The Significance of New Records of Benthic Red Algae (Rhodophyta) for Hainan Island (and China) between 1990 and 2016
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Times of Sampling in 1990 and 1992
2.2. Study Sites and Times of Sampling during 2008–2016
2.3. Collection, Conservation, and Floristic Analysis of Samples
3. Results
Floristic Analysis of New Findings of Red Algae in Hainan Island in 1990/1992 and during the Period 2008–2016
4. Discussion
- (1)
- The over-exploitation of the reef ecosystems, especially of algal grazing species of fish and invertebrates, developing from the 1950s–1970s [19].
- (2)
- The eutrophication of shallow waters (bays) in the 1990s–2000s, occurring as a result most obviously of sewage disposal associated with coastal urbanization linked to the increase in tourism, but also with the growth in agriculture and mariculture [13]. This conclusion is supported by the fact that the largest numbers of new finds of red algae in 2008–2016 were from the most polluted areas of the coast, from such localities as Sanya Bay and Ying Ge Hai.
- (3)
- The consequences of wider climate and environmental change, in particular the mass mortality of corals after bleaching such as occurred in the South China Sea in the summer of 1998 [17].
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, D.; Gagan, M.K.; Cheng, H.; Scott-Gagan, H.; Dykoski, C.A.; Edwards, R.L.; Su, R. Seasonal and interannual variability of the Mid-Holocene East Asian monsoon in coral δ18O records from the South China Sea. Earth Planet. Sci. Let. 2005, 237, 69–84. [Google Scholar] [CrossRef]
- Levitus, S.; Boyer, T. World Ocean Atlas 1994, vol. 4, Temperature, NOAA Atlas NESDIS 4; U.S. Government Printing Office: Washington, DC, USA, 1994; p. 117.
- Zhang, Q.; Xu, X.Z.; Long, X.M. A numerical study on internal tides in the northeast of the South China Sea. J. Trop. Oceanol. 1996, 14, 15–23. (In Chinese) [Google Scholar]
- Zhang, Q.; Shi, Q.; Chen, G.; Fong, T.C.; Wong, D.C.; Huang, H.; Wang, H.; Zhao, M. Status monitoring and health assessment of Luhuitou fringing reef of Sanya, Hainan, China. Chin. Sci. Bull. 2006, 51, 81–88. [Google Scholar] [CrossRef]
- Gurianova, E.F. Marine zoological expedition to Hainan Island. Vestnik AN SSSR 1959, 3, 89–92. (In Russian) [Google Scholar]
- Fiege, D.; Neumann, V.; Jinhe, L. Observations on coral reefs of Hainan Island, South China Sea. Mar. Pollut. Bull. 1994, 29, 84–89. [Google Scholar] [CrossRef]
- Hodgson, G.; Yau, E.P.M. Physical and biological controls of coral communities in Hong Kong. Proceedings of 8th International Coral Reef Symposium, Balboa, Panama, 24–29 June 1996; Smithsonian Tropical Research Institute: Gamnoa, Panama, 1997; pp. 459–464. [Google Scholar]
- Huang, L.M.; Tan, Y.H.; Song, X.Y.; Huang, X.P.; Wang, H.K.; Zhang, S.; Dong, J.D.; Chen, R.Y. The status of the ecological environment and a proposed protection strategy in Sanya Bay, Hainan Island, China. Mar. Pollut. Bull. 2003, 47, 180–186. [Google Scholar] [CrossRef]
- Zhang, G.; Que, H.; Liu, X.; Xu, H. Abalone mariculture in China. J. Shellfish Res. 2004, 23, 947–950. [Google Scholar]
- Tadashi, K.; Dai, C.F.; Park, H.S.; Huang, H.; Ang, P.O. Status of coral reefs in East and North Asia (China, Hong Kong, Taiwan, South Korea and Japan). In Status of coral reefs of the world: 2008; Wilkinson, C., Ed.; Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre: Townsville, Australia, 2008; pp. 145–158. [Google Scholar]
- Zhao, M.X.; Yu, K.F.; Zhang, Q.M.; Shi, Q. Spatial pattern of coral diversity in Luhuitou fringing reef, Sanya, China. Acta Ecol. Sin. 2008, 28, 1419–1428. [Google Scholar]
- Tseng, C.K. The past, present and future of phycology in China. Hydrobiologia 2004, 512, 11–20. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Xia, B.M.; Bartsch, I. Checklist of marine benthic green algae (Chlorophyta) on Hainan, a subtropical island off the coast of China: comparisons between the 1930s and 1990–2009 reveal environmental changes. Bot. Mar. 2011, 54, 523–535. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Huang, H.; Li, X.B. Seasonal changes in benthic algal communities of the upper subtidal zone in Sanya Bay (Hainan Island, China). J. Mar. Biol. Assoc. UK 2014, 91, 51–64. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Li, X.B.; Hansen, G.; Huang, H. Seasonal changes in the intertidal algal communities of Sanya Bay (Hainan Island, China). J. Mar. Biol. Assoc. UK 2014, 94, 879–893. [Google Scholar] [CrossRef]
- Titlyanova, T.; Titlyanov, E.; Xia, B.; Bartsch, I. New records of benthic marine green algae on Hainan Island, China. Nova Hedwigia 2012, 94, 441–470. [Google Scholar] [CrossRef] [PubMed]
- Titlyanova, T.V.; Titlyanov, E.A.; Kalita, T.L. Marine algal flora of Hainan Island: a comprehensive synthesis. Coastal Ecosystems 2014, 1, 28–53. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Xia, B.M.; Bartsch, I. Retrospective analysis of diversity and species composition of marine macroalgae of hainan island (China). Ocean Sci. J. 2016, 51, 1–22. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Belous, O.S.; Kalita, T.L. Inventory change (1990s–2010s) in the marine flora of Sanya Bay (Hainan Island, China). J. Mar. Biol. Assoc. UK 2015, 95, 461–470. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Li, X.B.; Kalita, T.L.; Huang, H. Recent (2008–2012) seaweed flora of Hainan Island, South China Sea. Mar. Biol. Res. 2015, 11, 540–550. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. World-wide electronic publication, National University of Ireland, Galway. Available online: http:// www.algaebase.org/ (accessed on 22 May 2017).
- Saunders, G.W.; Hommersand, M.H. Assessing red algal supraordinal diversity and taxonomy in the context of contemporary systematic data. Am. J. Bot. 2004, 91, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Catalogue of Life: 2010 Annual Checklist. Available online: http://www.catalogueoflife.org/annual-checklist/2010 (accessed on 15 February 2017).
- Liu, R. Checklist of marine biota of China Seas; China Scientific Book Service Co Ltd: Beijing, China, 2008; p. 1267. [Google Scholar]
 )—collection sites of two German-Chinese expeditions during October–December 1990 and March–April 1992; (+)—collection sites of Titlyanova, Titlyanov, and Li between 2008 and 2016.
)—collection sites of two German-Chinese expeditions during October–December 1990 and March–April 1992; (+)—collection sites of Titlyanova, Titlyanov, and Li between 2008 and 2016.
 )—collection sites of two German-Chinese expeditions during October–December 1990 and March–April 1992; (+)—collection sites of Titlyanova, Titlyanov, and Li between 2008 and 2016.
)—collection sites of two German-Chinese expeditions during October–December 1990 and March–April 1992; (+)—collection sites of Titlyanova, Titlyanov, and Li between 2008 and 2016.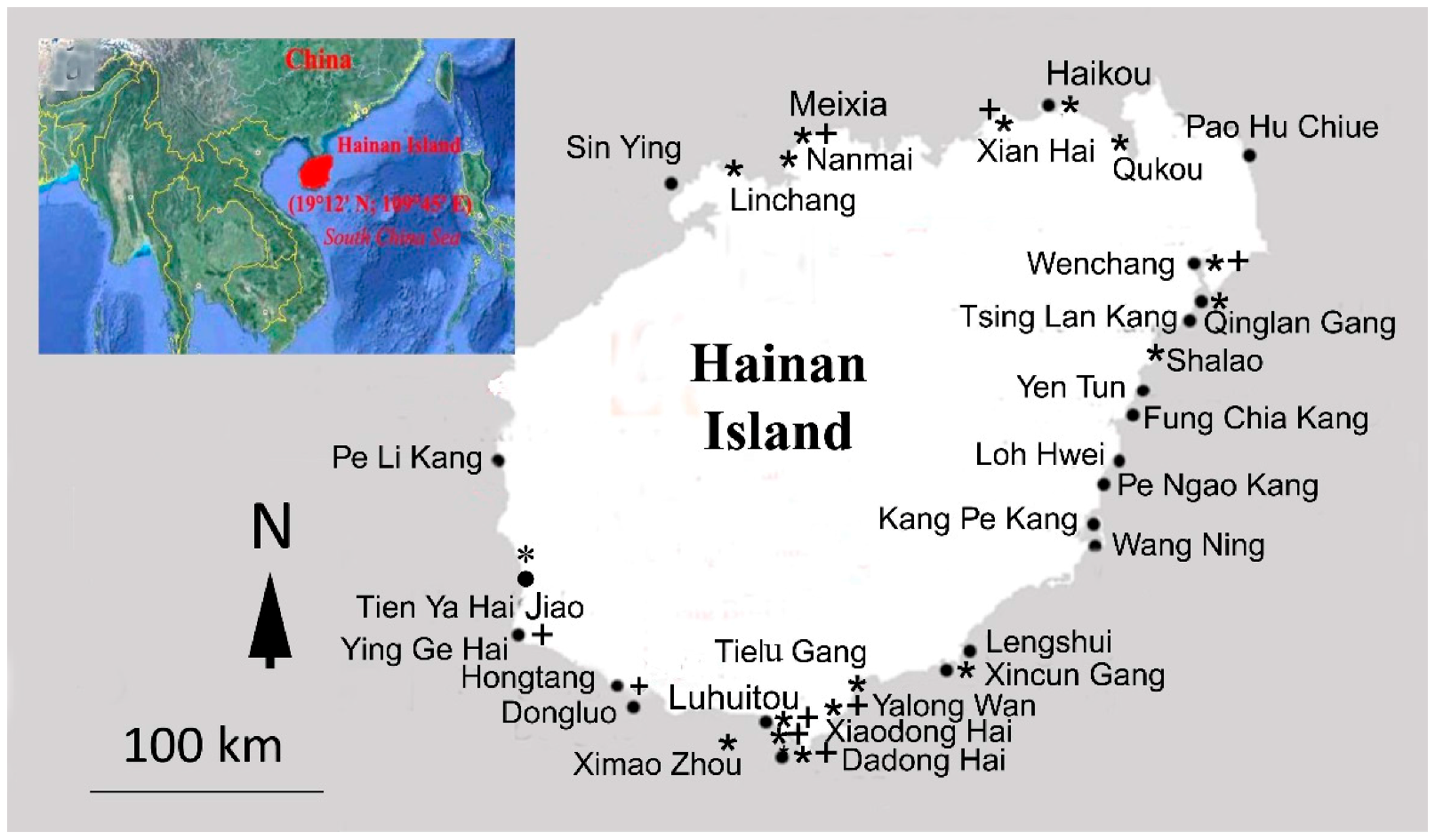






| List of Species, Varieties and Forms | Sr | Lf | Dg | Sampling Times and Locations of Finds |
|---|---|---|---|---|
| DIVISION RHODOPHYTA | ||||
| CLASS STYLONEMATOPHYCEAE | ||||
| ORDER STYLONEMATALES | ||||
| Family Stylonemataceae | ||||
| Bangiopsis dumontioides (P. Crouan & H. Crouan) V. Krishnmurthy | ♥♥ | Ep | T,S | (♥) Xh |
| Chroodactylon ornatum (C. Agardh) Basson | ♣ | Ep | T,S,M | (♣)Dh, Lc, Lh, Ty, Yw, Xh/(♥) Lh, Xh |
| Stylonema alsidii (Zanardini) K.M. Drew | ♣ | Ep | T,S,M | (♣)Dh, Lc, Lh, Mx, Nm, Qk, Sl, Ty, Yw, Xh, Xz/(♥)Dh, Mx, Lh, Wc, Yg, Yw, Xh |
| CLASS COMSPOGONOPHYCEAE | ||||
| ORDER ERYTHROPELTALES | ||||
| Family Erythrotrichiaceae | ||||
| Erythrotrichia carnea (Dillwyn) J. Agardh | ♣ | Ep | T,S,M,Ar,An | (♣)Dh, Lc, Lh, Nm, Qk, Sl, Ty, Yw, Xc, Xh, Xn, Xz/(♥)Dh, Lh, Mx, Wc, Yg, Yw, Xh |
| Erythrocladia irregularis Rosenvinge | ♣ | Ep | T,S,M,Ar,An | (♣)Ty, Xh/(♥)Lh, Wc, Yw, Xn |
| Porphyrostromium japonicum (Tokida) Kikuchi | ♣♣ | Ep | T,S,(I-P) | ♣Qk |
| Sahlingia subintegra (Rosenvinge) Kornmann | ♣ | Ep | T,S,M | (♣)Sl, Ty, Xc, Xh, /(♥)Lh, Mx, Wc, Yg, Yw, Xn |
| CLASS FLORIDEOPHYCEAE | ||||
| ORDER HILDENBRANDIALES | ||||
| Family Hildenbrandiaceae | ||||
| Hildenbrandia rubra (Sommerfelt) Meneghini | ♥ | HS, Cr | T,S,M,Ar, An | (♥)Dh, Lh, Mx, Xh |
| ORDER NEMALIALES | ||||
| Family Galaxauraceae | ||||
| Dichotomaria falcata (Kjellman) Kurihara & Masuda | ♣♣ | HS | T,S,(I-P) | (♣)Xz |
| Tricleocarpa cylindrica (J. Ellis & Solander) Huisman & Borowitzka | ♥ | HS | T,S | (♥)Lh, Xh |
| ORDER ACROCHAETIALES | ||||
| Family Acrochaetiaceae | ||||
| Acrochaetium catenulatum M. Howe | ♣♣ | Ep | T,S,M | (♣)Xh |
| Acrochaetium chaetomorphae (Tanaka & Pham-Hoàng Hô) Heerebout | ♥♥ | Ep | T,S,(I-P) | (♥)Dh, Lh |
| Acrochaetium secundatum (Lyngbye) Nägeli | ♣ | Ep | T,S,M | (♣)Nm, Yw/(♥)Lh, Yw |
| Acrochaetium subseriatum Børgesen | ♣♣ | Ep | T,S,(I-P) | (♣)Xh |
| ORDER COLACONEMATALES | ||||
| Family Colaconemataceae | ||||
| Colaconema bonnemaisoniae Batters | ♣ | Ep | T,S,M | (♣)Xh |
| Colaconema daviesii (Dillwyn) Stegenga | ♣ | Ep | T,S,M,Ar, An | (♣)Xh/(♥)Lh |
| ORDER CORALLINALES | ||||
| Family Corallinaceae | ||||
| Amphiroa foliacea J.V. Lamouroux | ♣ | HS | T,S,(I-P) | (♣)Lh, Sl/(♥)Dh, Lh, Wc, Xh |
| Hydrolithon boreale (Foslie) Y.M. Chamberlain | ♣ | Ep | T,S,M | (♣)Sl/(♥)Lh, Yw |
| Hydrolithon farinosum (J.V. Lamouroux) Penrose & Y.M. Chamberlain | ♣ | Ep | T,S,M | (♣)Dh, Lh, Qg, Qk, Sl, Ty, Yw Xh, Xz/(♥)Lh, Wc, Yw, Xh |
| Jania capillacea Harvey | ♣ | HS | T,S,(I-P) | (♣)Dh, Lh, Mx, Nm, Sl, Ty, Yw, |
| Xh, Xz, /(♥) Lh, Mx, Yg, Yw, Xh | ||||
| Jania longiarthra E.Y. Dawson | ♥ | HS | T,S | (♥)Xn |
| Jania pumila J.V. Lamouroux | ♣ | HS | T,S | (♣)Dh, Xc, Xh, Xz/(♥)Lh, Yw |
| Jania ungulata f. brevior (Yendo) Yendo | ♣ | HS | T,S,(I-P) | (♣)Lh, Xh/(♥)Dh, Wc, Yw, Xh |
| Lithophyllum tumidulum Foslie | ♥ | HS, Cr | T,S,(I-P) | (♥)Lh, Wc |
| Neogoniolithon megalocystum (Foslie) | ♥ | HS, Cr | T,S,M,(I-P) | (♥)Lh |
| Setchell & L.R. Mason | ||||
| Pneophyllum confervicola (Kützing) Y.M. Chamberlain | ♥♥ | HS, Cr | T,S,M | *Lh, Wc |
| Pneophyllum fragile Kützing | ♣ | HS, Cr | T,S,M | (♣)Dh, Lh, Mx, Nm, Sl, Ty, Yw, Xh, Xz/(♥)Lh, Mx, Wc, Yw |
| Family Lithothamniaceae | ||||
| Lithothamnion intermedium Kjellman | ♥ | HS, Cr | T,S,M | (♥)Yw |
| Lithothamnion phymatodeum Foslie | ♥ | HS, Cr | T,S,M,(I-P) | (♥)Lh |
| ORDER CERAMIALES | ||||
| Family Ceramiaceae | ||||
| Antithamnion antillanum Børgesen | ♣ | Ep | T,S | (♣)Sl/(♥)Dh, Lh |
| Antithamnionella breviramosa (E.Y. Dawson) Wollaston | ♥ | Fo | T,S | (♥) Xh |
| Antithamnionella elegans (Berthold) J.H. Price & D.M. John | ♥ | Ep | T,S,M | (♥)Lh, Yw, Xn |
| Antithamnionella spirographidis (Schiffner) E.M. Wollaston | ♣ | Ep | T,S,M | (♣)Sl |
| Centroceras japonicum Itono | ♥ | Ep | T,S,(I-P) | (♥)Lh, Yw |
| Centroceras minutum Yamada | ♣ | Ep | T,S,(I-P) | (♣)Sl, Xh, Xz/(♥)Lh |
| Ceramium aduncum Nakamura | ♣ | Ep | T,S,(I-P) | (♣)Nm, Xh/(♥)Lh |
| Ceramium borneense Weber-van Bosse | ♣ | Ep | T,S,(I-P) | (♣)Xh/(♥)Lh, Yw, Xh |
| Ceramium camouii E.Y. Dawson | ♥ | Ep, HS | T,S,(I-P) | (♥)Lh, Xh |
| Ceramium cimbricum H.E. Petersen | ♣ | Ep | T,S,M | (♣)Xh/(♥)Lh, Wc, Yg, Xh, Xn |
| Ceramium cingulatum Weber-van Bosse | ♣♣ | Ep | T,S | (♣)Dh, Lc, Lh, Nm, Sl, Ty, Yw, Xh, Xz, /(♥)Lh, Yg, Yw |
| Ceramium codii (H. Richards) Mazoyer | ♥♥ | Ep | T,S | (♥)Lh |
| Ceramium comptum Børgesen | ♥ | Ep | T,S | *Lh |
| Ceramium macilentum J. Agardh | ♣ | Ep | T,S,(I-P) | (♣)Sl, Xh, Xz/(♥)Lh, Xh, Xn |
| Ceramium marshallense E.Y. Dawson | ♣♣ | Ep, HS | T,S,(I-P) | (♣)Xh, Xz/(♥)Lh, Yg, Yw, Xh |
| Ceramium procumbens Setchell & N.L. Gardner | ♥♥ | Ep | T,S,(I- P) | (♥)Lh, Xh |
| Ceramium serpens Setchell & N.L. Gardner | ♥ | Ep | T,S | (♥)Xn |
| Ceramium tenerrimum (G. Martens) Okamura | ♣ | Ep | T,S | |
| Ceramium vagans P.C. Silva | ♣ | Ep | T,S | |
| Corallophila kleiwegii Weber-van Bosse | ♣ | Ep, HS | T,S,(I-P) | |
| Gayliella fimbriata (Setchell & N.L. Gardner) T.O. Cho & S.M. Boo | ♥ | Ep, | T,S,(I-P) | |
| Gayliella mazoyerae T.O. Cho, Fredericq & Hommersand | ♣ | Ep, HS | T,S | |
| Gayliella taylorii (E.Y. Dawson) T.O. Cho & S.M. Boo | ♥ | Ep | T,S | |
| Aglaothamnion cordatum (Børgesen) Feldmann-Mazoyer | ♥♥ | Ep | T,S | |
| Crouania attenuata (C. Agardh) J. Agardh | ♣ | Ep | T,S,M | |
| Family Delesseriaceae | ||||
| Taenioma perpusillum (J. Agardh) J. Agardh | ♣ | Ep, HS | T,S | |
| Family Rhodomelaceae | ||||
| Acanthophora aokii Okamura | ♥ | HS | T,S,(I-P) | |
| Bryocladia cervicornis (Kützing) F. Schmitz | ♥♥ | Ep | T,S,(I- P) | |
| Chondria dangeardii E.Y. Dawson | ♥♥ | Ep | T,S | |
| Chondria minutula Weber-van Bosse | ♥♥ | Ep | T,S,(I- P) | |
| Chondria pygmaea Garbary & Vandermeulen | ♥♥ | Ep | T,S | |
| Chondria repens Børgesen | ♣ | Ep | T,S,(I-P) | |
| Chondrophycus articulatus (C.K. Tseng) K.W. Nam | ♥ | HS | T,S,(I-P) | |
| Herposiphonia insidiosa (Greville ex J. Agardh) Falkenberg | ♥ | HS | T,S,(I-P) | |
| Herposiphonia parca Setchell | ♣ | Ep | T,S,(I-P) | |
| Herposiphonia secunda (C. Agardh) Ambronn | ♣ | Ep | T,S | |
| Herposiphonia secunda f. tenella (C. Agardh) M.J. Wynne | ♣♣ | HS | T,S | |
| Laurencia decumbens Kützing | ♥ | HS | T,S | |
| Laurencia pinnata Yamada | ♥ | HS | T,S,(I-P) | |
| Laurencia silvae J.F. Zhang & B.M. Xia | ♥ | Ep | T,S,(I- P) | |
| Lophosiphonia cristata Falkenberg | ♥♥ | Ep | T,S | |
| Melanothamnus ferulaceus (Suhr ex J. Agardh) Díaz-Tapia & Maggs | ♣ | Ep | T,S | |
| Melanothamnus pseudovillum (Hollenberg) Díaz-Tapia & Maggs | ♥♥ | Ep | T,S | |
| Melanothamnus savatieri (Hariot) Díaz-Tapia & Maggs | ♥♥ | Ep | T,S | |
| Palisada concreta (A.B. Cribb) K.W. Nam | ♥ | HS | T,S,(I-P) | |
| Palisada intermedia (Yamada) K.W. Nam | ♥ | HS | T,S | |
| Palisada parvipapillata (C.K. Tseng) K.W. Nam | ♣ | HS | T,S,(I-P) | |
| Palisada papillosa (C. Agardh) K.W. Nam | ♣ | HS | T,S,(I-P) | |
| Pleonosporium borreri (Smith) Nägeli | ♥♥ | Ep | T,S,M | |
| Polysiphonia exilis Harvey | ♥♥ | Ep | T,S | |
| Polysiphonia scopulorum Harvey | ♣ | Ep | T,S | |
| Polysiphonia scopulorum var. villum (J. Agardh) Hollenberg | ♣♣ | Ep | T,S | |
| Polysiphonia subtilissima Montagne | ♣ | Ep | T,S | |
| Tolypiocladia condensata (Weber-van Bosse) P.C. Silva | ♣♣ | HS | T,S,(I-P) | |
| Tolypiocladia glomerulata (C. Agardh) F. Schmitz | ♣ | HS, Ep | T,S,(I-P) | |
| Vertebrata reptabunda (Suhr) Diaz-Tapia & Maggs | ♣ | Ep | T,S | |
| Family Wrangeliaceae | ||||
| Gordoniella yonakuniensis (Yamada & T. Tanaka) Itono | ♣ | Ep | T,S,(I-P) | |
| Wrangelia argus (Montagne) Montagne | ♣ | HS, Ep | T,S | |
| Family Dasyaceae | ||||
| Heterosiphonia crispella (C. Agardh) M.J. Wynne | ♥♥ | Ep | T,S | |
| ORDER GELIDIALES | ||||
| Family Gelidiaceae | ||||
| Gelidium crinale (Hare ex Turner) Gaillon | ♣ | HS | T,S,M,An | |
| Gelidiophycus divaricatus (G. Martens) G.H. Boo, J.K. Park & S.M. Boo | ♣ | HS | T,S,(I-P) | (♣)Mx, Sl/(♥)Lh, Wc |
| Family Gelidiellaceae | ||||
| Gelidiella lubrica (Kützing) Feldmann & G. Hamel | ♣♣ | Ep | T,S | (♣)Xh |
| Parviphycus adnatus (E.Y. Dawson) B. Santelices | ♣♣ | Ep | T,S | (♣)Lh, Sl, Xh/(♥)Lh |
| Parviphycus pannosus (Feldmann) G. Furnari | ♣♣ | Ep | T,S | ♣Lh, Xh, Xz/*Dh, Lh, Wc, Yw, Xh |
| Family Pterocladiaceae | ||||
| Pterocladiella capillacea (S.G. Gmelin) Santelices & Hommersand | ♣ | HS | T,S,M | (♣)Xh/(♥)Lh, Xn |
| ORDER GIGARTINALES | ||||
| Family Cystocloniaceae | ||||
| Hypnea cenomyce J. Agardh | ♥ | HS | T,S | (♥)Lh, Mx, Yw |
| Hypnea chordacea Kützing | ♥ | HS | T,S,(I-P) | (♥)Yg |
| Hypnea esperi Bory | ♥ | HS | T,S,M,An | (♥)Lh, Yw |
| Hypnea musciformis var. esperi J. Agardh | ♣ | Ep | T,S,(I-P) | (♣)Dh, Lc, Nm, Xh |
| Hypnea nidulans Setchell | ♥ | HS | T,S,(I-P) | (♥)Lh |
| Hypnea valentiae (Turner) Montagne | ♥♥ | HS | T,S | (♥)Lh, Mx, Yg, Xh, Xn |
| Family Gigartinaceae | ||||
| Chondracanthus intermedius (Suringar) Hommersand | ♥ | HS, Cr | T,S,(I-P) | (♥)Mx, Xn |
| Chondracanthus tenellus (Harvey) Hommersand | ♥ | HS | T,S,(I-P) | (♥)Xn |
| Family Rhizophyllidaceae | ||||
| Portieria hornemannii (Lyngbye) P.C. Silva | ♣ | HS | T,S,(I-P) | (♣)Sl |
| ORDER PEYSSONNELIALES | ||||
| Family Peyssonneliaceae | ||||
| Peyssonnelia boergesenii Weber-van Bosse | ♥♥ | HS, Cr | T,S | (♥)Lh, Yw |
| Peyssonnelia inamoena Pilger | ♥♥ | HS, Cr | T,S | (♥)Lh, Yw |
| Peyssonnelia rubra (Greville) J. Agardh | ♣♣ | HS, Cr | T,S | (♣)Sl/(♥) Mx, Wc, Yw, Lh |
| ORDER GRACILARIALES | ||||
| Family Gracilariaceae | ||||
| Gracilaria changii (B.M. Xia & I.A. Abbott) I.A. Abbott, J. Zhang & B.M. Xia | ♣ | HS | T,S,(I-P) | (♣)Nm |
| Gracilaria coronopifolia J. Agardh | ♣ | HS | T,S,(I-P) | (♣)Xn/(♥)Lh, Xn |
| Gracilaria textorii (Suringar) De Toni | ♥ | HS | T,S | (♥)Xn |
| ORDER HALYMENIALES | ||||
| Family Halymeniaceae | ||||
| Grateloupia asiatica S. Kawaguchi & H.W. Wang | ♥ | HS | T,S | (♥)Yg, Xn |
| Grateloupia carnosa Yamada & Segawa | ♥ | HS | T,S,(I-P) | (♥)Yg |
| Grateloupia filicina (J.V. Lamouroux) C. Agardh | ♥ | HS | T,S,M,An | (♥)Lh, Yg, Xn |
| ORDER RHODYMENIALES | ||||
| Family Champiaceae | ||||
| Champia parvula (C. Agardh) Harvey | ♣ | HS | T,S,M | (♣)Yw, Xh, Xz/(♥)Dh, Lh, Yw, Xh |
| Champia vieillardii Kützing | ♣♣ | HS | T,S | (♣)Lh/(♥)Dh, Lh, Xh |
| Family Lomentariaceae | ||||
| Ceratodictyon scoparium (Montagne & Millardet) R.E. Norris | ♥♥ | HS | T,S | (♥)Dh, Lh, Wc, Xh |
| Lomentaria corallicola Børgesen | ♣♣ | HS | T,S | (♣)Sl, Xh/(♥)Lh, Dh |
| Family Rhodymeniaceae | ||||
| Rhodymenia intricata (Okamura) Okamura | ♥ | HS | T,S,(I-P) | (♥)Xn |
| Family Hymenocladiaceae | ||||
| Asteromenia anastomosans (Weber-van Bosse) G.W. Saunders, C.E. Lane, C.W. Schneider & Kraft | ♣ | HS | T,S | (♣)Qg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titlyanova, T.V.; Titlyanov, E.A.; Li, X.; Bartsch, I.; Xia, B. The Significance of New Records of Benthic Red Algae (Rhodophyta) for Hainan Island (and China) between 1990 and 2016. Diversity 2017, 9, 24. https://doi.org/10.3390/d9020024
Titlyanova TV, Titlyanov EA, Li X, Bartsch I, Xia B. The Significance of New Records of Benthic Red Algae (Rhodophyta) for Hainan Island (and China) between 1990 and 2016. Diversity. 2017; 9(2):24. https://doi.org/10.3390/d9020024
Chicago/Turabian StyleTitlyanova, Tamara V., Eduard A. Titlyanov, Xiubao Li, Inka Bartsch, and Bangmei Xia. 2017. "The Significance of New Records of Benthic Red Algae (Rhodophyta) for Hainan Island (and China) between 1990 and 2016" Diversity 9, no. 2: 24. https://doi.org/10.3390/d9020024
APA StyleTitlyanova, T. V., Titlyanov, E. A., Li, X., Bartsch, I., & Xia, B. (2017). The Significance of New Records of Benthic Red Algae (Rhodophyta) for Hainan Island (and China) between 1990 and 2016. Diversity, 9(2), 24. https://doi.org/10.3390/d9020024





