The Challenge of Managing Marine Biodiversity: A Practical Toolkit for a Cartographic, Territorial Approach
Abstract
:1. Introduction
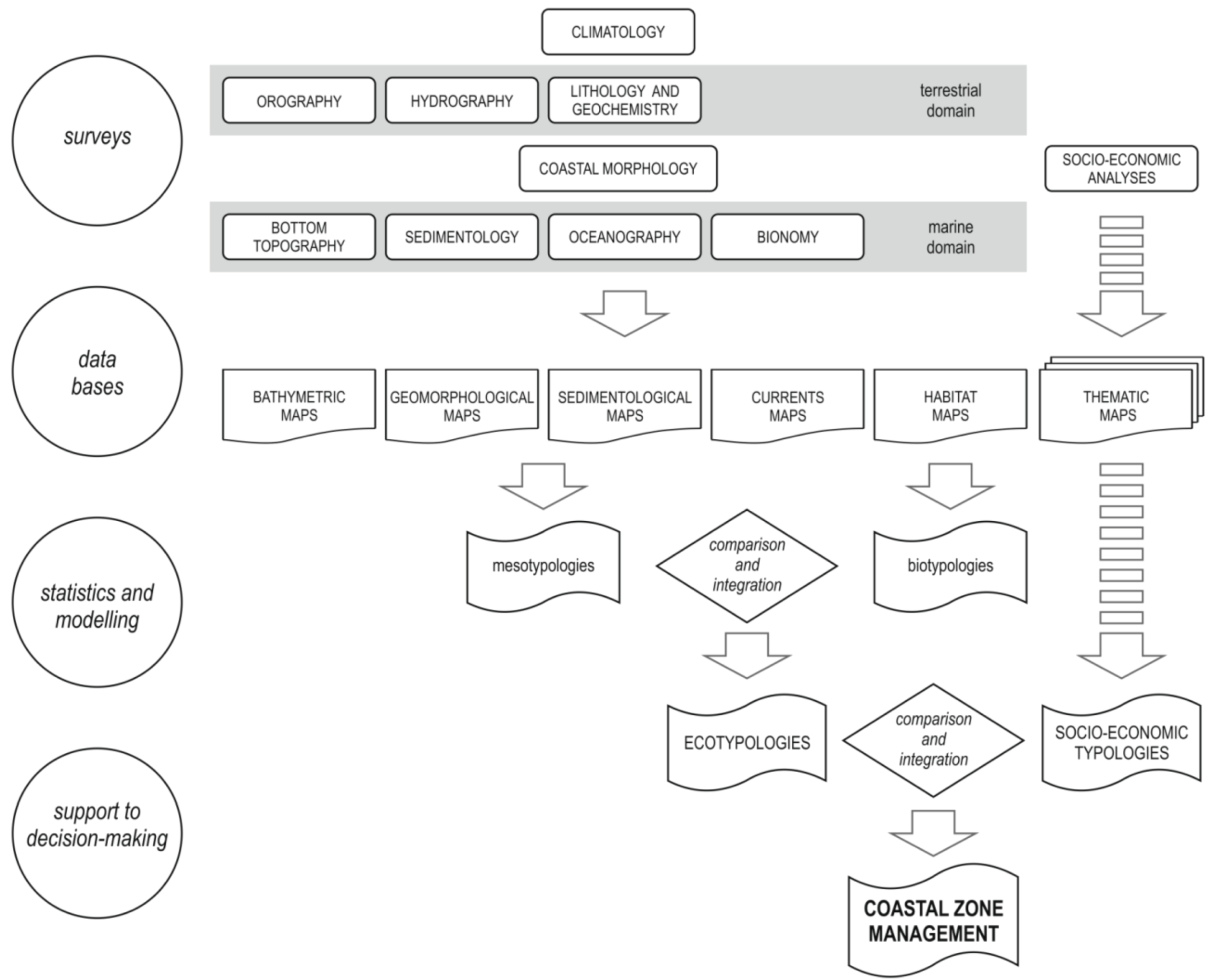
2. Methodological Aspects
2.1. Criteria and Definitions for Diagnostic Cartography
2.2. Map Production
| From Habitat Scores To Territory Scores | ||
|---|---|---|
| 1st criterion | cell score is the sum of the scores of all habitats contained in the cell | |
| advantage | the additional value of ecodiversity in accounted for | |
| disadvantage | do several low-value habitats equate one high-value habitat? | |
| 2nd criterion | cell score is the mean of the scores of all habitats contained in the cell | |
| advantage | several low-value habitats produce anyway low value-cells | |
| disadvantage | co-occurrence of low-value and high-value habitats ends in mediocre cells | |
| 3rd criterion | cell score corresponds to the maximum score of any single habitat contained in the cell | |
| advantage | the occurrence of high-value habitats is shown out | |
| disadvantage | ecodiversity is ignored | |
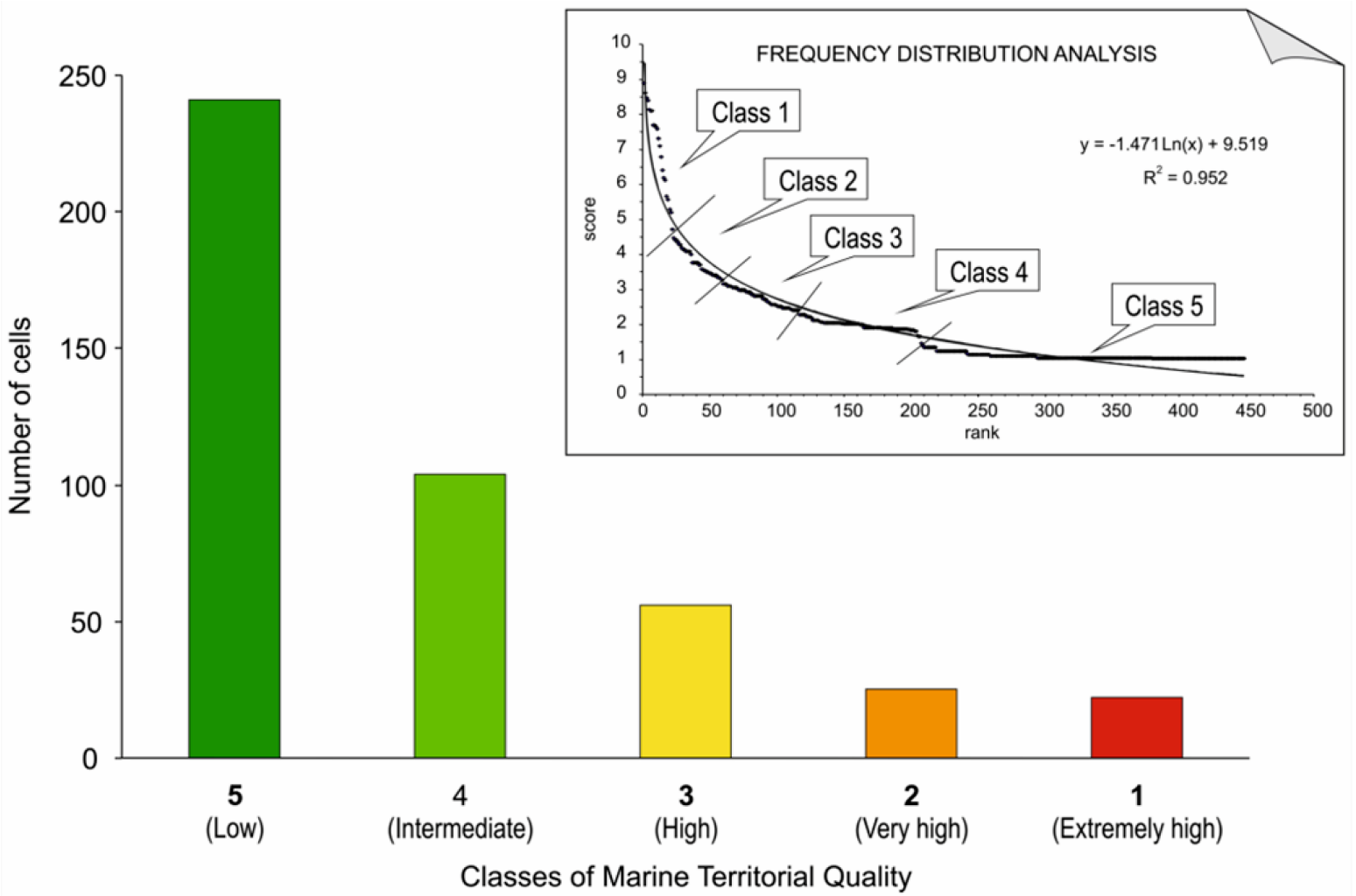
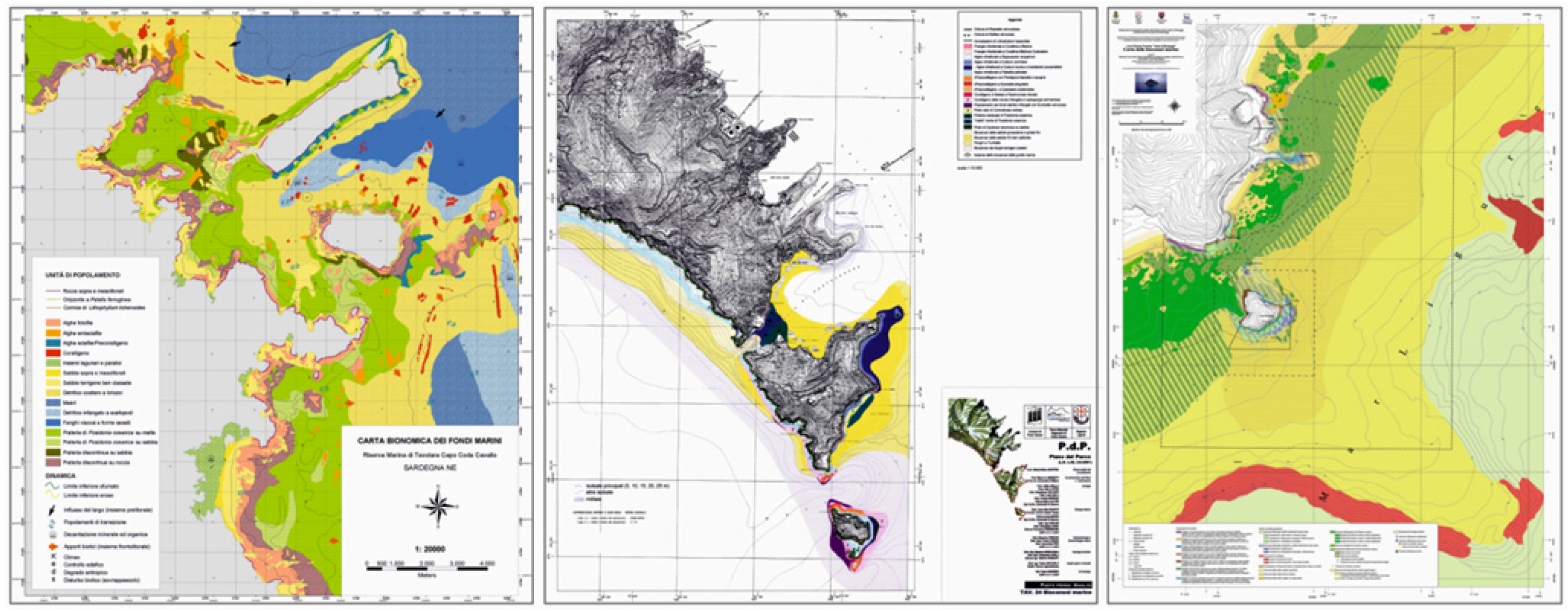
3. Use and Implications of Territorial Maps
3.1. Maps for Marine Territory Characterization
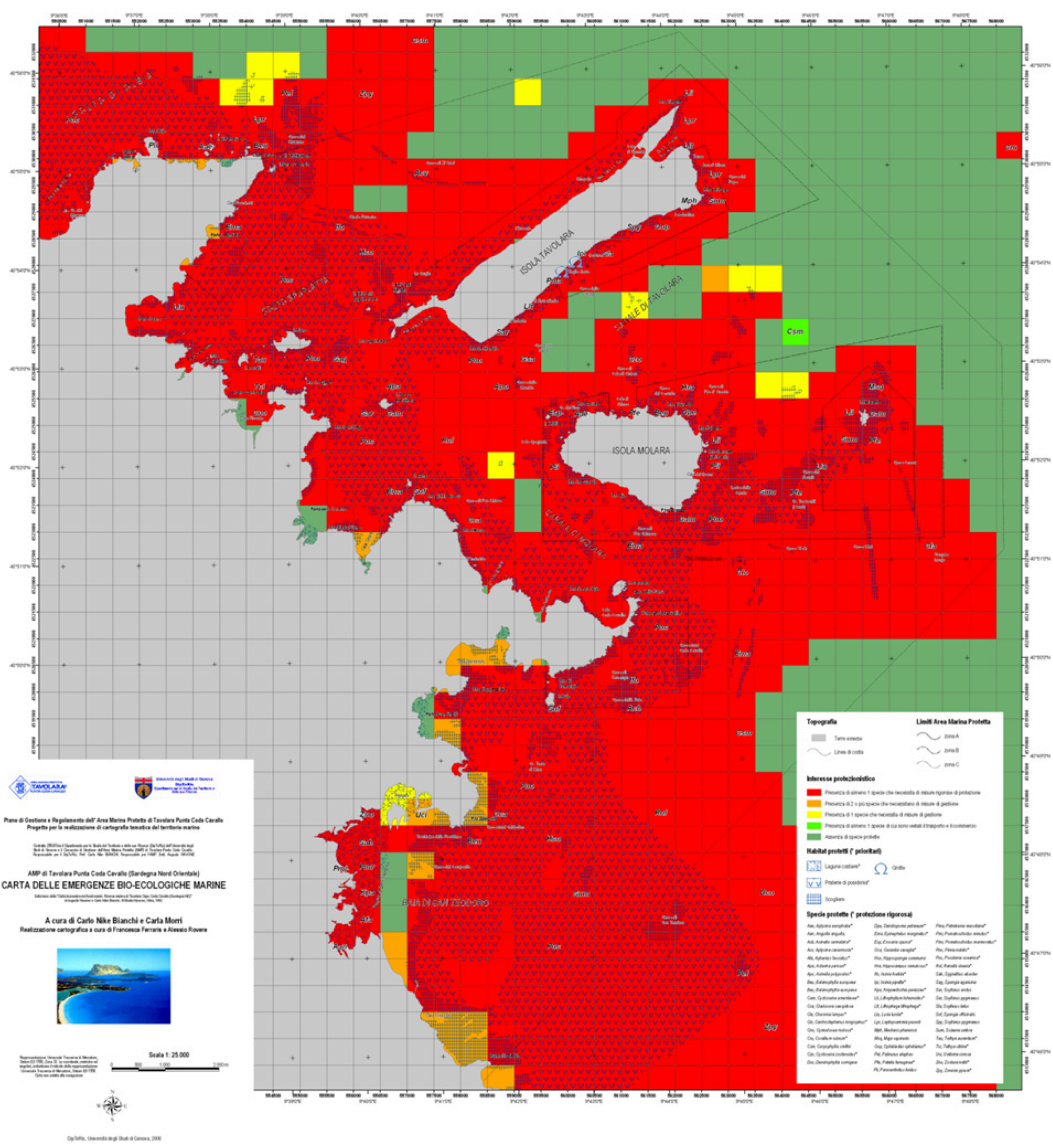
Map of Marine Natural Emergencies

- (1) Red: occurrence of at least one species of the first group (strict protection required);
- (2) Orange: occurrence of two or more species of the second group (management required);
- (3) Yellow: occurrence of one species of the second group (management required);
- (4) Light green: occurrence of at least one species of the third group (trade regulated);
- (5) Dark green: no protected species are present.
3.2. Maps for Marine Territory Evaluation
3.2.1. Map of Degradation and Risk
- (1) Red: very low degradation (little sign of human presence, marine ecosystems in excellent conditions);
- (2) Orange: low degradation (scarce human presence, limited fishing activity, marine ecosystems in near natural conditions);
- (3) Yellow: mean degradation (moderate human presence and fishing activity, marine ecosystems only slightly altered);
- (4) Light green: high degradation (high human presence and fishing activity, occurrence of anthropogenic waste and artefacts on the seafloor, marine ecosystems altered);
- (5) Dark green: very high degradation (harbours and/or completely urbanized areas, marine ecosystems deeply altered).

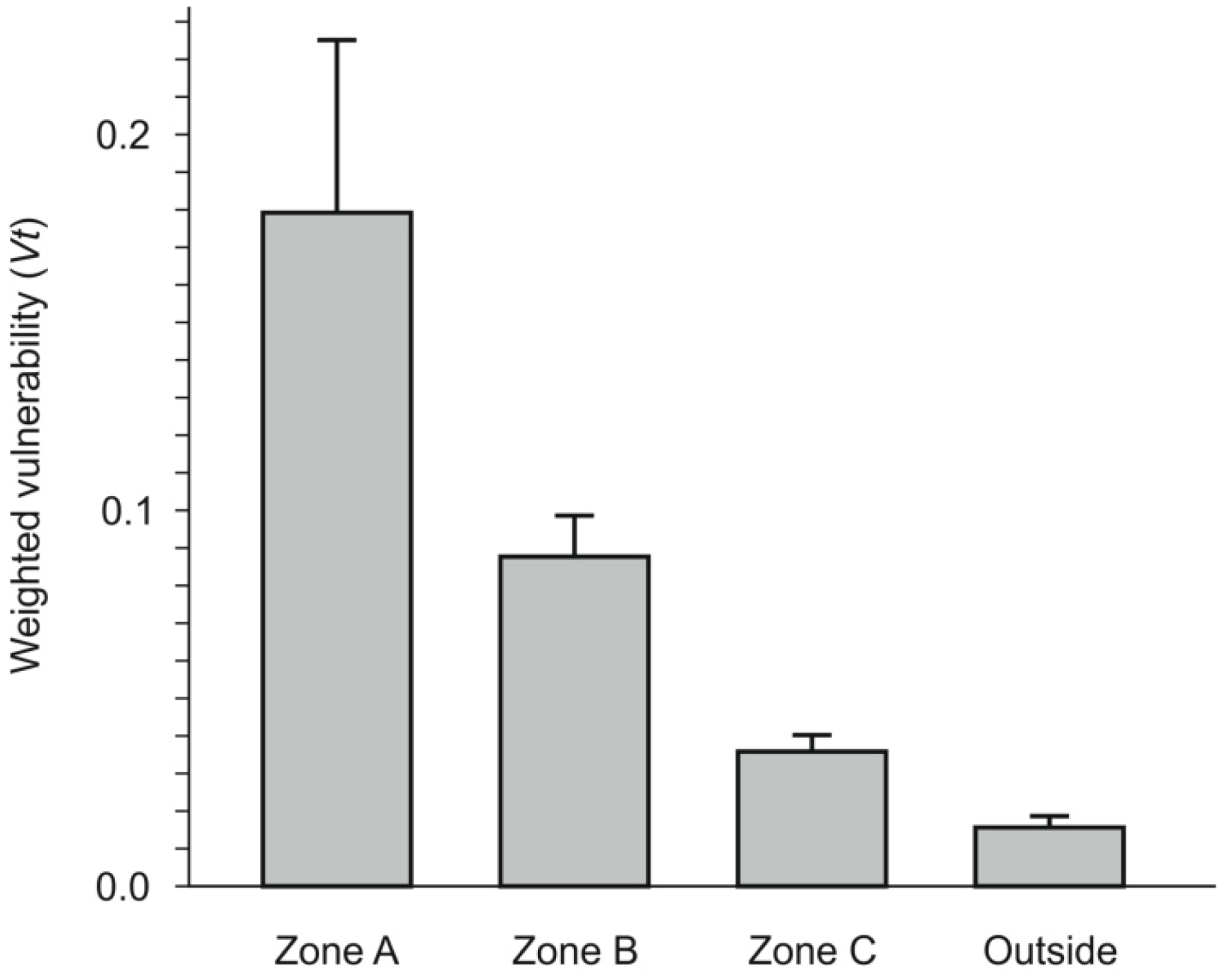
3.2.2. Weighted Vulnerability Map
| Code | Species | Higher taxon | Indication |
|---|---|---|---|
| Aca | Axinella cannabina | Porifera | sea warming |
| Ama | Aphelochaeta marioni | Annelida | rapid and/or intense settling |
| Amu | Aspidosiphon muelleri | Sipuncula | organic settling |
| Apo | Axinella polypoides | Porifera | sea warming |
| Avi | Arthrocladia villosa | Ochrophyta | bottom currents |
| Cca | Capitella capitata | Annelida | pollution |
| Cgi | Corbula gibba | Mollusca | organic and fine particle accumulation |
| Cno | Cymodocea nodosa | Tracheophyta | ecological substitution |
| Cra | Caulerpa racemosa | Chlorophyta | biological invasion |
| Cse | Chaetozone setosa | Annelida | inorganic settling |
| Csi | Colpomenia sinuosa | Ochrophyta | eutrophication |
| Csm | Caryophyllia smithii | Cnidaria | slow settling |
| Dar | Ditrupa arietina | Annelida | sediment instability |
| Dve | Dasycladus vermicularis | Chlorophyta | sea warming |
| Eve | Eunicella verrucosa | Cnidaria | turbidity |
| Evi | Eunice vittata | Annelida | habitat degradation |
| Gun | Glycera unicornis | Annelida | rapid and/or intense settling |
| Hmu | Hypnea musciformis | Rhodophyta | sea warming |
| Lby | Lithophyllum byssoides | Rhodophyta | bioconcretioning |
| Lin | Lithophyllum incrustans | Rhodophyta | overgrazing |
| Lla | Lumbrineris latreilli | Annelida | organic and fine particle accumulation |
| Lsa | Leptogorgia sarmentosa | Cnidaria | turbidity |
| Lst | Lithophyllum stictaeforme | Rhodophyta | bioconcretioning |
| Mci | Minuspio cirrifera | Annelida | organic settling |
| Mga | Mytilus galloprovincialis | Mollusca | eutrophication |
| Mli | Mesophyllum lichenoides | Rhodophyta | bioconcretioning |
| Mph | Madracis pharensis | Cnidaria | sea warming |
| Msp | Myrtea spinifera | Mollusca | organic and fine particle accumulation |
| Opa | Oculina patagonica | Cnidaria | biological invasion |
| Pam | Phyllangia americana mouchezii | Cnidaria | sea warming |
| Pcl | Paramuricea clavata | Cnidaria | benthic-pelagic coupling |
| Pdi | Pennaria disticha | Cnidaria | sea warming |
| Pfi | Paradialychone filicaudata | Annelida | habitat degradation |
| Pfu | Pseudochlorodesmis furcellata | Chlorophyta | sea warming |
| Ppa | Paralacydonia paradoxa | Annelida | organic and fine particle accumulation |
| Sco | Syllis cornuta | Annelida | sedimentary instability |
| Sde | Siphonoecetes dellavallei | Arthropoda | organic settling |
| Sem | Scoletoma emandibulata mabiti | Annelida | rapid and/or intense settling |
| Ser | Schizoporella errata | Bryozoa | eutrophication |
| Spe | Sporochnus pedunculatus | Ochrophyta | bottom currents |
| Tdi | Tellina distorta | Mollusca | sedimentary instability |
| Tfl | Thyasira flexuosa | Mollusca | mineral settling |
| Tfr | Tricleocarpa fragilis | Rhodophyta | warm waters |
| Tov | Timoclea ovata | Mollusca | habitat degradation |
| Uin | Ulva intestinalis | Chlorophyta | eutrophication |
| Ula | Ulva laetevirens | Chlorophyta | eutrophication |
- (1) Red: extremely high vulnerability;
- (2) Orange: very high vulnerability;
- (3) Yellow: high vulnerability;
- (4) Light green: mean vulnerability;
- (5) Dark green: low vulnerability.
3.2.3. Environmental Quality Map

- (1) Red: extremely high environmental quality;
- (2) Orange: very high environmental quality;
- (3) Yellow: high environmental quality;
- (4) Light green: mean environmental quality;
- (5) Dark green: low environmental quality.
3.2.4. Map of Susceptibility to Use

- (1) Red: availability always subordinated to conservation;
- (2) Orange: availability limited and always defined, sometimes impracticable;
- (3) Yellow: availability manifold but defined;
- (4) Light green: broad availability;
- (5) Dark green: maximum availability.

4. Final Remarks
4.1. Efficacy of the RAC-SPA Approach
4.2. Significance of Scale for Territorial Maps
4.3. Importance of Diachronic Cartography
4.4. Adoption of Territorial Cartography in MPA Management
4.5. Landscape Ecology and the Rediscovery of Bionomic Knowledge
4.6. MPAs as Field Laboratories for ICZM
Acknowledgments
References
- Jackson, J.B.C. Ecological extinction and evolution in the brave new ocean. Proc. Natl. Acad. Sci. USA 2008, 105, 11458–11465. [Google Scholar] [CrossRef]
- Craig, R.K. Marine biodiversity, Climate change, and governance of the oceans. Diversity 2012, 4, 224–238. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Sala, E. Unnatural oceans. Sci. Mar. 2001, 65, 273–281. [Google Scholar]
- Sanderson, E.W.; Malanding, J.; Levy, M.A.; Reford, K.H.; Waneebo, A.V.; Woolmer, G. The human footprint and the last of the wild. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Stachowitsch, M. Research on intact marine ecosystems: A lost era. Mar. Pollut. Bull. 2003, 46, 801–805. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar]
- Green, O.O.; Garmestani, A.S. Adaptive management to protect biodiversity: Best available science and the Endangered Species Act. Diversity 2012, 4, 164–178. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of ecosystem services: Putting the issue in perspective. Ecol. Econ. 1998, 25, 67–72. [Google Scholar] [CrossRef]
- Kareiva, P.; Watts, S.; McDonald, R.; Boucher, T. Domesticated nature: Shaping landscapes and ecosystems for human welfare. Science 2007, 316, 1866–1869. [Google Scholar] [CrossRef]
- Allison, G.W.; Lubchenco, J.; Carr, M.H. Marine reserves are necessary but not sufficient for marine conservation. Ecol. Appl. 1998, 8, S79–S92. [Google Scholar]
- Montefalcone, M.; Albertelli, G.; Morri, C.; Parravicini, V.; Bianchi, C.N. Legal protection is not enough: Posidonia oceanica meadows in marine protected areas are not healthier than those in unprotected areas of the northwest Mediterranean Sea. Mar. Pollut. Bull. 2009, 58, 515–519. [Google Scholar]
- Halpern, B.S. The impact of marine reserves: Do reserves work and does reserve size matter? Ecol. Appl. 2003, 13, S117–S137. [Google Scholar] [CrossRef]
- Mora, C.; Andrefouet, S.; Costello, M.J.; Kranenburg, C.; Rollo, A.; Veron, J.; Gaston, K.J.; Myers, R.A. Coral reefs and the global network of marine protected areas. Science 2006, 312, 1750–1751. [Google Scholar]
- Done, T.J.; Reichelt, R.E. Integrated coastal zone and fisheries ecosystem management: Generic goals and performance indices. Ecol. Appl. 1998, 8, S110–S118. [Google Scholar]
- Giakoumi, S.; Katsanevakis, S.; Vassilopoulou, V.; Panayotidis, P.; Kavadas, S.; Issaris, Y.; Kokkali, A.; Frantzis, A.; Panou, A.; Mavromati, G. Could European marine conservation policy benefit from systematic conservation planning? Aquat. Conserv. Mar. Freshw. Ecosyst. 2012. [Google Scholar] [CrossRef]
- Sano, M.; Gonzalez-Riancho, P.; Areizaga, J.; Medina, R. The strategy for coastal sustainability: A Spanish initiative for ICZM. Coast. Manage. 2010, 38, 76–96. [Google Scholar] [CrossRef]
- Fenberg, P.B.; Caselle, J.E.; Claudet, J.; Clemence, M.; Gaines, S.D.; García-Charton, J.A.; Gonçalves, E.J.; Grorud-Colvert, K.; Guidetti, P.; Jenkins, S.R.; et al. The science of European marine reserves: Status, efficacy, and future needs. Mar. Policy 2012, 36, 1012–1021. [Google Scholar] [CrossRef]
- Lester, S.E.; McLeod, K.L.; Tallis, H.; Ruckelshaus, M.; Halpern, B.S.; Levin, P.S.; Chavez, F.P.; Pomeroy, C.; McCay, B.J.; Costello, C.; et al. Science in support of ecosystem-based management for the US West Coast and beyond. Biol. Conserv. 2010, 143, 576–587. [Google Scholar] [CrossRef]
- Brodie, J.; Waterhouse, J. A critical review of environmental management of the “not so Great” Barrier Reef. Estuar. Coast. Shelf Sci. 2012, 104-105, 1–22. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Stelzenmüller, V.; South, A.; Sørensen, T.K.; Jones, P.J.S.; Kerr, S.; Badalamenti, F.; Anagnostou, C.; Breen, P.; Chust, G.; et al. Ecosystem-based marine spatial management: Review of concepts, policies, tools, and critical issues. Ocean Coast. Manag. 2011, 54, 807–820. [Google Scholar] [CrossRef]
- Toonen, R.J.; Andrews, K.R.; Baums, I.B.; Bird, C.E.; Concepcion, G.T.; Daly-Engel, T.S.; Eble, J.A.; Faucci, A.; Gaither, M.R.; Iacchei, M.; et al. Defining boundaries for ecosystem-based management: A multispecies case study of marine connectivity across the Hawaiian Archipelago. J. Mar. Biol. 2011, 2011, 460173. [Google Scholar]
- Salomidi, M.; Katsanevakis, S.; Borja, Á.; Braeckman, U.; Damalas, D.; Galparsoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: A stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci. 2012, 13, 49–88. [Google Scholar]
- Laane, R.W.P.M.; Slijkerman, D.; Vethaak, A.D.; Schobben, J.H.M. Assessment of the environmental status of the coastal and marine aquatic environment in Europe: A plea for adaptive management. Estuar. Coast. Shelf Sci. 2012, 96, 31–38. [Google Scholar]
- Bianchi, C.N.; Zurlini, G. Criteri e prospettive di una classificazione ecotipologica dei sistemi marini costieri italiani. Acqua Aria 1984, 8, 785–796. [Google Scholar]
- Pesch, R.; Schmidt, G.; Schroeder, W.; Weustermann, I. Application of CART in ecological landscape mapping: Two case studies. Ecol. Indic. 2011, 11, 115–122. [Google Scholar]
- Gilman, E. Guidelines for coastal and marine site-planning and examples of planning and management intervention tools. Ocean Coast. Manag. 2002, 45, 377–404. [Google Scholar] [CrossRef]
- Rojas-Nazar, Ú.; Gaymer, C.F.; Squeo, F.A.; Garay-Flühmann, R.; López, D. Combining information from benthic community analysis and social studies to establish no-take zones within a multiple uses marine protected area. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 74–86. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Sgorbini, S.; Zurlini, G. Essai de cartographie benthique du golfe de Gaète (Mer Tyrrhénienne, Italie) à l'aide de la “trend-surface analysis”. Rapp. Comm. Int. Mer Médit. 1985, 29, 221–226. [Google Scholar]
- Bianchi, C.N.; Ardizzone, G.D.; Belluscio, A.; Colantoni, P.; Diviacco, G.; Morri, C.; Tunesi, L. Benthic cartography. Biol. Mar. Mediterr. 2004, 11, 347–370. [Google Scholar]
- Bianchi, C.N. From bionomic mapping to territorial cartography, or from knowledge to management of marine protected areas. Biol. Mar. Mediterr. 2008, 14, 22–51. [Google Scholar]
- Riegl, B.; Korrubel, J.L.; Martin, C. Mapping and monitoring of coral communities and their spatial patterns using a surface-based video method from a vessel. Biol. Mar. Sci. 2001, 692, 869–880. [Google Scholar]
- Willis, K.J.; Jeffers, E.S.; Tovar, C.; Long, P.R.; Caithness, N.; Smit, M.G.D.; Hagemann, R.; Collin-Hansen, C.; Weissenberger, J. Determining the ecological value of landscapes beyond protected areas. Biol. Conserv. 2012, 147, 3–12. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Cinelli, F.; Morri, C. La carta bionomica dei mari toscani: Introduzione, criteri informativi e note esplicative. Atti Soc. Tosc. Sci. Nat. Mem. A 1996, 102, 255–270. [Google Scholar]
- Crain, C.M.; Halpern, B.S.; Beck, M.W.; Kappel, C.V. Understanding and managing human threats to the coastal marine environment. Ann. NY Acad. Sci. 2009, 1162, 39–62. [Google Scholar] [CrossRef]
- Mee, L. Between the Devil and the Deep Blue Sea: The coastal zone in an Era of globalisation. Estuar. Coast. Shelf Sci. 2012, 96, 1–8. [Google Scholar] [CrossRef]
- Newton, A.; Carruthers, T.J.B.; Icely, J. The coastal syndromes and hotspots on the coast. Estuar. Coast. Shelf Sci. 2012, 96, 39–47. [Google Scholar] [CrossRef]
- Cocito, S.; Bianchi, C.N.; Degl'Innocenti, F.; Forti, S.; Morri, C.; Sgorbini, S.; Zattera, A. Esempio di utilizzo di descrittori ambientali nell'analisi ecologica del paesaggio sommerso marino costiero. Atti Soc. Ital. Ecol. 1991, 13, 65–68. [Google Scholar]
- Cocito, S.; Bianchi, C.N. Ordinamento e classificazione di descrittori ambientali nel parco marino Penisola del Sinis-Isola di Mal di Ventre. Oebalia 1992, 17, 495–501. [Google Scholar]
- Pittman, S.; Kneib, R.; Simenstad, C.; Nagelkerken, I. Seascape ecology: Application of landscape ecology to the marine environment. Mar. Ecol. Prog. Ser. 2011, 427, 187–302. [Google Scholar] [CrossRef]
- Ball, I.R.; Possingham, H.P.; Watts, M. Marxan and relatives: Software for spatial conservation prioritisation. In Spatial Conservation Prioritisation: Quantitative Methods and Computational Tools; Moilanen, A., Wilson, K.A., Possingham, H.P., Eds.; University Press: Oxford, UK, 2009; pp. 185–195, Chapter 14. [Google Scholar]
- Teixeira, H.; Borja, Á.; Weisberg, S.B.; Ranasinghe, J.A.; Cadie, D.B.; Dauer, D.M.; Dauvin, J.C.; Degraer, S.; Diaz, R.J.; Grémare, A.; et al. Assessing coastal benthic macrofauna community condition using best professional judgement - developing consensus across North America and Europe. Mar. Pollut. Bull. 2010, 60, 589–600. [Google Scholar] [CrossRef]
- Parravicini, V.; Rovere, A.; Vassallo, P.; Micheli, F.; Montefalcone, M.; Morri, C.; Paoli, C.; Albertelli, G.; Fabiano, M.; Bianchi, C.N. Understanding relationships between conflicting human uses and ecosystem status: A geospatial modeling approach. Ecol. Indic. 2012, 19, 253–263. [Google Scholar]
- Klein, C.J.; Chan, A.; Kircher, L.; Cundiff, A.J.; Gardner, N.; Hrovat, Y.; Scholtz, A.; Kendall, B.E.; Airamé, S. Striking a balance between biodiversity conservation and socioeconomic viability in the design of Marine Protected Areas. Conserv. Biol. 2003, 22, 691–700. [Google Scholar]
- Pandian, P.K.; Ruscoe, J.P.; Shields, M.; Side, J.C.; Harris, R.E.; Kerr, S.A.; Bullen, C.R. Seabed habitat mapping techniques: An overview of the performance of various systems. Mediterr. Mar. Sci. 2009, 10, 29–43. [Google Scholar]
- Seascape Evaluation Assessment and Mapping. Available online: http://www.seamap.it/?page_id=876 (Accessed on 23 November 2012).
- Bianchi, C.N.; Zattera, A. Alcune considerazioni sulla gestione della fascia costiera. Notiz. Soc. It. Biol. Mar. 1986, 10, 25–29. [Google Scholar]
- Vallega, A. Fundamentals of Integrated Coastal Management; Springer: Berlin, Germany, 1999. [Google Scholar]
- Bedulli, D.; Bianchi, C.N.; Morri, C.; Zurlini, G. Caratterizzazione biocenotica e strutturale del macrobenthos delle coste pugliesi. In Indagine Ambientale Del Sistema Marino Costiero Della Regione Puglia; Viel, M., Zurlini, G., Eds.; ENEA: Roma, Italy, 1986; pp. 227–255. [Google Scholar]
- Viel, M.; Bianchi, C.N.; Morri, C.; Peroni, C.; Putti, M.; Rossi, G. Il sistema marino costiero livornese: Aspetti biosedimentologici. In Atti Del Primo Convegno Sullo Stato Dell'ambiente a Livorno; Butta, R., Specchia, A., Eds.; O. Debatte & F.: Livorno, Italy, 1987; Volume 1, pp. 167–175. [Google Scholar]
- Damiani, V.; Bianchi, C.N.; Ferretti, O.; Bedulli, D.; Morri, C.; Viel, M.; Zurlini, G. Risultati di una ricerca ecologica sul sistema marino costiero pugliese. Thalassia Salentina 1988, 18, 153–169. [Google Scholar]
- Damiani, V.; Peroni, C.; Bianchi, C.N.; Rossi, G.; Viel, M.; Morri, C. Ricerche biosedimentologiche sul sistema marino antistante la costa livornese: Risultati preliminari. Acqua Aria 1988b, Volume Speciale, 21–25. [Google Scholar]
- Damiani, V.; Bianchi, C.N.; Sgorbini, S.; Abbate, M.; Morri, C. Caratteristiche ecologiche del tratto marino antistante l'estuario del fiume Magra e interazioni tra fiume e mare. In Studio Ambientale Del Fiume Magra; Abbate, M., Damiani, V., Eds.; ENEA: Roma, Italy, 1989; pp. 203–217. [Google Scholar]
- Ferretti, O.; Niccolai, I.; Bianchi, C.N.; Tucci, S.; Morri, C.; Veniale, F. An environmental investigation of a marine coastal area: Gulf of Gaeta (Tyrrhenian Sea). Hydrobiologia 1989, 176/177, 171–187. [Google Scholar] [CrossRef]
- Delbono, I.; Micheli, I.; Bianchi, C.N.; Ferretti, O.; Morri, C.; Peirano, A. The national coastal plan: First biosedimentological results in a test area in the Ligurian Sea (NW Italy). Rapp. Comm. Int. Mer Médit. 2001, 36, 379. [Google Scholar]
- Lasagna, R.; Montefalcone, M.; Albertelli, G.; Corradi, N.; Ferrari, M.; Morri, C.; Bianchi, C.N. Much damage for little advantage: Field studies and morphodynamic modelling highlighted the environmental impact of an apparently small coastal mismanagement. Estuar. Coast. Shelf Sci. 2011, 94, 255–262. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Baron, J.; Melillo, J.M.; Naiman, R.J.; O’Malley, R.I.; Orians, G.; Palmer, M.A.; Pfaff, A.S.P.; Running, S.W.; Sala, O.E. Aggregate measures of ecosystem services: Can we take the pulse of nature? Front. Ecol. Environ. 2005, 3, 56–59. [Google Scholar] [CrossRef]
- Manson, S.M. Validation and verification of multi-agent models for ecosystem management. In Complexity and Ecosystem Management: The Theory and Practice of Multi-Agent Approaches; Janssen, M., Ed.; Edward Elgar: Northampton, MA, USA, 2003; pp. 63–74. [Google Scholar]
- Dauvin, J.C.; Bellan, G.; Bellan-Santini, D. The need for clear and comparable terminology in benthic ecology. Part I. Ecological concepts. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 432–445. [Google Scholar] [CrossRef]
- Dauvin, J.C.; Bellan, G.; Bellan-Santini, D. The need for clear and comparable terminology in benthic ecology. Part II. Application of the European Directives. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 446–456. [Google Scholar] [CrossRef]
- Chemello, R.; Russo, G.F. Una metodica per la valutazione della qualità ambientale nelle Aree Marine Protette; Valtrend: Napoli, Italy, 2001. [Google Scholar]
- Muxika, I.; Borja, Á.; Bonne, W. The suitability of the marine biotic index (AMBI) to new impact sources along European coasts. Ecol. Indic. 2005, 5, 19–31. [Google Scholar]
- Van Hoey, G.; Borja, Á.; Birchenough, S.; Buhl-Mortensen, L.; Degraer, S.; Fleischer, D.; Kerckhof, F.; Magni, P.; Muxika, I.; Reiss, H.; Schröder, A.; Zettler, M.L. The use of benthic indicators in Europe: From the Water Framework Directive to the Marine Strategy Framework Directive. Mar. Pollut. Bull. 2010, 60, 2187–2196. [Google Scholar] [CrossRef]
- Teixeira, H.; Weisberg, S.B.; Borja, Á.; Ranasinghe, J.A.; Cadien, D.B.; Velarde, R.G.; Lovell, L.L.; Pasko, D.; Phillips, C.A.; Montagne, D.E.; et al. Calibration and validation of the AZTI’s Marine Biotic Index (AMBI) for Southern California marine bays. Ecol. Indic. 2012, 12, 84–95. [Google Scholar]
- Gobert, S.; Sartoretto, S.; Rico-Raimondino, V.; Andral, B.; Chery, A.; Lejeune, P.; Boissery, P. Assessment of the ecological status of Mediterranean French coastal waters as required by the Water Framework Directive using the Posidonia oceanica Rapid Easy Index: PREI. Mar. Pollut. Bull. 2009, 58, 1727–1733. [Google Scholar] [CrossRef]
- Montefalcone, M. Ecosystem health assessment using the Mediterranean seagrass Posidonia oceanica: A review. Ecol. Indic. 2009, 9, 595–604. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. Ecological evaluation of transitional and coastal waters: A marine benthic macrophytes-based model. Mediterr. Mar. Sci. 2001, 2, 45–65. [Google Scholar]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; de Torres, M. A new methodology based on littoral community cartography for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Bardat, J.; Bensettiti, F.; Hindermeyer, X. Approche méthodologique de l’évaluation d’espaces naturels: Exemple de l’application de la directive habitats en France. Écologie 1997, 28, 45–59. [Google Scholar]
- Bellan-Santini, D.; Bellan, G.; Bitar, G.; Harmelin, J.G.; Pergent, G. Handbook for Interpreting Types of Marine Habitat for the Selection of Sites to Be Included in the National Inventories of Natural Sites of Conservation Interest; RAC SPA: Tunis, Tunisia, 2002. [Google Scholar]
- Relini, G. Nuovi contributi per la conservazione della biodiversità marina in Mediterraneo. Biol. Mar. Mediterr. 2000, 7, 173–211. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Nature conservation, Marine Protected Areas, sustainable development and the flow of invasive species to the Mediterranean Sea. Sci. Rep. Port Cros Natl. Park 2005, 21, 29–54. [Google Scholar]
- Orrù, P.; Panizza, V.; Ulzega, A. Submerged geomorphosites in the marine protected areas of Sardinia (Italy): Assessment and improvement. Quatern. 2005, 18, 167–174. [Google Scholar]
- Rovere, A.; Vacchi, M.; Parravicini, V.; Morri, C.; Bianchi, C.N.; Firpo, M. Bringing geoheritage underwater: Methodological approaches to evaluation and mapping. In Mapping Geoheritage; Regolini-Bissig, G., Reynard, E., Eds.; Institut de Géographie, Travaux et Recherches: Lausanne, Switzerland, 2010; Volume 35, pp. 65–80. [Google Scholar]
- Rovere, A.; Vacchi, M.; Parravicini, V.; Bianchi, C.N.; Zouros, N.; Firpo, M. Bringing geoheritage underwater: Definitions, methods, and application in two Mediterranean marine areas. Environ. Earth Sci. 2011, 64, 133–142. [Google Scholar] [CrossRef]
- Rovere, A.; Parravicini, V.; Firpo, M.; Morri, C.; Albertelli, G.; Bianchi, C.N. Emergenze naturalistiche dell’Area Marina Protetta di Bergeggi (SV): Integrazione di aspetti biologici, ecologici e geomorfologici. Biol. Mar. Mediterr. 2007, 14, 86–87. [Google Scholar]
- Rovere, A.; Parravicini, V.; Firpo, M.; Morri, C.; Bianchi, C.N. Combining geomorphologic, biological and accessibility values for marine natural heritage evaluation and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 541–552. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Ballesteros, E.; Ben Maiz, N.; Boisset, F.; Bouladier, E.; Cinelli, F.; Cirik, S.; Cormaci, M.; Jeudy de Grissac, A.; Laborel, J.; et al. Livre Rouge ‘Gérard Vuigner’ des végétaux, peuplements et paysages marins menacés de Méditerranée; UNEP MAP (Technical Report Series No. 43): Athens, Greece, 1990. [Google Scholar]
- Boudouresque, C.F.; Avon, M.; Gravez, V. Les espèces marines à protéger en Méditerranée; GIS Posidonie: Marseille, France, 1991. [Google Scholar]
- Boudouresque, C.F.; Beaubrun, P.C.; Relini, G.; Templado, J.; van Klaveren, M.C.; van Klaveren, P.; Walmsley, J.G. Critères de sélection et liste révisée des espèces en danger et menacées (marines et saumâtres) en Méditerranée; GIS Posidonie: Marseille, France, 1996. [Google Scholar]
- Possingham, H.P.; Grantham, H.; Rondinini, C. How can you conserve species that haven’t been found? J. Biogeogr. 2007, 34, 758–759. [Google Scholar] [CrossRef]
- Ridder, B. The naturalness versus wildness debate: Ambiguity, inconsistency, and unattainable objectivity. Restor. Ecol. 2007, 15, 8–12. [Google Scholar] [CrossRef]
- Reza, M.I.H.; Abdullah, S.A. Regional Index of Ecological Integrity: A need for sustainable management of natural resources. Ecol. Indic. 2011, 11, 220–229. [Google Scholar]
- Machado, A. An index of naturalness. J. Nat. Conserv. 2004, 12, 95–110. [Google Scholar] [CrossRef]
- Gobster, P.H.; Nassauer, J.I.; Daniel, T.C.; Fry, G. The shared landscape: What does aesthetics have to do with ecology? Landsc. Ecol. 2007, 22, 959–972. [Google Scholar] [CrossRef]
- Diviacco, G. Indagine sulla qualità dell’ambiente naturale costiero marino in Liguria. Stato di conservazione delle biocenosi bentiche costiere; Regione Liguria, Ufficio Parchi e Aree Protette: Genova, Italy, 1998. [Google Scholar]
- Bianchi, C.N.; Morri, C. Indicatori biologici ed ecologici nell’ambiente marino. In Studi per la creazione di strumenti di gestione costiera: Golfo del Tigullio; Ferretti, O., Ed.; ENEA: La Spezia, Italy, 2003; pp. 111–120. [Google Scholar]
- Hobday, A.J. Sliding baselines and shuffling species: Implications of climate change for marine conservation. Mar. Ecol. 2011, 32, 392–403. [Google Scholar] [CrossRef]
- Goberville, E.; Beaugrand, G.; Sautour, B.; Tréguer, P. Evaluation of coastal perturbations: A new mathematical procedure to detect changes in the reference state of coastal systems. Ecol. Indic. 2011, 11, 1290–1300. [Google Scholar]
- Borja, Á.; Dauer, D.M.; Grémare, A. The importance of setting targets and reference conditions in assessing marine ecosystem quality. Ecol. Indic. 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Skondras, N.A.; Karavitis, C.A.; Gkotsis, I.I.; Scott, P.J.B.; Kaly, U.L.; Alexandris, S.G. Application and assessment of the Environmental Vulnerability Index in Greece. Ecol. Indic. 2011, 11, 1699–1706. [Google Scholar]
- Borja, Á. The European water framework directive: A challenge for nearshore, coastal and continental shelf research. Cont. Shelf Res. 2005, 25, 1768–1783. [Google Scholar] [CrossRef]
- Muxika, I.; Borja, Á.; Bald, J. Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 16–29. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Carstensen, J.; Carvalho, L.; Elliott, M.; Feld, C.K.; Heiskanen, A.S.; Johnson, R.K.; Moe, J.; Pont, D.; et al. The European Water Framework Directive at the age of 10: A critical review of the achievements with recommendations for the future. Sci. Total Environ. 2010, 408, 4007–4019. [Google Scholar] [CrossRef] [Green Version]
- Borja, Á.; Elliott, M.; Carstensen, J.; Heiskanen, A.S.; van de Bund, W. Marine management—Towards an integrated implementation of the European Marine Strategy Framework and the Water Framework Directives. Mar. Pollut. Bull. 2010, 60, 2175–2186. [Google Scholar] [CrossRef]
- Gatti, G.; Montefalcone, M.; Rovere, A.; Parravicini, V.; Morri, C.; Albertelli, G.; Bianchi, C.N. Seafloor integrity down the harbor waterfront: The coralligenous shoals off Vado Ligure (NW Mediterranean). Adv. Oceanogr. Limnol. 2012, 3, 51–67. [Google Scholar] [CrossRef]
- Rice, J.; Arvanitidis, C.; Borja, Á.; Frid, C.; Hiddink, J.G.; Krause, J.; Lorance, P.; Ragnarsson, S.A.; Skold, M.; Trabucco, B.; et al. Indicators for Sea-floor Integrity under the European Marine Strategy Framework Directive. Ecol. Indic. 2012, 12, 174–184. [Google Scholar]
- Simboura, N.; Zenetos, A.; Pancucci-Papadopoulou, M.A.; Reizopoulou, S.; Streftaris, N. Indicators for the Sea-floor Integrity of the Hellenic Seas under the European Marine Strategy Framework Directive: Establishing the thresholds and standards for Good Environmental Status. Mediterr. Mar. Sci. 2012, 13, 140–152. [Google Scholar]
- Tunesi, L.; Moss, D.; Evans, D.; Mo, G.; Di Martino, V. The Mediterranean marine habitats in the EuNIS European system. Biol. Mar. Mediterr. 2006, 13, 224–225. [Google Scholar]
- Swaney, D.P.; Humborg, C.; Emeis, K.; Kannen, A.; Silvert, W.; Tett, P.; Pastres, R.; Solidoro, C.; Yamamuro, M.; Hénocque, Y.; et al. Five critical questions of scale for the coastal zone. Estuar. Coast. Shelf Sci. 2012, 96, 9–21. [Google Scholar] [CrossRef]
- Parravicini, V.; Donato, M.; Rovere, A.; Montefalcone, M.; Albertelli, G.; Bianchi, C.N. Indagine preliminare sul coralligeno dell’area di Bergeggi (SV): Tipologie ed ipotesi sul suo mantenimento. Biol. Mar. Mediterr. 2007, 14, 162–163. [Google Scholar]
- Bianchi, C.N. Il monitoraggio della biodiversità nelle Aree Marine Protette: Considerazioni scientifiche e metodologiche. Notiz. Soc. Ital. Biol. Mar. 2002, 41, 89–95. [Google Scholar]
- Bianchi, C.N. Climate change and biological response in the marine benthos. Proc. Assoc. Ital. Oceanol. Limnol. 1997, 12, 3–20. [Google Scholar]
- Bianchi, C.N.; Boero, F.; Fonda Umani, S.; Morri, C.; Vacchi, M. Successione e cambiamento negli ecosistemi marini. Biol. Mar. Mediterr. 1998, 5, 117–135. [Google Scholar]
- Montefalcone, M.; Parravicini, V.; Bianchi, C.N. Quantification of coastal ecosystem resilience. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Academic Press: Waltham, MA, USA, 2011; Volume 10, (Ecohydrology and Restoration), pp. 49–70, Chapter 3. [Google Scholar]
- Barsanti, M.; Delbono, I.; Ferretti, O.; Peirano, A.; Bianchi, C.N.; Morri, C. Measuring change of Mediterranean coastal biodiversity: Diachronic mapping of the meadow of the seagrass Cymodocea nodosa (Ucria) Ascherson in the Gulf of Tigullio (Ligurian Sea, NW Mediterranean). Hydrobiologia 2007, 580, 35–41. [Google Scholar] [CrossRef]
- Montefalcone, M.; Rovere, A.; Parravicini, V.; Albertelli, G.; Morri, C.; Bianchi, C.N. Evaluating change in seagrass meadows: A time-framed comparison of Side Scan Sonar maps. Aquat. Bot. 2012. [Google Scholar] [CrossRef]
- Leriche, A.; Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Denis, J. A one century suite of seagrass bed maps: Can we trust ancient maps? Estuar. Coast. Shelf Sci. 2004, 59, 353–362. [Google Scholar] [CrossRef]
- Montefalcone, M.; Baudana, M.; Venturini, S.; Lasagna, R.; Bianchi, C.N.; Albertelli, G. Distribuzione spaziale delle praterie di Posidonia oceanica nell’Area Marina Protetta di Portofino. Biol. Mar. Mediterr. 2006, 13, 90–91. [Google Scholar]
- Montefalcone, M.; Giovannetti, E.; Lasagna, R.; Parravicini, V.; Morri, C.; Albertelli, G. Analisi diacronica della prateria di Posidonia oceanica nell’Area Marina Protetta di Bergeggi. Biol. Mar. Mediterr. 2007, 14, 82–83. [Google Scholar]
- Montefalcone, M.; Parravicini, V.; Vacchi, M.; Albertelli, G.; Ferrari, M.; Morri, C.; Bianchi, C.N. Human influence on seagrass habitat fragmentation in NW Mediterranean Sea. Estuar. Coast. Shelf Sci. 2010, 86, 292–298. [Google Scholar] [CrossRef]
- Villa, F.; Tunesi, L.; Agardy, T. Zoning marine protected areas through spatial multiple-criteria analysis: The case of the Asinara Island national marine reserve of Italy. Conserv. Biol. 2002, 16, 1–12. [Google Scholar] [CrossRef]
- Kappel, C.V.; Halpern, B.S.; Martone, R.G.; Micheli, F.; Selkoe, K.A. In the zone: Comprehensive ocean protection. Issues Sci. Technol. 2009, 25, 33–44. [Google Scholar]
- Guidetti, P. The importance of experimental design in detecting the effects of protection measures on fish in Mediterranean MPAs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2002, 12, 619–634. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Importanza della fauna bentonica per il monitoraggio della biodiversità nelle Aree Marine Protette. In Le Aree Marine Protette del Mediterraneo; Carrada, G.C., Coiro, P., Russo, G.F., Eds.; Electa: Napoli, Italy, 2003; pp. 125–134. [Google Scholar]
- Merino, G.; Maynou, F.; Boncoeur, J. Bioeconomic model for a three-zone Marine Protected Area: A case study of Medes Islands (northwest Mediterranean). ICES J. Mar. Sci. 2009, 66, 147–154. [Google Scholar]
- Farina, A. Principles and methods in landscape ecology; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Bastian, O. Landscape ecology—towards a unified discipline? Landsc. Ecol. 2002, 16, 757–766. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Catra, M.; Giaccone, G.; Morri, C. Il paesaggio marino costiero: Ambienti e diversità. In Mediterraneo: Ambienti, paesaggio, diversità; Cosentino, A., La Posta, A., Maggiore, A.M., Tartaglini, N., Eds.; Téchne: Milano, Italy, 2005; pp. 30–61. [Google Scholar]
- Bianchi, C.N.; Morri, C. L’approccio bionomico per la caratterizzazione e la zonazione dell’ambiente marino costiero: Una rassegna introduttiva. Proc. Ital. Assoc. Oceanol. Limnol. 2001, 14, 401–434. [Google Scholar]
- Morri, C.; Bellan-Santini, D.; Giaccone, G.; Bianchi, C.N. Principles of bionomy: Definition of assemblages and use of taxonomic descriptors (macrobenthos). Biol. Mar. Mediterr. 2004, 11 (Suppl. 1), 573–600. [Google Scholar]
- Bianchi, C.N.; Ceppodomo, I.; Galli, C.; Sgorbini, S.; Dell'Amico, F.; Morri, C. Benthos dei mari toscani. I: Livorno—Isola d'Elba (crociera Enea 1985). In Arcipelago Toscano. Studio oceanografico, sedimentologico, geochimico e biologico; Ferretti, O., Immordino, F., Damiani, V., Eds.; ENEA: Roma, Italy, 1993; pp. 263–290. [Google Scholar]
- Bianchi, C.N.; Ceppodomo, I.; Niccolai, I.; Aliani, S.; de Ranieri, S.; Abbiati, M.; Dell'Amico, F.; Morri, C. Benthos dei mari toscani. II: Isola d'Elba—Montecristo (crociera Enea 1986). In Arcipelago Toscano. Studio oceanografico, sedimentologico, geochimico e biologico; Ferretti, O., Immordino, F., Damiani, V., Eds.; ENEA: Roma, Italy, 1993; pp. 291–315. [Google Scholar]
- Bianchi, C.N.; Ceppodomo, I.; Cocito, S.; Aliani, S.; Dell'Amico, F.; Cattaneo-Vietti, R.; Morri, C. Benthos dei mari toscani. III: La Spezia—Livorno (crociera Enea 1987). In Arcipelago Toscano. Studio oceanografico, sedimentologico, geochimico e biologico; Ferretti, O., Immordino, F., Damiani, V., Eds.; ENEA: Roma, Italy, 1993; pp. 317–337. [Google Scholar]
- Boudouresque, C.F.; Avon, M.; Pergent-Martini, C. Qualité du milieu marin: Indicateurs biologiques et physico-chimiques; GIS Posidonie: Marseille, France, 1993. [Google Scholar]
- Dauvin, J.C.; Bellan, G.; Bellan-Santini, D. Benthic indicators: From subjectivity to objectivity—where is the line? Mar. Pollut. Bull. 2010, 60, 947–953. [Google Scholar] [CrossRef]
- UNEP MAP, Guidelines for the development of ecological status and stress reduction indicators for the Mediterranean region. UNEP MAP, Technical Reports Series No. 154: Athens, Greece, 2004.
- Magni, P.; Hyland, J.; Manzella, G.; Rumohr, H.; Viaroli, P.; Zenetos, A. Indicators of stress in the marine benthos; UNESCO, IOC Workshop Report No. 195, IMC Special Publication: Paris, France, 2005. [Google Scholar]
- Tunesi, L.; Diviacco, G. Environmental and socio-economic criteria for the establishment of marine coastal parks. Int. J. Environ. Stud. 1993, 43, 253–259. [Google Scholar] [CrossRef]
- Mandelik, Y.; Roll, U.; Fleischer, A. Cost-efficiency of biodiversity indicators for Mediterranean ecosystems and the effects of socio-economic factors. J. Appl. Ecol. 2010, 47, 1179–1188. [Google Scholar] [CrossRef]
- Schultz, E.T.; Johnston, R.J.; Segerson, K.; Besedin, E.Y. Integrating ecology and economics for restoration: Using ecological indicators in valuation of ecosystem services. Restor. Ecol. 2012, 20, 304–310. [Google Scholar]
- Foley, M.M.; Halpern, B.S.; Micheli, F.; Armsby, M.H.; Caldwell, M.R.; Crain, C.M.; Prahler, E.; Rohr, N.; Sivas, D.; Beck, M.W.; et al. Guiding ecological principles for marine spatial planning. Mar. Policy 2010, 34, 955–966. [Google Scholar] [CrossRef]
- McCay, B.J.; Jones, P.J.S. Marine protected areas and the governance of marine ecosystems and fisheries. Conserv. Biol. 2011, 25, 1130–1133. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Climate change and biological response in Mediterranean Sea ecosystems—a need for broad-scale and long-term research. Ocean Challenge 2004, 13, 32–36. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bianchi, C.N.; Parravicini, V.; Montefalcone, M.; Rovere, A.; Morri, C. The Challenge of Managing Marine Biodiversity: A Practical Toolkit for a Cartographic, Territorial Approach. Diversity 2012, 4, 419-452. https://doi.org/10.3390/d4040419
Bianchi CN, Parravicini V, Montefalcone M, Rovere A, Morri C. The Challenge of Managing Marine Biodiversity: A Practical Toolkit for a Cartographic, Territorial Approach. Diversity. 2012; 4(4):419-452. https://doi.org/10.3390/d4040419
Chicago/Turabian StyleBianchi, Carlo Nike, Valeriano Parravicini, Monica Montefalcone, Alessio Rovere, and Carla Morri. 2012. "The Challenge of Managing Marine Biodiversity: A Practical Toolkit for a Cartographic, Territorial Approach" Diversity 4, no. 4: 419-452. https://doi.org/10.3390/d4040419
APA StyleBianchi, C. N., Parravicini, V., Montefalcone, M., Rovere, A., & Morri, C. (2012). The Challenge of Managing Marine Biodiversity: A Practical Toolkit for a Cartographic, Territorial Approach. Diversity, 4(4), 419-452. https://doi.org/10.3390/d4040419





