; University of Chicago: Chicago, IL, USA, 2009; pp. 265–280. ISBN 9780226253305. [Google Scholar]
- Luo, J.; Jiang, T.; Lu, G.; Wang, L.; Wang, J.; Feng, J. Bat conservation in China: Should protection of subterranean habitats be a priority? Oryx 2013, 47, 526–531. [Google Scholar] [CrossRef]
- Olival, K.J.; Hosseini, P.R.; Zambrana-Torrelio, C.; Ross, N.; Bogich, T.L.; Daszak, P. Host and viral traits predict zoonotic spillover from mammals. Nature 2017, 546, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Brook, C.E.; Dobson, A.P. Bats as ‘special’reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015, 23, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Walker, P.J.; Poon, L.L. Mass extinctions, biodiversity and mitochondrial function: Are bats ‘special’as reservoirs for emerging viruses? Curr. Opin. Virol. 2011, 1, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.D.; O’Shea, T.J.; Hayman, D.T.; Wood, J.L.; Cunningham, A.A.; Gilbert, A.T.; Mills, J.N.; Webb, C.T. Network analysis of host–virus communities in bats and rodents reveals determinants of cross-species transmission. Ecol. Lett. 2015, 18, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Turmelle, A.S.; Olival, K.J. Correlates of viral richness in bats (order Chiroptera). Ecohealth 2009, 6, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.; Olival, K.J.; Bumrungsri, S.; Siriaroonrat, B.; Bourgarel, M.; Morand, S. Parasite and viral species richness of Southeast Asian bats: Fragmentation of area distribution matters. Int. J. Parasitol. Parasites Wildl. 2014, 3, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Maganga, G.D.; Bourgarel, M.; Vallo, P.; Dallo, T.D.; Ngoagouni, C.; Drexler, J.F.; Drosten, C.; Nakouné, E.R.; Leroy, E.M.; Morand, S. Bat distribution size or shape as determinant of viral richness in African bats. PLoS ONE 2014, 9, e100172. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.D.; Hayman, D.T.S.; O’Shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.C.; Mills, J.N.; Timonin, M.E.; Willis, C.K.R.; Cunningham, A.A.; et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc. R. Soc. Lond. B Biol. Sci. 2013, 280, 20122753. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Li, J.-L.; Yang, X.-L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Towner, J.S.; Pourrut, X.; Albariño, C.G.; Nkogue, C.N.; Bird, B.H.; Grard, G.; Ksiazek, T.G.; Gonzalez, J.-P.; Nichol, S.T.; Leroy, E.M. Marburg virus infection detected in a common African bat. PLoS ONE 2007, 2, e764. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Zhang, Y.-Z.; Jiang, R.-D.; Guo, H.; Zhang, W.; Li, B.; Wang, N.; Wang, L.; Waruhiu, C.; Zhou, J.-H. Genetically diverse filoviruses in Rousettus and Eonycteris spp. bats, China, 2009 and 2015. Emerg. Infect. Dis. 2017, 23, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; Palacios, G.; Vázquez-Morón, S.; González, F.; Dopazo, H.; Molero, F.; Juste, J.; Quetglas, J.; Savji, N.; de la Cruz Martínez, M. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 2011, 7, e1002304. [Google Scholar] [CrossRef]
- Anthony, S.J.; Johnson, C.K.; Greig, D.J.; Kramer, S.; Che, X.; Wells, H.; Hicks, A.L.; Joly, D.O.; Wolfe, N.D.; Daszak, P. Global patterns in coronavirus diversity. Virus Evol. 2017, 3, vex012. [Google Scholar] [CrossRef] [PubMed]
- Altringham, J.D. Bats: From Evolution to Conservation; Oxford University Press: Chicago, IL, USA, 2011; ISBN 978-0-1992-0712-1. [Google Scholar]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Amman, B.R.; Carroll, S.A.; Reed, Z.D.; Sealy, T.K.; Balinandi, S.; Swanepoel, R.; Kemp, A.; Erickson, B.R.; Comer, J.A.; Campbell, S.; et al. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012, 8, e1002877. [Google Scholar] [CrossRef]
- Plowright, R.K.; Eby, P.; Hudson, P.J.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.A.; Martin, G. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. Lond. B Biol. Sci. 2015, 282, 20142124. [Google Scholar] [CrossRef]
- Streicker, D.G.; Turmelle, A.S.; Vonhof, M.J.; Kuzmin, I.V.; McCracken, G.F.; Rupprecht, C.E. Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science 2010, 329, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, R.; Halpin, K.; Hyatt, A.D.; Daszak, P.; Mungall, B.A. Henipavirus susceptibility to environmental variables. Virus Res. 2008, 132, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Peiris, J.; Lam, S.; Poon, L.; Yuen, K.; Seto, W. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.M.; Muller, L.K.; Russell, R.E.; O’Connor, M.; Lindner, D.L.; Blehert, D.S. Distribution and environmental persistence of the causative agent of white-nose syndrome, Geomyces destructans, in bat hibernacula of the eastern United States. Appl. Environ. Microbiol. 2013, 79, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Jurado, V.; Laiz, L.; Rodriguez-Nava, V.; Boiron, P.; Hermosin, B.; Sanchez-Moral, S.; Saiz-Jimenez, C. Pathogenic and opportunistic microorganisms in caves. Int. J. Speleol. 2010, 39, 2. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Sintunawa, C.; Kaewpom, T.; Khongnomnan, K.; Olival, K.J.; Epstein, J.H.; Rodpan, A.; Sangsri, P.; Intarut, N.; Chindamporn, A. Group C betacoronavirus in bat guano fertilizer, Thailand. Emerg. Infect. Dis. 2013, 19, 1349. [Google Scholar] [CrossRef] [PubMed]
- Seltmann, A.; Corman, V.M.; Rasche, A.; Drosten, C.; Czirják, G.Á.; Bernard, H.; Struebig, M.J.; Voigt, C.C. Seasonal fluctuations of astrovirus, but not coronavirus shedding in bats inhabiting human-modified tropical forests. Ecohealth 2017, 14, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Clements, R.; Sodhi, N.S.; Schilthuizen, M.; Ng, P.K. Limestone karsts of Southeast Asia: Imperiled arks of biodiversity. AIBS Bull. 2006, 56, 733–742. [Google Scholar] [CrossRef]
- Kingston, T. Research priorities for bat conservation in Southeast Asia: A consensus approach. Biodivers. Conserv. 2010, 19, 471–484. [Google Scholar] [CrossRef]
- Mildenstein, T.; Tanshi, I.; Racey, P.A. Exploitation of bats for bushmeat and medicine. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C., Kingston, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 325–375. ISBN 978-3-3192-5220-9. [Google Scholar]
- Mann, S.L.; Steidl, R.J.; Dalton, V.M. Effects of cave tours on breeding Myotis velifer. J. Wildl. Manag. 2002, 66, 618–624. [Google Scholar] [CrossRef]
- Bausch, D.G.; Borchert, M.; Grein, T.; Roth, C.; Swanepoel, R.; Libande, M.L.; Talarmin, A.; Bertherat, E.; Muyembe-Tamfum, J.J.; Tugume, B.; Colebunders, R. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 2003, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- Albarino, C.G.; Shoemaker, T.; Khristova, M.L.; Wamala, J.F.; Muyembe, J.J.; Balinandi, S.; Campbell, S.; Cannon, D.; Gibbons, A.; Bergeron, E. Genomic analysis of filoviruses associated with four viral hemorrhagic fever outbreaks in Uganda and the Democratic Republic of the Congo in 2012. Virology 2013, 442, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Timen, A.; Koopmans, M.P.; Vossen, A.C.; van Doornum, G.J.; Günther, S.; Van den Berkmortel, F.; Verduin, K.M.; Dittrich, S.; Emmerich, P.; Osterhaus, A.D.; et al. Response to imported case of Marburg hemorrhagic fever, the Netherlands. Emerg. Infect. Dis. 2009, 15, 1171. [Google Scholar] [CrossRef] [PubMed]
- ICTV. Master Species List 2016 v1.3. Available online: https://talk.ictvonline.org/files/master-species-lists/m/msl (accessed on 6 June 2017).
- IUCN. The IUCN Red List of Threatened Species Vol. 2017–1. Available online: http://www.iucnredlist.org/ (accessed on 1 June 2017).
- PREDICT Consortium. PREDICT Preliminary Test Results. Available online: http://data.predict.global/ (accessed on 1 June 2017).
- Jones, K.E.; Bielby, J.; Cardillo, M.; Fritz, S.A.; O’Dell, J.; Orme, C.D.L.; Safi, K.; Sechrest, W.; Boakes, E.H.; Carbone, C. PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 2009, 90, 2648. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Bini, L.M.; Rangel, T.F.; Morales-Castilla, I.; Olalla-Tárraga, M.Á.; Rodríguez, M.Á.; Hawkins, B.A. On the selection of phylogenetic eigenvectors for ecological analyses. Ecography 2012, 35, 239–249. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Sant’Ana, C.E.R.; Bini, L.M. An eigenvector method for estimating phylogenetic inertia. Evolution 1998, 52, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Bininda-Emonds, O.R.; Cardillo, M.; Jones, K.E.; MacPhee, R.D.; Beck, R.M.; Grenyer, R.; Price, S.A.; Vos, R.A.; Gittleman, J.L.; Purvis, A. The delayed rise of present-day mammals. Nature 2007, 446, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.A.; Bininda-Emonds, O.R.; Purvis, A. Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett. 2009, 12, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.B. Order Chiroptera. In Mammal Species of the World; Wilson, D.E., Reeder, D.M., Eds.; The John Hopkins Press: Baltimore, MD, USA, 2005; pp. 312–529. [Google Scholar]
- Gómez, J.M.; Nunn, C.L.; Verdú, M. Centrality in primate–parasite networks reveals the potential for the transmission of emerging infectious diseases to humans. Proc. Natl. Acad. Sci. USA 2013, 110, 7738–7741. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef]
- Halpin, K.; Young, P.; Field, H.; Mackenzie, J. Isolation of Hendra virus from Pteropid bats: A natural reservoir of Hendra virus. J. Gen. Virol. 2000, 81, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Broders, H.G.; Quinn, G.M.; Forbes, G.J. Species status, and the spatial and temporal patterns of activity of bats in southwest Nova Scotia, Canada. Northeast. Nat. (Steuben) 2003, 10, 383–398. [Google Scholar] [CrossRef]
- Kingston, T. Response of bat diversity to forest disturbance in Southeast Asia: Insights from long-term research in Malaysia. In Bat Evolution, Ecology, and Conservation; Adams, R.A., Pedersen, S.C., Eds.; Springer: New York, NY, USA, 2013; pp. 169–185. ISBN 978-1-4614-7397-8. [Google Scholar]
- Fenton, M.B.; Simmons, N.B. Bats: A World of Science and Mystery; University of Chicago Press: Chicago, IL, USA, 2015; ISBN 978-0-2260-6512-0. [Google Scholar]
- Schneeberger, K.; Czirják, G.Á.; Voigt, C.C. Measures of the constitutive immune system are linked to diet and roosting habits of neotropical bats. PLoS ONE 2013, 8, e54023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Kosoy, M.; Olival, K.J.; Dittmar, K. Horizontal transfers and gene losses in the phospholipid pathway of Bartonella reveal clues about early ecological niches. Genome Biol. Evol. 2014, 6, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Fleming, T.H. The role of frugivorous bats in tropical forest succession. Biol Rev Camb Philos. Soc. 2007, 82, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Bumrungsri, S.; Sripaoraya, E.; Chongsiri, T.; Sridith, K.; Racey, P.A. The pollination ecology of durian (Durio zibethinus, Bombacaceae) in southern Thailand. J. Trop. Ecol. 2009, 25, 85–92. [Google Scholar] [CrossRef]
- Bumrungsri, S.; Harbit, A.; Benzie, C.; Carmouche, K.; Sridith, K.; Racey, P. The pollination ecology of two species of parkia (Mimosaceae) in southern Thailand. J. Trop. Ecol. 2008, 24, 467–475. [Google Scholar] [CrossRef]
- Petchmunee, K. Economic Valuation and Learning Process Construction: A Case Study of the Cave Nectarivorous Bat (Eonycteris spelaea dobson). MSc Thesis (Environmental Science), Prince of Songkla University, Hat Yai, Thailand, 2008. (In Thai with English Abstract). [Google Scholar]
- Lau, S.K.; Li, K.S.; Tsang, A.K.; Shek, C.-T.; Wang, M.; Choi, G.K.; Guo, R.; Wong, B.H.; Poon, R.W.; Lam, C.S. Recent transmission of a novel alphacoronavirus, bat coronavirus HKU10, from Leschenault’s rousettes to pomona leaf-nosed bats: First evidence of interspecies transmission of coronavirus between bats of different suborders. J. Virol. 2012, 01305–01312. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Zamudio, M.; Rojas-Anaya, E.; Kolokotronis, S.-O.; Taboada, B.; Loza-Rubio, E.; Méndez-Ojeda, M.L.; Arias, C.F.; Osterrieder, N.; Greenwood, A.D. Bats, primates, and the evolutionary origins and diversification of mammalian gammaherpesviruses. MBio 2016, 7, e01425–e01416. [Google Scholar] [CrossRef] [PubMed]
- Kreuder Johnson, C.; Hitchens, P.L.; Smiley Evans, T.; Goldstein, T.; Thomas, K.; Clements, A.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; Karesh, W.B.; et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 2015, 5, 14830. [Google Scholar] [CrossRef] [PubMed]
- Longdon, B.; Brockhurst, M.A.; Russell, C.A.; Welch, J.J.; Jiggins, F.M. The evolution and genetics of virus host shifts. PLoS Pathog. 2014, 10, e1004395. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Krasnov, B.R.; Mouillot, D. Host specificity in phylogenetic and geographic space. Trends Parasitol. 2011, 27, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Brierley, L.; Vonhof, M.J.; Olival, K.J.; Daszak, P.; Jones, K.E. Quantifying global drivers of zoonotic bat viruses: A process-based perspective. Am. Nat. 2016, 187, E53–E64. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.D.; Dick, C.W.; Dittmar, K. Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). J. Trop. Ecol. 2007, 23, 177–189. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Yang, J.; Jin, Q. DBatVir: The database of bat-associated viruses. Database 2014, 2014, bau021. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; Ren, X.; He, G.; Zhang, J.; Yang, J.; Qian, Z.; Dong, J.; Sun, L.; Zhu, Y. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 2016, 10, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Towner, J.S.; Amman, B.R.; Sealy, T.K.; Carroll, S.A.R.; Comer, J.A.; Kemp, A.; Swanepoel, R.; Paddock, C.D.; Balinandi, S.; Khristova, M.L. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009, 5, e1000536. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).