Abstract
Predatory release has long been considered a potential contributor to population outbreaks of crown-of-thorns starfish (CoTS; Acanthaster spp.). This has initiated extensive searches for potentially important predators that can consume large numbers of CoTS at high rates, which are also vulnerable to over-fishing or reef degradation. Herein, we review reported predators of CoTS and assess the potential for these organisms to exert significant mortality, and thereby prevent and/or moderate CoTS outbreaks. In all, 80 species of coral reef organisms (including fishes, and motile and sessile invertebrates) are reported to predate on CoTS gametes (three species), larvae (17 species), juveniles (15 species), adults (18 species) and/or opportunistically feed on injured (10 species) or moribund (42 species) individuals within reef habitats. It is clear however, that predation on early life-history stages has been understudied, and there are likely to be many more species of reef fishes and/or sessile invertebrates that readily consume CoTS gametes and/or larvae. Given the number and diversity of coral reef species that consume Acanthaster spp., most of which (e.g., Arothron pufferfishes) are not explicitly targeted by reef-based fisheries, links between overfishing and CoTS outbreaks remain equivocal. There is also no single species that appears to have a disproportionate role in regulating CoTS populations. Rather, the collective consumption of CoTS by multiple different species and at different life-history stages is likely to suppress the local abundance of CoTS, and thereby mediate the severity of outbreaks. It is possible therefore, that general degradation of reef ecosystems and corresponding declines in biodiversity and productivity, may contribute to increasing incidence or severity of outbreaks of Acanthaster spp. However, it seems unlikely that predatory release in and of itself could account for initial onset of CoTS outbreaks. In conclusion, reducing anthropogenic stressors that reduce the abundance and/or diversity of potential predatory species represents a “no regrets” management strategy, but will need to be used in conjunction with other management strategies to prevent, or reduce the occurrence, of CoTS outbreaks.
1. Introduction
Adult crown-of-thorns starfish (CoTS; Acanthaster sp.) have numerous long, very sharp and toxic spines (Figure 1). In addition, the dermal tissues of CoTS (and all of their organs) contain high concentrations of chemicals, including saponins [1,2] and plancitoxins [3], which are both unpalatable [4] and highly toxic [5,6,7]. Intuitively therefore, one might expect that these starfish are effectively protected and largely immune from predation (e.g., [8]). In reality, there are few organisms that are completely immune to predation at any or all stages of their life cycle. Rather, well-developed anti-predatory defences reduce the range of predators to which prey species are vulnerable [9], but may or may not affect overall rates of predation and the extent to which prey populations are controlled by predators. Accordingly, there is an increasing number of coral reef organisms (fishes and invertebrates) reported to predate on CoTS [10,11], including some predators (e.g., Arothron pufferfishes) that feed almost exclusively on adult CoTS when they are in abundant supply (e.g., during outbreaks). Such predators may be important in supressing the abundance of prey species [12] as well as influencing the behaviour, habitat-associations, and population dynamics of even well-armoured and/or chemically defended prey species (e.g., [13]).

Figure 1.
Adult crown-of-thorns starfish are defended against predators by numerous long, very sharp and toxic spines. Photographic credit: Scott Ling, Dick’s Reef, Swains Region, southern Great Barrier Reef (22°18′ S, 152°39′ E).
Despite their physical and chemical defences, post-settlement stages (juvenile and adults) of CoTS often exhibit injuries, largely manifested as missing arms [11,14,15]. These injuries are believed to occur when predators are only able to remove one or a few arms before the starfish escapes or avoids further damage by hiding within the reef matrix [15]. If however, there are high rates of partial predation at specific reef locations [11] then it is expected at least some CoTS will also be killed outright and/or consumed in entirety. The cryptic nature and nocturnal behaviour of CoTS, especially when small (<12 cm diameter) or at low densities [11,16] further suggests that they must be highly vulnerable to predators. In controlled experiments, survivorship of laboratory reared Acanthaster spp. settled to natural substrates is effectively zero, owing to very high rates of predation by naturally occurring predators [17,18,19]. Recent research also demonstrates that CoTS larvae are highly vulnerable to predation [20], despite having the highest concentrations of anti-predator chemicals (discussed later). Cowan et al. [20] showed that CoTS larvae are readily consumed by many common planktivorous damselfishes, and often in preference to other asteroid larvae.
While there is now general acceptance that CoTS are vulnerable to predation (e.g., [11,21]), on-going controversies relate to whether known predators would ever be capable of regulating CoTS populations, and mitigating, if not preventing outbreaks. More specifically, attention is focussed on whether anthropogenic impacts (via fishing or habitat degradation) have supressed the abundance of key predators, thereby accounting for the seemingly recent and/or increasing occurrence of CoTS outbreaks [10].
1.1. The Predator Removal Hypothesis
The predator removal hypothesis was one of the first hypotheses proposed to account for CoTS outbreaks [22]. Following trophic-cascade concepts as a result of ecological extinction of functional echinoderm predators such as sea otters (e.g., [23,24,25]), lobsters [26] and large benthic predatory fishes [27], this hypothesis (like many other hypotheses put forward in the 1960s and 1970s, such as the nutrient enrichment hypothesis, e.g., [28,29,30]) is predicated on the idea that CoTS outbreaks are an unnatural phenomenon, caused by anthropogenic modification and degradation of coral reef environments [31]. The initial formulation of the predator removal hypothesis related to apparent overfishing of the giant triton (Charonia tritonis) on the GBR in the decades immediately preceding the first documented outbreak of CoTS in 1962 [22]. Notably, ~10,000 giant tritons were removed from the GBR each year from 1947 to 1960 by trochus fishermen and commercial shell collectors [22]. Densities of triton must have been significant to sustain this level of removal, or at the very least, much higher than they are now. While there is no empirical data on their abundance, C. tritonis are exceedingly rare on the GBR, and have been since the 1960s, perhaps reflecting the legacy of excessive removals in the 1950s [32].
Endean [22] argued that the effective loss of giant triton from reefs in the northern GBR relaxed normally strong regulatory pressure on abundance of juvenile and sub-adult CoTS, leading to increased abundance of large adult starfish that were capable of initiating outbreaks by virtue of their massive combined reproductive output. Adding weight to this hypothesis, outbreaks of CoTS were reported from other locations (e.g., Fiji and Western Samoa) where C. tritonis had also been extensively harvested, whilst outbreaks had not been reported in areas (e.g., Malaysia, Philippines and Taiwan) where C. tritonis were abundant [22]. The ability of C. tritonis to provide the sufficient top-down control necessary to regulate CoTS populations has since been questioned (e.g., [33]) largely based on their generally low rates of feeding and the apparent reluctance to eat CoTS when provided with alternative prey.
Though the role of giant triton in regulating abundances of CoTS (past, present, or future) is still not resolved, the predator removal hypothesis has evolved through time to place increasing emphasis on fish predators. Attention has focused on large predatory fishes capable of consuming adult starfish (e.g., [33,34,35]), which are targeted by fisheries and/or have declined in abundance due to localized fishing activities. Explicit and direct evidence that any of the major fisheries target species (e.g., coral trout, Plectropomus sp.) are significant predators of crown-of-thorns starfish is meagre [34]. However, some studies [33,36,37] have reported increased incidence and/or severity of outbreaks of CoTS along gradients of increasing fishing effort. On the GBR, Sweatman [37] showed that reefs open to fishing were seven times more likely to experience an outbreak of crown-of-thorns starfish (57% of reefs affected) compared to reefs effectively closed to fishing within no-take marine reserves (8% of reefs affected). While the mechanistic basis of these patterns has not been critically tested, increasing evidence of links between fishing and starfish outbreaks [36,37] has fuelled significant interest in predation, both to understand the cause(s) and ultimately manage CoTS outbreaks.
1.2. Objectives of This Review
The purpose of this review is to synthesise existing knowledge of potentially important CoTS predators, considering their individual and collective capacity to influence population dynamics of CoTS. There is an ever-increasing list of putative predators (e.g., [11,38,39], Table 1), largely based on anecdotal observations of different coral reef organisms (mainly fishes) feeding on dead or dying CoTS within reef environments. Our intention in this review is to differentiate between organisms that opportunistically feed on dead or injured CoTS (scavengers), versus those predators that feed on live and healthy starfish and either kill them outright or reduce their individual fitness and/or reduce population level fitness by altering patterns in abundance and distribution. It is possible, for example, that the mere presence of benthic predators could disperse adult CoTS that might otherwise aggregate to spawn, and thereby reduce fertilization success. Moreover, this review will explicitly consider potential predators at different stages in the life cycle of CoTS, especially pre-settlement (e.g., gametes, larvae) and early post-settlement life stages, which is quite possibly the most significant bottleneck in their life-history [40,41,42]. Where possible, we report or derive estimates of the rates of mortality due to predation across different life-history stages of CoTS.

Table 1.
Species that feed on different life stages and states of health of Acanthaster spp. “F” denotes that the particular predator has been directly observed feeding on a particular life stage in the field, which also includes where starfish were made unnaturally available; “L” denotes where feeding is inferred based on studies in a laboratory/aquarium; “G” denotes that Acanthaster remains have been recovered from the stomach of the predator; I = not directly witnessed.
Having established the range of putative CoTS predators, this review will consider empirical and theoretical evidence that supports (or refutes) the potential role of predators in moderating (if not preventing) CoTS outbreaks. If predation underlies observed differences in the incidence or severity of outbreaks across gradients of fishing pressure [36,37], we would expect to find that the specific predators would be significantly more abundant in areas with little or no fishing, with corresponding increases in effective rates of predation on juvenile and/or adult CoTS within these areas. Persistent controversy around the role of predation in regulating abundance of CoTS (e.g., [11,36,37]) highlights many deficiencies in previous research approaches and points to the definitive need for experimental studies that explicitly test the mechanistic underpinnings of the predator removal hypothesis.
2. Known Predators of Crown-of-Thorns Starfish
A total of 80 species of coral reef organisms are reported to feed on CoTS, including 24 motile and sessile invertebrates versus 56 species of coral reef fishes (Table 1). However, most species have been observed feeding on moribund and dead individuals in the field, while observations of predation on healthy, uninjured starfish are comparatively rare. Similarly, field observations of species feeding on the gametes of CoTS are also extremely limited, and field observations of predation upon larvae are simply not feasible. Gut content analysis has also been largely unsuccessful in identifying putative predatory species (e.g., [43]). However, there have been significant advances in the field of environmental DNA (eDNA) analysis in recent years [44] and it is likely that this technique could be utilised to both identify previously unknown predators, and establish the frequency with which known predators actually consume CoTS. One suggested method to do this would be to collect faeces of presumed CoTS predators and test this for presence of CoTS DNA. However, there are limitations to this technique. Most notably, it is not possible to distinguish between particular life stages of prey species, nor whether specific prey species were alive or dead when consumed [44], which is important in understanding the role of predators in structuring populations of CoTS. Such experiments should be supplemented with benthic surveys to confirm presence of juvenile or adult starfish, and plankton tows to confirm presence/absence of CoTS larvae (see [45]). This could provide an indication of the CoTS life stage from which DNA found in predator faeces has originated, and would be particularly beneficial for predators such as damselfish which may prey upon both pre- and post-settlement life stages (e.g., [20,28]).
2.1. Pre-Settlement Predation
Most of the putative CoTS predators feed or scavenge on post-settlement life stages (juveniles and adults) compared to pre-settlement stages (Figure 2 and Figure 3). However, this probably reflects limited research directed at identifying potential predators on CoTS eggs and larvae and/or difficulties in documenting predation on these early life-history stages. Coral reefs typically support very high abundance and diversity of planktivorous species, including many different reef fishes (e.g., [20,28,47,63]) as well as sessile invertebrates, such as corals [8], which may consume CoTS propagules during spawning, as well as feeding on CoTS larvae when they settle. CoTS are one of the most fecund invertebrates [64], with very high fertilisation rates [65,66], but intuitively, most eggs and larvae must fail to survive. As for other marine species with planktonic larvae, significant rates of pre-settlement mortality are also likely to arise due to predation [67,68]. Yet, given their exceptional reproductive potential [64], even small changes in the proportion of larvae that survive and settle will lead to vast differences in the absolute number of juvenile and adult starfish.
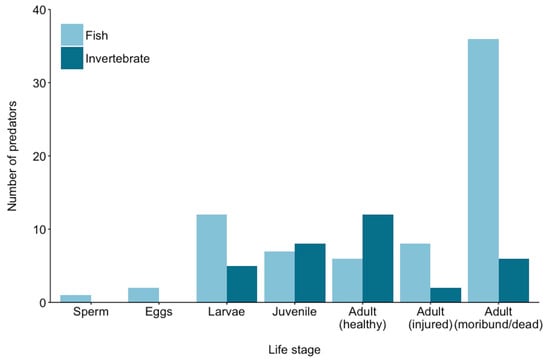
Figure 2.
Putative predators of crown-of-thorns starfish across each major life stage. Predators at each life stage are not mutually exclusive. This figure is based on references from Table 1.
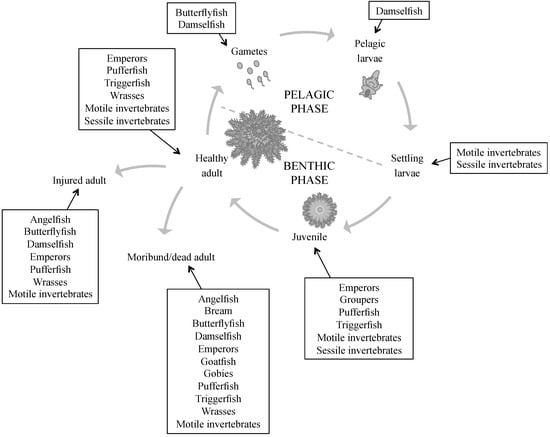
Figure 3.
Main predatory groups acting at different life stages of crown-of-thorns starfish. This figure is based on references from Table 1.
Early studies suggested that CoTS eggs and larvae were largely immune from predation due to unpalatable chemical defences (saponins) contained within [4]. However, more recent examination of predation upon both eggs [69] and larvae [20] reveals that these are indeed readily consumed by a range of highly abundant, planktivorous damselfishes. Given that this group of predators can be extremely abundant on coral reefs, it is likely that they play an important role in reducing the proportion of CoTS that survive through to settlement, and high densities of damselfishes should be considered important for the buffering capacity of coral reefs. Given that all the planktivorous damselfishes tested in the recent studies [20,69] consumed CoTS material, albeit to varying extents, it is highly likely that there are more predators of the pre-settlement stages that are yet to be identified. Furthermore, the actual suite of predators that prey upon the early life stages of CoTS is likely to span a far greater taxonomic range, from benthic species such as those already identified (e.g., [8,19], Table 1) to large pelagic fishes, such as manta rays and whale sharks.
2.2. Post-Settlement Predation
A key component of the vulnerability of larvae is their susceptibility to predation during settlement. Larvae preferentially settle in habitats with fine-scale topographic complexity [49], which is likely to be an adaptation to minimise early post-settlement mortality. However, a wide range of potential predators are abundant within the reef matrix (e.g., [11,17,39,70]). Benthic predators and filter feeders, including corals such as Pocillopora damicornis, may exact a heavy toll on the settling larvae of coral reef asteroids, including Acanthaster spp. [8]. Keesing and Halford [47] estimate mortality at settlement and during metamorphosis to be in excess of 85%. Furthermore, Cowan et al. [19] observed that 55% of brachiolaria larvae that settled to rubble with naturally attached crustose coralline had undergone metamorphosis after 48-h when substrates had been cleaned of potential predators, compared to 0% when polychaete predators were present. If predation represents a significant threat to settling larvae, this should have provided sufficient selective pressure for the evolution of behavioural mechanisms to evade predators, as seen in juveniles and adults in low-density populations. Cowan et al. [19] used static choice chambers to determine whether such mechanisms are present to assist larvae in avoiding settlement on or near predators, revealing that larvae are able to both detect, and respond to, the presence of predators within settlement substrates; larvae were attracted to rubble with naturally attached crustose coralline algae that had been cleaned of predators, but were deterred from these substrates when polychaete predators were present. Whether similar mechanisms are present to enable juveniles to detect and actively avoid predators in the reef matrix remains to be tested.
Following settlement, many marine organisms experience very high early post-settlement mortality (e.g., asteroids [8]; bivalves [71]; fish [72]; corals [73]), to the extent that this is a recognised demographic bottleneck for some taxa [73]. Although Sweatman’s [50] measurements of predation by fishes on laboratory-reared juvenile CoTS (25–79 mm diameter) revealed very low predation rates (0.13% per day), predation on juvenile CoTS is due mainly to epifaunal invertebrate predators, which are highly abundant on coral reefs (e.g., [17,70]). For example, survivorship of laboratory reared CoTS that are settled to freshly collected rubble is effectively zero, owing to very high rates of predation on newly settled larvae by naturally occurring predators (e.g., polychaetes) within the rubble [17].
In the eastern Pacific, Glynn [39] demonstrated that the harlequin shrimp Hymenocera picta and a polychaete worm Pherecardia striata were significant predators of CoTS [39,59,74]. Moreover, there have not been any outbreaks, or persistently high numbers of CoTS on reefs in Panama, where these two predators are found in high abundance, and where populations of alternative prey (ophidiasterids) are scarce. Although both field [74] and laboratory studies [43,74,75] demonstrate that the shrimp has difficulties attacking larger and more active CoTS, it is expected that they are highly effective predators of smaller, more cryptic CoTS within the reef matrix [39]. The observed preference of H. picta for other asteroid prey species, particularly smaller ophidiasterids [43], and the strong preference of P. striata for crustacean tissue over tissue from CoTS [39] emphasises how the relative scarcity of alternative prey may be an important factor influencing the capacity of a potential predator to manipulate the population dynamics of CoTS [74]. If they are important in regulating populations of CoTS, this may occur within a relatively restricted geographical area.
The polyp Pseudocorynactis sp. may also play an important role in the population control of CoTS [62] in areas where it occurs in high abundances, such as Sogod Bay, Philippines. Pseudocorynactis sp. prey on a range of echinoderms and has been observed ingesting adult CoTS up to 250 mm in diameter [62]. Furthermore, Pseudocorynactis sp. preferentially settles under coral ledges and in reef crevices, where it likely predates on cryptic juvenile and sub-adult CoTS [62].
3. Rates of Predation on Crown-of-Thorns Starfish
Understanding of the importance of predation in the population dynamics of CoTS is significantly constrained by a lack of empirical data on background mortality rates and natural predation rates. Quantifying mortality rates of CoTS in the field is tractable, but requires significant investment to follow the fate of a large number of uniquely tagged or recognizable individuals (sensu [76]) across a broad size range of individuals and in different habitats. The biggest limitation to such experiments is the limited capacity to tag CoTS (e.g., [77]). Previous methods used to distinguish individual starfish (staining, branding, tagging and dyeing) were only effective for days to weeks, limiting any capacity to get meaningful long-term data on rates of mortality [77]. Identifying more permanent tagging solutions is essential, but even short-term tagging and tethering experiments could yield important information about the vulnerability of CoTS to predation.
Short-term tagging and tethering experiments have been used to effectively estimate or compare predation vulnerability for a range of echinoderms, especially echinoids or urchins (e.g., [76,78,79]). These studies typically use very short observations (≤3 days) to estimate predation rates, though some recent studies have been conducted over several months; Ling and Johnson [76] successfully tagged and tethered the urchin Centrostephanous rodgersii, and measured subsequent survivorship >100 days. At present, there are few direct, quantitative estimates of predation or mortality rates for CoTS in the field, which are critical for establishing the importance of predation in limiting the densities of starfish at individual reefs [21].
Previous research on CoTS predators has focused on estimating maximum rates of starfish consumption by individual predators and extrapolating this to account for natural densities of these predators (e.g., [28,80,81]). This is based on the belief that effective control of outbreak populations is fundamentally reliant on high rates of predation to compensate for rapid population growth of CoTS when outbreaks begin. However, it is not the rate of feeding per se that is important in determining whether a predator can effectively regulate prey densities [12], but changes in the rates of predation in response to spatial and temporal gradients in prey abundance. Predators that are capable of consuming large numbers of CoTS, by virtue of their high abundance and/or individual capacity to consume large numbers of CoTS [20], may be important in suppressing local CoTS densities. Cowan et al. [20] showed that a wide range of planktivorous damselfishes will feed on CoTS larvae, some of which (D. aruanus and P. amboinensis) have very high satiation limits. The capacity of these fishes to consume large numbers of CoTS larvae, combined with high densities of these fishes (as well as many other planktivorous fishes that may also readily feed on CoTS larvae) could be critical in limiting settlement rates [42,82] and thereby moderating the local severity of outbreaks. It is unlikely however, that these fishes could actually prevent an outbreak from ever occurring, unless either (i) they selectively target CoTS larvae (over other potentially more abundant prey) even at very low prey densities, and thereby prevent initiation of outbreaks; or (ii) their combined feeding capacity exceeds the very large number of CoTS larvae that can cause the rapid and pronounced onset of some outbreaks.
Early research on putative CoTS predators largely dismissed the importance of generalist reef predators, suggesting instead that important predators would have to be highly specialized (preferentially feeding on CoTS to the exclusion of almost all other potential prey) and feed on adult CoTS [80]. Importantly, if there are specialist predators that are highly effective in finding and killing CoTS even when they occur at very low densities, then it may be these predators that are key in preventing outbreaks from ever occurring [82]. However, effective CoTS predators must be sufficiently generalist to consume alternative prey [82] and thereby sustain themselves during non-outbreak periods when CoTS are scarce. If predation (or lack thereof) is a potential cause of CoTS outbreaks, it seems that we should also be focusing on predators that target pre-settlement, settlement, and post-settlement pre-reproductive stages [41,42]. Most notably, predation by benthic invertebrates on newly settled starfish appears, at present, to be the most significant bottleneck in their life-history [42], but this may be largely attributable to underestimates of predation rates on CoTS during other life-stages.
Quantitative data on predation rates is rare and in most cases comes from experimental studies that aim to determine maximum predation rates by specific organisms (e.g., [17,20,28,47,61]), or modelling efforts that predict the rate of predation needed to prevent outbreaks (e.g., [33,41,82,83]). Based on caging experiments, the triton shell C. tritonis is estimated to consume 0.7 adult starfish per week [28], however attacks are not always fatal [55] and this predator prefers to feed on other starfish when given a choice [28]. Furthermore, pre-fishing numbers of C. tritonis remain largely unknown and it is unlikely that this invertebrate was ever present in sufficiently high numbers to prevent outbreaks [84,85]. Starfish numbers are persistently low in areas where the corallimorph P. hoplites [61] or harlequin shrimp H. picta together with the lined fireworm P. striata [59] are abundant. Consumption rates of adult starfish (up to 340 mm diameter) by P. hoplites are estimated to be 29.5 g day−1 [61]. In the eastern Pacific, Glynn [59] reported that 5%–6% of the CoTS were being attacked by H. picta at any time, and 0.6% of the starfish population were preyed upon by both H. picta and P. striata. Approximately 50% of CoTS being attacked by H. picta ultimately died, compared to close to 100% for CoTS being attacked by both H. picta and P. striata, and these two predators are particularly effective in regulating numbers of juvenile starfish [59]. Keesing and Halford [17] measured significant mortality rates on post-metamorphic starfish (5.05% day−1 on 1-month old starfish and 0.85% day−1 on 4-month old starfish), due to predation by epibenthic fauna contained within dead coral rubble. This is much higher than the attack rate on juvenile starfish (1%–1.5%) that McCallum [40] suggested would be sufficient to limit the occurrence of CoTS outbreaks, based on demographic modelling. Cowan et al. [20] conducted a series of laboratory experiments, measuring maximal predation rates by a wide range of planktivorous damselfishes upon CoTS larvae, reporting consumption rates ranging from 14 larvae h−1 by C. viridis, up to 158 larvae h−1 by D. aruanus.
Ormond and Campbell [43] suggest that the predatory behaviour of large fishes (the pufferfish A. hispidus, and triggerfishes P. flavimarginatus and B. viridescens) may have the capacity to control densities of adult CoTS in the Red Sea, and may be capable of disbanding aggregations in their early stages. It is however, unlikely that these species would be effective in controlling CoTS populations across the entire Indo-Pacific region as they are not universally common [86]. However, triggerfishes (particularly, Balistapus undulatus) may nonetheless be important maintaining the structure of coral reef ecosystems, by predating on rock-boring urchins [87]. There may be other large predatory fishes capable of regulating densities of adult CoTS; Keesing and Halford [47] observed the pufferfish A. stellatus to be capable of consuming adult starfish (20 cm diameter) in less than 10 min. Further, Ormond et al. [33] reported greater mean densities of lethrinids and large fish predators from the Red Sea, where no major outbreaks of CoTS were known to have occurred, compared to the GBR, where two cycles of large scale outbreaks had occurred [33]. Further, within the GBR, mean predatory fish densities were found to be reduced on reefs that were experiencing major outbreaks [33]. Fish species examined were commercially targeted or frequently caught as bycatch, and variations in population densities of predatory fish between sites was compatible with fisheries data on fishing intensity, therefore the pattern of difference in CoTS populations could be explained by differing fishing intensities between locations [33].
3.1. Sub-Lethal Injuries
Discussions to this point have focused on instances of whole animal or lethal predation, but sublethal (or partial) predation is often very apparent and well documented among echinoderms [88,89]. For CoTS, very high proportions of adults (up to 60%) have evidence of recent injuries (most apparent as missing arms), which is attributed to partial predation [15]. Even if the predation is not immediately fatal, sublethal attacks may still have an important influence on population dynamics. In the short term, open injuries and exposed internal organs may increase the likelihood of pathogenic infections and disease transmission among individuals [90,91,92] and can also increase susceptibility to further attacks [39]. Even if starfish effectively repair injuries caused by partial predation, effective declines in the size of individuals caused by sub-lethal predation will reduce food intake [88,93,94]. Crown-of-thorns starfish also regrow damaged or missing arms [28,95], which will require re-allocation of nutrients to regeneration, which could otherwise have been used for immune defence [11], reproduction or somatic growth [88,93]. The removal of arm tips, and consequent removal of the eyespot, may additionally result in reduced foraging efficiency due to the loss of vision, which is important for navigating between reef structures and locating prey [96].
There are strongly opposing views about the inferences of high incidence of partial predation in populations of crown-of-thorns starfish. In general, high incidence of sub-lethal predation has been considered to be generally reflective of high intensity of predation (e.g., [11,15]), such that high rates of partial mortality serve as a proxy for high levels of overall mortality. In the Philippines, Rivera-Posada et al. [11] showed that the incidence of injuries on CoTS was highest within a no-take marine reserve, and supporting information on the high diversity and abundance of reef fishes corroborate the idea that overall intensity of predation would likely be much higher inside versus outside of this reserve. Conversely, high incidence of partial mortality may reflect low intensity of predation pressure [16] because when predation is intense it would be expected that most predation events would result in complete mortality. As such, high incidences of starfish with partial injuries point to the strong regenerative capacity of starfish [14] and may suggest that predation is predominantly sub-lethal for crown-of-thorns starfish. Schoener [97] suggested that there is no reason to expect any relationship between rates of injuries versus rates of predation-driven mortality, because the efficiency of predation (the proportion of attacks that cause death) is independent of the attack rate or incidence of predation. For the asteroid Asterias amurensis, high density populations have been observed to be completely annihilated by an incursion of thousands of spider crabs (Leptomithrax gaimardii) that moved to shallow reefs in large numbers [89]. Additionally, where crab incursions involved fewer individuals, high rates of sub-lethal injury (~70% of starfish population injured) occurred independently of mass predator-driven mortality [89]. In this example, the high mobility of spider crabs can lead to overwhelming local impacts on starfish populations but this impact is highly variable across space and time [89]. Ultimately, a dedicated research project is needed to test the relationship between rates of partial versus complete predation mortality. However, in the absence of any empirical data on overall rates of predation mortality, the incidence of injuries serves as the best proxy to test for variation in vulnerability to predation among locations and with size of starfish [15].
A common trend among echinoderm species is for the incidence of sub-lethal injuries to decline with increasing body size [14,78,88,89,93,98] reflecting general declines in the vulnerability to predation. Accordingly, Messmer et al. [14] observed a clear linear decline in both rate and severity of predation with increasing starfish size. Rivera-Posada et al. [11] reported highest incidence of arm damage in the intermediate (11–20 cm diameter) size class however. This pattern may be explained by changes in behavioural and physical characteristics with increasing size, whereby intermediate sized individuals may have greatest exposure to predators, while smaller starfish (<10 cm) tend to remain hidden but are also more likely to be completely consumed [16]. Reduced incidence of sublethal predation in largest individuals (≥21 cm) may be explained by increased length of spines [11] and satiation of predators following removal of a smaller proportion of the total body mass. Disparity between the trends observed in these studies may be a result of differing sample size or differing suites of predators between the locations on the GBR [14] versus in the Philippines [11].
Sub-lethal and/or ‘trait-mediated’ effects of predators [89] can include alteration of behaviour and spatial patterns in echinoderms, in addition to changes in adult abundances and local spatial distributions, which are important for the reproductive ecology of free-spawning marine invertebrates as they influence rates of fertilization success [99]. For CoTS, fertilization success may be close to 100% when spawning individuals are adjacent to each other [100], thus any predator that is capable of dissipating an aggregation, or sufficiently reducing adult density, is likely to have a significant impact on zygote production. Humans have been shown to indirectly alter spatial distributions of asteroids, leading to much higher rates of fertilization in human-driven hotspots of zygote production [97]. In this way, sub-lethal predatory effects, leading to reduced individual reproductive performance, in combination with alteration to spatial configurations at the time of spawning may impact on zygote production for asteroids [99], potentially influencing the occurrence of secondary outbreaks for CoTS [101].
3.2. Population Modelling
Population simulation models provide a means of exploring the possible role of predation (or other natural causes of mortality) in regulating populations of CoTS (e.g., [82]), especially given little or no empirical data on satiation levels and feeding efficiency of potential predators or predation rates on larval and juvenile CoTS. Simulation models indicate that changes to predation rates during the pre-reproductive, post-settlement stage may be particularly relevant in understanding the dynamics of CoTS populations [40,41,82]. Notably, given their exceptional reproductive capacity, small changes in proportional survivorship or settlement success of CoTS larvae will result in large changes in adult abundance. Accordingly, McCallum [41] argues that relatively subtle changes in the abundance of predators (e.g., caused by exploitation) and/or predation rates, will reduce the level of local recruitment required to overcome (or satiate) predators.
The potential ecological importance of predation as a regulatory factor upon CoTS populations depends largely upon the ability of predators to find and consume prey [102]. Quantification of the functional response, described by the intake rate of prey as a function of prey density [103], is a common method that provides insight into the dynamics of predator–prey systems [104,105,106]. Functional responses may be classified into three types. A type I response assumes a linear increase in the intake rate with increasing food density, generally up to a maximum value, beyond which the intake rate is constant [107]. Type II is characterized by a decelerating intake rate [107] and assumes that the predator is limited by its ability to process food [108,109]. Type III is associated with an accelerating intake rate, associated with prey switching behaviour (preferential consumption of the most common type of prey [110]), up to a saturation point [107]. Predators that exhibit prey-switching behaviour, feeding more frequently on CoTS in response to a marked increase in their local abundance, may be capable of dissipating an aggregation in its early stages [53]. In order to persist when population densities of CoTS are low, predators should be able to take a range of prey, exhibiting an increased feeding response in reaction to a rapid increase in the CoTS population [53]. Predators exhibiting these type II and type III functional responses [103] are typical of vertebrate predators, reinforcing the focus on fish, and are supported in McCallum’s [15,82] population models. In laboratory feeding experiments on Acanthaster spp. larvae, planktivorous damselfishes exhibit primarily a type II functional response, indicating their capacity to consume sufficient larvae to suppress settlement rates when larvae are already scarce, thereby contributing to very low natural densities of CoTS recorded outside of outbreak periods [20]. However, very high densities of larvae, which are a necessary condition for the rapid and pronounced onset of outbreaks, are likely to swamp even the combined consumption capacity of all planktivorous reef fishes [40].
4. Conclusions
Crown-of-thorns starfish are vulnerable to predation from a wide range of coral reef organisms, and at all stages of their life cycle. Despite identification of potentially key predators, and groups of predators, natural predation rates in both outbreaking and non-outbreaking populations remain largely unknown. This considerably limits our understanding of the role of predation in structuring the population dynamics of CoTS and of approaches to managing their often-devastating impacts on coral reef ecosystems. Whilst predation is likely to be important in suppressing settlement rates and contributing to naturally low densities of CoTS, the initiation and spread of outbreaks cannot, at present, be definitively attributed to changes (presumably declines) in the abundance of predators and/or changes in predation rates (e.g., [111]). Babcock et al. [111] showed that there are likely to be multiple factors that contribute to outbreaks of CoTS, such that a diverse range of management strategies will be required to prevent, or reduce the occurrence, of outbreaks. Maximising the number and diversity of putative CoTS predators is nonetheless, a “no regrets” strategy to minimise the risk of CoTS outbreaks and increase the resilience of coral reef ecosystems, generally. In the meantime, further research into potential predators, as well as estimates of predation and mortality across all life-stages of CoTS, is still warranted. More specifically, rates of pre- and post-settlement predation should be explicitly compared along known gradients in abundance of putative CoTS predators (e.g., inside and outside of marine reserves with marked differences in the abundance and diversity of fishes that feed on CoTS). New technologies provide improved opportunities to explore spatial and temporal variation in the demographics of CoTS populations, for example, DNA screening of diets for large numbers of potential CoTS predators [44,112], plus increased potential to tag and track benthic species within reef environments [113]. Such novel approaches along with remote sensing techniques will provide new insights into changes in the population dynamics and/or environmental conditions during the onset of population outbreaks.
Acknowledgments
This research was supported by the National Environmental Science Programme Tropical Water Quality Hub, as well as Crown-of-Thorns Starfish Research Grants awarded to M.P., Z.L.C. and V.M. by Lizard Island Research Stations and the Australian Museum. Discussions with Peter Doherty and David Westcott were critical to the development of ideas put forward in this review.
Author Contributions
All authors contributed equally to the concept and layout of this review. Z.L.C. compiled and analysed the data; Z.L.C. and M.P. wrote the paper; S.L. and V.M. edited the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howden, M.E.H.; Lucas, J.S.; McDuff, M.; Salathe, R. Chemical defences of Acanthaster planci. In Crown-of-Thorns Starfish Seminar Proceedings; Australian Government Publishing Service: Canberra, Australia, 1975; pp. 67–79. [Google Scholar]
- Barnett, D.; Dean, P.W.; Hart, R.J.; Lucas, J.S.; Salathe, R.; Howden, M.E.H. Determination of contents of steroidal saponins in starfish tissues and study of their biosynthesis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 90, 141–145. [Google Scholar] [CrossRef]
- Shiomi, K.; Yamamoto, S.; Yamanaka, H.; Kikuchi, T. Purification and characterization of a lethal factor in venom from the crown-of-thorns starfish (Acanthaster planci). Toxicon 1988, 26, 1077–1083. [Google Scholar] [CrossRef]
- Lucas, J.; Hart, R.; Howden, M.; Salathe, R. Saponins in eggs and larvae of Acanthaster planci (L.) (Asteroidea) as chemical defences against planktivorous fish. J. Exp. Mar. Bio. Ecol. 1979, 40, 155–165. [Google Scholar] [CrossRef]
- Mackie, A.M.; Singh, H.T.; Fletcher, T.C. Studies on the cytolytic effects of seastar (Marthasterias glacialis) saponins and synthetic surfactants in the plaice Pleuronectes platessa. Mar. Biol. 1975, 29, 307–314. [Google Scholar] [CrossRef]
- Shiomi, K.; Yamamoto, S.; Yamanaka, H.; Kikuchi, T.; Konno, K. Liver damage by the crown-of-thorns starfish (Acanthaster planci) lethal factor. Toxicon 1990, 28, 469–475. [Google Scholar] [CrossRef]
- Shiomi, K.; Midorikawa, S.; Ishida, M.; Nagashima, Y.; Nagai, H. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon 2004, 44, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Early life histories of coral reef asteroids, with special reference to Acanthaster planci (L.). In Biology and Geology of Coral Reefs; Jones, O., Endean, R., Eds.; Academic Press: New York, NY, USA, 1973; pp. 369–387. [Google Scholar]
- Bertness, M.D.; Garrity, S.D.; Levings, S.C. Predation pressure and gastropod foraging: A tropical-temperate comparison. Evolution 1981, 35, 995–1007. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Rivera-Posada, J.; Caballes, C.F.; Pratchett, M.S. Size-related variation in arm damage frequency in the crown-of-thorns sea star, Acanthaster planci. J. Coast. Life Med. 2014, 2, 187–195. [Google Scholar]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 343–366. [Google Scholar] [CrossRef]
- Pekár, S.; Liíznarová, E.; Řezáč, M. Suitability of woodlice prey for generalist and specialist spider predators. Ecol. Entomol. 2015, 41, 123–130. [Google Scholar] [CrossRef]
- Messmer, V.; Pratchett, M.; Chong-Seng, K. Variation in incidence and severity of injuries among crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 2016. under review. [Google Scholar]
- McCallum, H.I.; Endean, R.; Cameron, A.M. Sublethal damage to Acanthaster planci as an index of predation pressure. Mar. Ecol. Prog. Ser. 1989, 56, 29–36. [Google Scholar] [CrossRef]
- Birkeland, C.; Lucas, J.S. Acanthaster Planci: Major Management Problem of Coral Reefs; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Keesing, J.; Halford, A. Field measurement of survival rates of juvenile Acanthaster planci, techniques and preliminary results. Mar. Ecol. Prog. Ser. 1992, 85, 107–114. [Google Scholar] [CrossRef]
- Zann, L.; Brodie, J.; Berryman, C.; Naqasima, M. Recruitment, ecology, growth and behavior of juvenile Acanthaster planci (L.) (Echinodermata: Asteroidea). Bull. Mar. Sci. 1987, 41, 561–575. [Google Scholar]
- Cowan, Z.-L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Benthic predators influence microhabitat preferences and settlement success of crown-of-thorns starfish larvae (Acanthaster cf. solaris). Diversity 2016, 8. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 2016, 35, 1253–1262. [Google Scholar] [CrossRef]
- Moran, P.J. The Acanthaster phenomenon. Oceanogr. Mar. Biol. 1986, 24, 379–480. [Google Scholar]
- Endean, R. Report on Investigations Made into Aspects of the Current Acanthaster planci (Crown of Thorns) Infestations of Certain Reefs of the Great Barrier Reef; Queensland Department of Primary Industries (Fisheries Branch): Brisbane, Australia, 1969.
- Estes, J.A.; Palmisano, J.F. Sea otters: Their role in structuring nearshore communities. Science 1974, 185, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Smith, N.S.; Palmisano, J.F. Sea otter predation and community organization in the western Aleutian Islands, Alaska. Ecology 1978, 59, 822–833. [Google Scholar] [CrossRef]
- Estes, J.A.; Duggins, D.O. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol. Monogr. 1995, 65, 75–100. [Google Scholar] [CrossRef]
- Ling, S.D.; Johnson, C.R.; Frusher, S.D.; Ridgway, K.R. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl. Acad. Sci. USA 2009, 106, 22341–22345. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Pearson, R.G.; Endean, R. A Preliminary Study of the Coral Predator Acanthaster planci (L.) (Asteroidea) on the Great Barrier Reef; Queensland Department of Harbours and Marine: Brisbane, Queensland, Australia, 1969; Volume 3, pp. 27–55.
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Potts, D.C. Crown-of-thorns starfish—Man-induced pest or natural phenomenon? In The Ecology of Pests: Some Australian Case Histories; Kitching, R., Jones, R., Eds.; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1981; pp. 55–86. [Google Scholar]
- Endean, R. Population explosions of Acanthaster planci and associated destruction of hermatypic corals in the Indo-West Pacific region. In Biology and Geology of Coral Reefs; Jones, O.A., Endean, R., Eds.; Academic Press: New York, NY, USA, 1973; pp. 389–438. [Google Scholar]
- Ormond, R.; Bradbury, R.; Bainbridge, S.; Fabricius, K.; Keesing, J.; de Vantier, L.; Medlay, P.; Steven, A. Test of a model of regulation of crown-of-thorns starfish by fish predators. In Acanthaster and the Coral Reef: A Theoretical Perspective; Bradbury, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 189–207. [Google Scholar]
- Sweatman, H.P. Commercial fishes as predators of adult Acanthaster planci. In Proceedings of the 8th International Coral Reef Symposium, Panama City, Panama, 24–29 June 1996; Lessios, H.A., Macintyre, I.G., Eds.; Volume 1, pp. 617–620.
- Mendonça, V.M.; Al Jabri, M.M.; Al Ajmi, I.; Al Muharrami, M.; Al Areimi, M.; Al Aghbari, H.A. Persistent and expanding population outbreaks of the corallivorous starfish Acanthaster planci in the Northwestern Indian Ocean: Are they through overfishing in coral reefs, or a response to a changing environment? Zool. Stud. 2010, 49, 108–123. [Google Scholar]
- Dulvy, N.K.; Freckleton, R.P.; Polunin, N.V.C. Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol. Lett. 2004, 7, 410–416. [Google Scholar] [CrossRef]
- Sweatman, H. No-take reserves protect coral reefs from predatory starfish. Curr. Biol. 2008, 18, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J. Preliminary observations of the decomposition of crown-of-thorns starfish, Acanthaster planci (L.). Coral Reefs 1992, 11, 115–118. [Google Scholar] [CrossRef]
- Glynn, P.W. An amphinoid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western pacific coral reefs. Bull. Mar. Sci. 1984, 35, 54–71. [Google Scholar]
- McCallum, H.I. Effects of predation on organisms with pelagic larval stages: Models of metapopulations. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Choat, J.H., Barnes, D., Borowitzka, M.A., Coll, J.C., Davies, P.J., Flood, P., Hatcher, B.G., Hopley, D., Hutchings, P.A., Kinsey, D., et al., Eds.; Volume 2, pp. 101–106.
- McCallum, H.I. Effects of predation on Acanthaster: Age-structure metapopulation models. In Acanthaster and the Coral Reef: A Theoretical Perspective; Bradbury, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 208–219. [Google Scholar]
- Morello, E.B.; Plagányi, É.E.; Babcock, R.C.; Sweatman, H.; Hillary, R.; Punt, A.E. Model to manage and reduce crown-of-thorns starfish outbreaks. Mar. Ecol. Prog. Ser. 2014, 512, 167–183. [Google Scholar] [CrossRef]
- Ormond, R.F.; Campbell, A.C. Formation and breakdown of Acanthaster planci aggregations in the Red Sea. In Proceedings of the 2nd International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1973; Cameron, A.M., Cambell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; Volume 1, pp. 595–619.
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Uthicke, S.; Doyle, J.; Duggan, S.; Yasuda, N.; Mckinnon, A.D. Outbreak of coral-eating crown-of-thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Pratchett, M.S.; Aguilar, C.; Grand, A.; Caballes, C.F. Bile salts and the single-shot lethal injection method for killing crown-of-thorns sea stars (Acanthaster planci). Ocean Coast. Manag. 2014, 102, 383–390. [Google Scholar] [CrossRef]
- Keesing, J.; Halford, A. Importance of postsettlement processes for the population dynamics of Acanthaster planci (L.). Mar. Freshw. Res. 1992, 43, 635–651. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Rivera-Posada, J. Controlling outbreaks of the coral-eating crown-of-thorns starfish using a single injection of common household vinegar. Coral Reefs 2016, 35, 223–228. [Google Scholar] [CrossRef]
- Lucas, J. Environmental influences on the early development of Acanthaster planci (L.). In Crown-of-Thorns Starfish Seminar Proceedings; Australian Government Publishing Service: Canberra, Australia, 1975; pp. 109–121. [Google Scholar]
- Sweatman, H.P.A. A field study of fish predation on juvenile crown-of-thorns starfish. Coral Reefs 1995, 14, 47–53. [Google Scholar] [CrossRef]
- Birdsey, R. Large Reef Fishes as Potential Predators of Acanthaster Planci: A Pilot Study by Alimentary Tract Analysis of Predatory Fishes from Reefs Subject to Acanthaster Feeding; Report to the Great Barrier Reef Marine Park Authority: Townsville, Australia, 1988; unpublished.
- Endean, R. Destruction and recovery of coral reef communities. In Biology and Geology of Coral Reefs; Jones, O., Endean, R., Eds.; Academic Press: New York, NY, USA, 1976; Volume 3, Biology 2; pp. 215–254. [Google Scholar]
- Ormond, R.; Campbell, A.; Head, S.; Moore, R.; Rainbow, P.; Saunders, A. Formation and breakdown of aggregations of the crown-of-thorns starfish, Acanthaster planci (L.). Nature 1973, 246, 167–169. [Google Scholar] [CrossRef]
- Owens, D. Acanthaster planci starfish in Fiji: Survey of incidence and biological studies. Fiji Agric. J. 1971, 33, 15–23. [Google Scholar]
- Chesher, R.H. Acanthaster planci: Impact on Pacific Coral Reefs; Final report to U.S. Department of the Interior; Westinghouse Research Laboratories: Pittsburgh, PA, USA, 1969; p. 151. [Google Scholar]
- Randall, J.E.; Head, S.M.; Sanders, A.P. L. Food habits of the giant humphead wrasse, Cheilinus undulatus (Labridae). Environ. Biol. Fishes 1978, 3, 235–238. [Google Scholar] [CrossRef]
- Alcala, A.C. The sponge crab Dromidiopsis dormia as a predator of the crown of thorns starfish. Silliman J. 1974, 21, 174–177. [Google Scholar]
- Wickler, W.; Seibt, U. Das Verhalten von Hymenocera picta Dana, einer Seesterne fressenden Garnele (Decapoda, Natantia, Gnathophyllidae). Z. Tierpsychol. 1970, 27, 352–368. [Google Scholar] [CrossRef]
- Glynn, P.W. Acanthaster population regulation by a shrimp and a worm. In Proceedings of the 4th International Coral Reef Symposium, Manilla, Philippines, 18–22 May 1981; Gomez, E.D., Birkeland, C.E., Buddemeier, R.W., Johannes, R.E., Marsh, J.A., Jr., Tsuda, R.T., Eds.; 1982; Volume 2, pp. 607–612. [Google Scholar]
- Brown, T.W. Starfish menaces coral reefs. Hemisphere 1970, 14, 31–36. [Google Scholar]
- Bos, A.R.; Mueller, B.; Gumanao, G.S. Feeding biology and symbiotic relationships of the corallimorpharian Paracorynactis hoplites (Anthozoa: Hexacorallia). Raffles Bull. Zool. 2011, 59, 245–250. [Google Scholar]
- Bos, A.R.; Gumanao, G.S.; Salac, F.N. A newly discovered predator of the crown-of-thorns starfish. Coral Reefs 2008, 27. [Google Scholar] [CrossRef]
- Ciarapica, G.; Passeri, L. An overview of the maldivian coral reefs in Felidu and North Male Atoll (Indian Ocean): Platform drowning by ecological crises. Facies 1993, 28, 33–65. [Google Scholar] [CrossRef]
- Babcock, R.C.; Milton, D.A.; Pratchett, M.S. Relationships between size and reproductive output in the crown-of-thorns starfish. Mar. Biol. 2016, 163, 1–7. [Google Scholar] [CrossRef]
- Babcock, R.C. Spawning behaviour of Acanthaster planci. Coral Reefs 1990, 9, 124. [Google Scholar] [CrossRef]
- Babcock, R.C.; Mundy, C.N. Reproductive biology, spawning and field fertilization rates of Acanthaster planci. Aust. J. Mar. Freshw. Res. 1992, 43, 525–534. [Google Scholar] [CrossRef]
- Bailey, K.M.; Houde, E.D. Predation on eggs and larvae of marine fishes and the recruitment problem. Adv. Mar. Biol. 1989, 25, 1–83. [Google Scholar]
- Fabricius, K.E.; Metzner, J. Scleractinian walls of mouths: Predation on coral larvae by corals. Coral Reefs 2004, 23, 245–248. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Ling, S.D.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Inter-specific variation in potential importance of planktivorous damselfishes as predators of Acanthaster sp. eggs. Coral Reefs 2016. under review. [Google Scholar]
- Keesing, J.K.; Wiedermeyer, W.L.; Okaji, K.; Halford, A.R.; Hall, K.C.; Cartwright, C.M. Mortality rates of juvenile starfish Acanthaster planci and Nardoa spp. measured on the Great Barrier Reef, Australia and in Okinawa, Japan. Oceanol. Acta 1996, 19, 441–448. [Google Scholar]
- Roegner, G.C.; Mann, R. Early recruitment and growth of the American oyster Crassostrea virginica (Bivalvia: Ostreidae) with respect to tidal zonation and season. Mar. Ecol. Prog. Ser. Oldend. 1995, 117, 91–101. [Google Scholar] [CrossRef]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Chong-Seng, K.M.; Graham, N.A.J.; Pratchett, M.S. Bottlenecks to coral recovery in the Seychelles. Coral Reefs 2014, 33, 449–461. [Google Scholar] [CrossRef]
- Glynn, P.W. Interactions between Acanthaster and Hymenocera in the field and laboratory. In Proceedings: the 3rd International Coral Reef Symposium, Miami, Florida, USA; Taylor, D.L., Ed.; University of Miami: Miami, FL, USA, 1977; Volume 1, pp. 209–216. [Google Scholar]
- Wickler, W. Biology of Hymenocera picta Dana. Micronesica 1973, 9, 225–230. [Google Scholar]
- Ling, S.D.; Johnson, C.R. Marine reserves reduce risk of climate-driven phase shift by reinstating size-and habitat-specific trophic interactions. Ecol. Appl. 2012, 22, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Individual recognition and phenotypic variability in Acanthaster planci (Echinodermata: Asteroidea). Coral Reefs 1982, 1, 89–94. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Muthiga, N.A. Patterns of predation on a sea urchin, Echinometra mathaei (de Blainville), on Kenyan coral reefs. J. Exp. Mar. Bio. Ecol. 1989, 126, 77–94. [Google Scholar] [CrossRef]
- Bonaviri, C.; Fernández, T.V.; Badalamenti, F.; Gianguzza, P.; Di Lorenzo, M.; Riggio, S. Fish versus starfish predation in controlling sea urchin populations in Mediterranean rocky shores. Mar. Ecol. Prog. Ser. 2009, 382, 129–138. [Google Scholar] [CrossRef]
- Potts, D. Crown-of-thorns starfish—Man-induced pest or natural phenomenon? In The Ecology of Pests: Some Australian Case Histories; Kitching, R.E., Jones, R.E., Eds.; CSIRO: Melbourne, Australia, 1982; pp. 55–86. [Google Scholar]
- Chesher, R.H. Destruction of Pacific corals by the sea star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef] [PubMed]
- McCallum, H.I. Predator regulation of Acanthaster planci. J. Theor. Biol. 1987, 127, 207–220. [Google Scholar] [CrossRef]
- McCallum, H. Completing the circle: Stock-recruitment relationships and Acanthaster. Mar. Freshw. Res. 1992, 43, 653–662. [Google Scholar] [CrossRef]
- Ormond, R.F.G.; Campbell, A.C. Observations on Acanthaster planci and other coral reef echinoderms in the Sudanese Red Sea. In Symposia of the Zoological Society of London; Academic Press: London, UK, 1971; Volume 28, pp. 433–454. [Google Scholar]
- McClanahan, T.R. Kenyan coral reef-associated gastropod fauna: A comparison between protected and unprotected reefs. Mar. Ecol. Prog. Ser. 1989, 53, 11–20. [Google Scholar] [CrossRef]
- Endean, R. Acanthaster planci infestations of reefs of the Great Barrier Reef. In Proceedings: the 3rd International Coral Reef Symposium, Miami, Florida, USA; Taylor, D.L., Ed.; University of Miami: Miami, FL, USA, 1977; Volume 1, pp. 185–191. [Google Scholar]
- McClanahan, T.R.; Muthiga, N.A. Similar impacts of fishing and environmental stress on calcifying organisms in Indian Ocean coral reefs. Mar. Ecol. Prog. Ser. 2016, 560, 87–103. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Vasquez, J. The effect of sublethal predation on the biology of echinoderms. Oceanol. Acta 1996, 19, 431–440. [Google Scholar]
- Ling, S.D.; Johnson, C.R. Native spider crab causes high incidence of sub-lethal injury to the introduced seastar Asterias amurensis. In Echinoderms in a Changing World: Proceedings of the 13th International Echinoderm Conference, January 5–9 2009, University of Tasmania, Hobart Tasmania, Australia; CRC Press: New York, NY, USA, 2012; pp. 195–201. [Google Scholar]
- Rivera-Posada, J.A.; Pratchett, M.; Cano-Gómez, A.; Arango-Gómez, J.D.; Owens, L. Injection of Acanthaster planci with thiosulfate-citrate-bile-sucrose agar (TCBS). I. Disease induction. Dis. Aquat. Organ. 2011, 97, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Owens, L.; Caballes, C.F.; Pratchett, M.S. The role of protein extracts in the induction of disease in Acanthaster planci. J. Exp. Mar. Bio. Ecol. 2012, 429, 1–6. [Google Scholar] [CrossRef]
- Caballes, C.F.; Schupp, P.J.; Pratchett, M.S.; Rivera-Posada, J.A. Interspecific transmission and recovery of TCBS-induced disease between Acanthaster planci and Linckia guildingi. Dis. Aquat. Organ. 2012, 100, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M. Arm loss and regeneration in Asteroidea (Echinodermata). Echinoderm Res. 1991, 1992, 39–52. [Google Scholar]
- Lawrence, J.M. Energetic costs of loss and regeneration of arms in stellate echinoderms. Integr. Comp. Biol. 2010, 50, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Messmer, V.; Pratchett, M.S.; Clark, T.D. Capacity for regeneration in crown-of-thorns starfish, Acanthaster planci. Coral Reefs 2013, 32, 461. [Google Scholar] [CrossRef]
- Sigl, R.; Steibl, S.; Laforsch, C. The role of vision for navigation in the crown-of-thorns seastar, Acanthaster planci. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Schoener, T.W. Inferring the properties of predation and other injury-producing agents from injury frequencies. Ecology 1979, 60, 1110–1115. [Google Scholar] [CrossRef]
- Marrs, J.; Wilkie, I.C.; Sköld, M.; Maclaren, W.M.; McKenzie, J.D. Size-related aspects of arm damage, tissue mechanics, and autotomy in the starfish Asterias rubens. Mar. Biol. 2000, 137, 59–70. [Google Scholar] [CrossRef]
- Ling, S.D.; Johnson, C.R.; Mundy, C.N.; Morris, A.; Ross, D.J. Hotspots of exotic free-spawning sex: Man-made environment facilitates success of an invasive seastar. J. Appl. Ecol. 2012, 49, 733–741. [Google Scholar] [CrossRef]
- Benzie, J.A.H.; Black, K.P.; Moran, P.J.; Dixon, P. Small-scale dispersion of eggs and sperm of the crown-of-thorns starfish (Acanthaster planci) in a shallow coral reef habitat. Biol. Bull. 1994, 186, 153–167. [Google Scholar] [CrossRef]
- Endean, R. Acanthaster planci on the Great Barrier Reef. In Proceedings of the 2nd International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1973; Cameron, A.M., Cambell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; Volume 1, pp. 563–576.
- Hassell, M.P. The Dynamics of Arthropod Predator-Prey Systems; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Holling, C. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef]
- Abrams, P.A. The effects of adaptive behavior on the Type-2 functional response. Ecology 1990, 71, 877–885. [Google Scholar] [CrossRef]
- Buckel, J.A.; Stoner, A.W. Functional response and switching behaviour of young-of-the-year piscivorus bluefish. J. Exp. Mar. Bio. Ecol. 2000, 245, 25–41. [Google Scholar] [CrossRef]
- Nilsson, P.A.; Ruxton, G.D. Temporally fluctuating prey and interfering predators: A positive feedback. Anim. Behav. 2004, 68, 159–165. [Google Scholar] [CrossRef]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Kaspari, M. Prey preparation and the determinants of handling time. Anim. Behav. 1990, 40, 118–126. [Google Scholar] [CrossRef]
- Baker, D.J.; Stillman, R.A.; Smith, B.M.; Bullock, J.M.; Norris, K.J. Vigilance and the functional response of granivorous foragers. Funct. Ecol. 2010, 24, 1281–1290. [Google Scholar] [CrossRef]
- Murdoch, W.W. Switching in general predators: Experiments on predator specificity and stability of prey populations. Ecol. Monogr. 1969, 39, 335–354. [Google Scholar] [CrossRef]
- Babcock, R.C.; Dambacher, J.M.; Morello, E.B.; Plagányi, É.E.; Hayes, K.R.; Sweatman, H.P.A.; Pratchett, M.S. Assessing different causes of crown-of-thorns starfish outbreaks and appropriate responses for management on the Great Barrier Reef. PLoS ONE 2016, 11, e0169048. [Google Scholar] [CrossRef] [PubMed]
- Redd, K.S.; Ling, S.D.; Frusher, S.D.; Jarman, S.; Johnson, C.R. Using molecular prey detection to quantify rock lobster predation on barrens-forming sea urchins. Mol. Ecol. 2014, 23, 3849–3869. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, L.D.; Babcock, R.C.; Hyndes, G.A. Movements of the western rock lobster (Panulirus cygnus) within shallow coastal waters using acoustic telemetry. Mar. Freshw. Res. 2008, 59, 603–613. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).