On the Breeds of Cattle—Historic and Current Classifications
Abstract
: Classification of cattle breeds contributes to our understanding of the history of cattle and is essential for an effective conservation of genetic diversity. Here we review the various classifications over the last two centuries and compare the most recent classifications with genetic data. The classifications devised during the 19th to the late 20th century were in line with the Linnaean taxonomy and emphasized cranial or horn morphology. Subsequent classifications were based on coat color, geographic origin or molecular markers. Several theories were developed that linked breed characteristics either to a supposed ancestral aurochs subspecies or to a presumed ethnic origin. Most of the older classifications have now been discarded, but have introduced several Latin terms that are still in use. The most consistent classification was proposed in 1995 by Felius and emphasizes the geographic origin of breeds. This is largely in agreement with the breed clusters indicated by a biochemical and molecular genetic analysis, which reflect either groups of breeds with a common geographic origin or single breeds that have expanded by export and/or crossbreeding. We propose that this information is also relevant for managing the genetic diversity of cattle.1. Introduction
Represented by a worldwide population of about 1.4 billion animals, cattle are our most important livestock species. As the major source of milk, meat, hides and draught power, cattle may be considered as multi-purpose livestock. In addition, since their domestication, they have played a major role in human culture by participating in fighting games, racing and religious ceremonies. Because of the animal's size, the husbandry of cattle requires a more organized management than the keeping of other livestock, which may well have made a major contribution to the growing complexity and stratification of early agricultural societies [1]. As with other domestic species, their dispersal over different continents and adaptation to various environments has led to the development of many types of cattle [1]. This wide variety of characteristics evolved over thousands of years, but was accentuated by the development of well defined, specialized and genetically isolated breeds during the last centuries.
After World War II and even more in the last quarter of the 20th century, this process has resulted in the global use of only a few of the most productive of these specialized breeds, which expanded at the expense of local, seemingly less productive populations. There is now a growing awareness that the diversity of cattle should be conserved and local breeds should be protected from extinction, although commercial interests still promote the ‘industrial’ breeds. However, the modern breeding techniques such as artificial insemination, cryopreservation and cloning by which the productive breeds expanded may also contribute to the conservation of local breeds. In order to make an optimal choice during conservation programs, it is essential to describe the relationships between breeds and the current diversity in the form of a consistent and comprehensive classification.
Here we review and compare the various classifications of cattle that have been proposed since the 19th century until recently. The first classifications were inspired by Linnaean taxonomy and emphasized cranial or horn morphology. Subsequent classifications were based on coat color, geographic origin or molecular markers. Several theories were developed that linked breed characteristics either to a supposed ancestral aurochs subspecies or to a presumed ethnic origin. Most of the older classifications can now be shown to have had serious shortcomings, but have introduced several Latin terms that are still in use. The most systematic classification was proposed in 1995 by Felius [2], which emphasized the geographic origin of breeds and is largely in agreement with the breed clusters indicated by biochemical and molecular genetic analyses.
2. On the Classification of Organisms
In general, classification is an attempt to devise a well defined ordering of the objects that are being studied. For living species this is achieved by grouping similar organisms together in a non-overlapping hierarchical arrangement. This is the core activity of the science of systematics, which by classifying organisms describes the diversity of organisms and infers their evolutionary relationships. The first classification of living creatures was developed by the classical scholar Aristotle, who distinguished species by habitat and means of reproduction and divided animals into higher and lower classes [3]. Linnaeus in 1758 [4] laid the foundation of the modern biological classification with the introduction of a binary nomenclature (genus name followed by species name) and a definitive species concept. By creating a hierarchy of orders, families, tribes, genera, species and subspecies for all sorts of organisms known at the time, Linnaeus founded the sciences of systematics and taxonomy.
Because the concept of evolution as proposed by Darwin became only accepted after 1859, the Linnaean classification was meant to be static: all species were as created by God: “Thus the man gave names to all cattle, to the birds of heaven, and to every wild animal” (Genesis 2:20 [5]). However, several pre-Darwinian scholars had already separated biblical and natural history. Buffon [6] proposed even in 1749 that the 200–300 mammalian species known by that time had evolved over a 10,000 year period from the degeneration of about 40 basic forms and in 1809 Lamarck [7] published an evolutionary theory involving the inheritance of acquired properties. After the Darwinian revolution, it became common to interpret the classification of a group of organisms in the same group as an indication of common ancestry. The classification of domestic animals with their wild ancestor species—like Bos taurus and Bos indicus with Bos primigenius and Bos namadicus, respectively—is most obvious. As we will show below, the lower-level classification of the various types of cattle is less unambiguous.
3. Why it is Useful to Classify Cattle
Classification of the hundreds of cattle breeds orders a large, seemingly chaotic variety in both appearance and performance into a consistent scheme. Placing breeds and varieties into well-defined groups reveals relations between types, subtypes, breeds and varieties. This information may be relevant for various reasons:
Relationships between breeds allow a reconstruction of their history. Lack of documentation on the history of cattle breeding has created room for unfounded fiction, which, once printed, has often been amplified into a general belief. For instance, the longhorned Salers cattle are assumed to have descended directly from local aurochs that are depicted with similar horns in the nearby caves of Lascaux, but molecular evidence shows a close relationship with Alpine cattle.
A classification may point out the uniqueness of a breed, which may be relevant to conservation. For some 20 years there has been an increasing interest in the preservation of local breeds, not only because genetic diversity may become irreversibly lost, but also because the breeds are perceived to belong to the cultural and historic heritage.
Breed classification will also promote a better appreciation of the value of local breeds, often adapted to their environment and suitable for extensive management. This would prevent a counterproductive introduction of highly productive breeds in regions suitable only for extensive management, which has been practised since the mid-20th century on an unforgivably wide scale. Rehabilitation and revaluation of locally adapted breeds will not only result in a sustainable conservation, but also improve agricultural production under local conditions.
4. Why it is Difficult to Classify Cattle
During the last two centuries several kinds of classifications have been developed in order to identify types and breeds of cattle. Several criteria have been used, such as coat color, horn size, cranial types, geography, (presumed) origin, and purpose or combinations of these. However, this nearly always resulted in a simplification that only described a part of a complex reality. This not only makes such classifications largely arbitrary, but also diminishes its usefulness as described above.
Several factors complicate the classification of cattle. Most of these apply to any subspecies classification, but for domestic animals the continual intervention of man and our perceptions of breeds introduce additional complications.
4.1. Unknown History
Written records on the history of cattle older than the 18th century are scant or do not exist. We do know that most European breeds are not older than the period of the industrial revolution, when systematic selective breeding started. Many so-called ‘land cattle breeds’ or ‘land races’ are ascribed an ancient origin, or advertised as: “known in the region since times immemorial”, but are relatively new. Early records are available only for a few breed types, such as the English White Park Cattle and possibly the Chianina, similar to the cattle from Lucania described by Virgil in the first century AD [8]. However, there is little documented information on the diversity of cattle before breed formation, on the influence of migrations [1] and on the genetic roots of the current breeds. Presumably, genetic exchange among cattle populations was common and depended on their geographic proximity.
4.2. Gradual Differences between Breeds
Differences between breeds are not as absolute as between species, as for instance the clear-cut difference between cattle, yak and bison. Breeds not only originated relatively recently from a common gene pool, but genetic isolation is rarely absolute (see below). Even the demarcation of zebu and taurine cattle, which evolved from two different sources and are clearly different in morphology, adaptation and behavior, is arbitrary since many intermediate types are known and several breeds have been developed by taurine-indicine crossbreeding [2].
4.3. Genetic Exchange between Breeds
As mentioned already, gene flow between neighboring regions was likely to be common before breed formation in the 18th century, but clearly did not stop when cattle were partitioned into breeds. More often than not, the history of breeds mentions deliberate upgrading in order to improve production characteristics by using bulls of other populations from the same or a different country [2]. For instance, the British Shorthorn was a popular breeding sire for many European breeds in the 19th century. Now the Dairy Shorthorn has itself been crossed with Red Holstein and Danish Red, resulting in the Blended Red and White Shorthorn, while only few traditional Beef Shorthorn lines have remained pure. In other cases upgrading was minimal and transitional, like the use of British Shorthorn in the French Charolais, now one of the foremost beef breeds, or the introgression of Brown Swiss in Danish Red.
4.4. Multiple Origins of Breeds
Several breeds have absorbed other breeds or local varieties. A few examples:
- -
The well-known Southwest-French Blonde d'Aquitaine and the Swiss-German Simmental-Fleckvieh were both formed by amalgamating several local strains.
- -
Heck cattle, claimed to be a revival of the wild aurochs, were developed by a few generations of crossbreeding of dairy, dual-purpose and primitive looking breeds.
- -
American and Australian cattle breeders, who are less inhibited by traditional preferences than their European colleagues, have created numerous synthetic breeds by combining European and Asian breeds from different origins [2].
4.5. Variation Within a Breed; Allopatric Development
Varieties within breeds may be more important than differences between separate breeds. For instance, the Belgian White-Blue breed includes an extremely heavy double-muscled type, a less heavy double muscled type and a dual-purpose type. For a few ‘cosmopolitan’ breeds, systematic breeding has led to ‘allopatric development’: populations are taken to another region, such as the New World, are developed in their new environment and then pass on their newly acquired characteristics to the original ancestor population. In North America the dual-purpose Swiss Brown was reformed into a single-purpose dairy breed, called Brown-Swiss, and has now influenced its parental stock. The most well-known example is of course the American development of the black-pied dairy Dutch-Friesian, already reputed because of its high milk production, into the highly productive Holstein, which then changed the Friesian-type cattle into the Holstein-Friesian, all over Europe.
4.6. Changes over Time
Several breeds are different now from what they were only 20 years ago. In fact, selective breeding has accelerated the evolution of cattle to the point that the last two centuries saw more changes in appearance and production than the preceding millennia [1]. Breeding objectives are not fixed, but follow changes, for example new preferences and requirements of consumers. By the late 19th century, Dutch-Friesian cattle were of a large, refined, single-purpose dairy type; in the 1930′s they were mainly of a stronger, courser type; and in the 1950s they were of a small, deep bodied dual-purpose type. Today pure Dutch-Friesians are of a medium, milky dual-purpose type. In Holstein-Friesian, selection of the quantity of milk has changed to a preference for high protein content.
5. Historic Classifications
5.1. Overview
In the early 19th to the late 20th century, the Linnaean style of taxonomy with its emphasis on differences in morphology led to classifications that were based on cranial shapes and the length and curving of the horns. This could be linked to comparisons of excavated fossilized cattle skulls by archaeologists and zoologists of the 19th century. In this period presumed basic forms were granted Latin names, several of which are still in use. Appendix 1 in the supplementary information lists the various Latin terms that have been introduced by various authors. The most influential cranial classifications were from the German-speaking school.
Coat color was used as a criterion for classifications from 1896 and this continued until 1993. Around 1900 the morphological classifications of cattle were correlated with a supposed historic origin, assuming that different peoples or tribes kept their own types of cattle.
For Iberian cattle breeds, standards were hardly defined until the mid-20th century with the Lidia fighting cattle being the only exception. Breeds were classified according to external type, color pattern and regional origin. Iberian authors assumed a descent from various types of aurochs in order to explain the different types of cattle [9-11].
In the 20th century the attention shifted to the economic importance of breeds. European breeds were described per country or continental region and those considered of little value were ignored. A limited number of highly productive breeds expanded at the cost of many local breeds. It was not before the late 1960s that new interest arose in local breeds and the conservation of genetic resources. This led to the compilation of livestock breed databases (reviewed by Groeneveld et al. [12]). In 1995 Felius published a nearly complete cattle breed encyclopedia with a classification based on a combination of geographic origin and morphological type.
Meanwhile, progress in genetics led to molecular classifications. After the biochemical studies of Baker and Manwell from 1980 [13], based on limited numbers of genetic markers, the last decade saw the analysis of more comprehensive breed panels with DNA-based markers [12]. These are now being superseded by high-throughput SNP genotyping and even genomic sequencing.
Below, the various classifications of cattle are discussed in more detail. These are not only interesting from a historical point of view, but also reflect the diverse regional or national perceptions of the diversity of cattle.
5.2. Cranial Horn-Type Classifications
From the late 18th century archaeozoologists became interested in the origin of domestic cattle. Assuming that the crania of cattle had stayed relatively unchanged in the course of history, different cranial types of Neolithic cattle were considered as archetypes of domestic cattle. In what probably was the first book on British cattle breeds, Youatt [14] presented in 1834 a classification based on the length of the horns as the most convenient classification: the long-horns, the middle-horns, the short-horns and polled cattle. Irish Cattle were added as a geographical group.
In 1843 Owen introduced the term brachyceros for shorthorned cattle [15], but in 1846 renamed it Bos longifrons. The Neolithic shorthorned cattle type was described in great detail by Rütimeyer (1867, [16]), who is considered as the founder of domestic animal archaeozoology. Rütimeyer [16] examined many cattle fossils and identified two aurochs species: Bos primigenius [17] and an early form of Indian aurochs denoted as Bos namadicus [18], which he (incorrectly) presumed to be the parental form of the Bos primigenius. He also proposed that shorthorned cattle represented the oldest and most widespread form of domestic cattle (Bos taurus) of Neolithic Europe, the origin of which had to be sought in Asia. On the contrary, Adametz [19] considered in 1898 the brachyceros as a genuine European wild form, but Leithner ([20], cited by [21]) assumed in 1926 a descent from local primigenius animals. In the course of time it became clear that all European cattle have predominantly an Asian origin and that the brachyceros/longifrons phenotype emerged after domestication.
Crania excavated in Norway by Nilsson (1849, [22]) were considered as yet another type of aurochs, Bos frontosus. However, Rütimeyer [16] considered it as a domestic variation and reserved the term frontosus for a cranial form in domestic cattle as observed in Swiss Fleckvieh (Simmental): Bos taurus frontosus. Although Dawkins in 1866 pointed out that several Bos taurus frontosus—brachyceros (longifrons) intermediates coexisted during the Neolithic period [23], Rütimeyer's work initiated the skull type theory as an instrument for the determination of evolutionary origin and breed classification. This was adopted particularly by the authors of the German-speaking school, who developed their classifications on the basis of the most characteristic skulls, but ignored the intermediate types of crania [24].
Inspired by Rütimeyer [16] and Nathusius [25], Wilckens [26] based in 1876 the first classification of cattle breeds on measurements of the skulls. He compared the bones of the skull and summarized his results in schedules and tables. He also introduced the term brachycephalus after the Bos taurus brachycephalus, a cranial type excavated in Italy and dating back to the Roman period. His survey covered only Central-European breeds, a few Dutch and German lowland breeds, the Galloway, Ayrshire and Shorthorn and classified cattle into strictly separate breed groups, according to four basic cranial types (Figure 1, Tables 1(A) and S1).
Several scientists elaborated or modified this classification. In 1912 Werner [27] used the term B.t. longifrons (long-headed) instead of brachyceros and elaborated the classification of Wilckens with a detailed regional subdivision in Rasse and Unterrasse, each given a Latin name (Table S2). Note that in agreement with Rütimeyer [16], Wilckens [26], Werner [27], Adametz [30] and Dürst [32] classified the productive lowland dairy breeds in the same primigenius group as the steppe cattle.
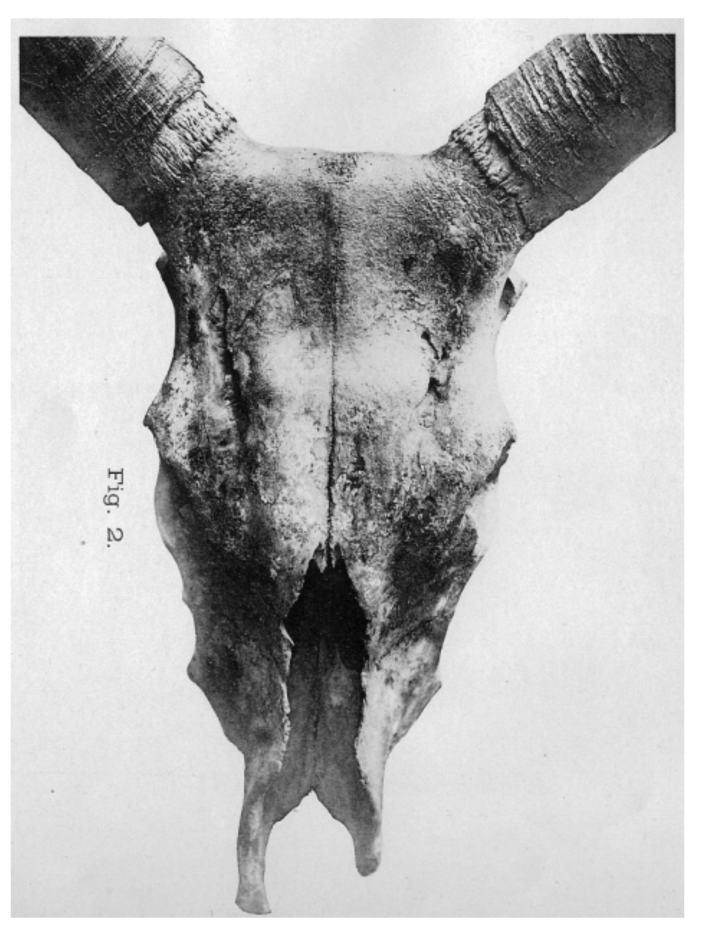
After excavating a hornless cranium, Arenander [33] proposed in 1898 another ancestral type, Bos akeratos for hornless aurochs, which he assumed to be the original European aurochs and the ancestor of both polled and horned cattle. This was still referred to in 1928 by Auld [34], but was not generally accepted (e.g., see [35]). The term akeratos was adopted in 1931, not as an aurochs variant, but as a basic form by Dürst [32], who examined a large number of ancient and modern crania from Europe, Egypt and Mesopotamia. Dürst [28,36] also added a long-horned B.t. macroceros type to the classification of Wilckens (Figure 2 and Table 1(B)). This included both Western and Eastern African together with Iberian crania, all of which he found to be similar.
Keller [29] in 1905 combined the type of cranium and horns with coat color and geographic origin. Like Rütimeyer [16] he believed that the primigenius type cattle descended from the European aurochs and that shorthorned cattle have an Asian origin. Also in agreement with Rütimeyer [16], the primigenius group included in addition to the steppe and lowland cattle the frontosus type. In his system (Table S3), the brachyceros/longifrons (shorthorned), brachycephalus (short-headed) and akeratos (hornless) types were sister taxa of the indicus (zebu), africanus (sanga) and longicornis (longhorned sanga), all preceded by Bos sondaicus, as he believed these types to be of banteng origin. This idea, as well as the belief that the brachyceros type in the course of time had lost its hump, did not gain much ground, but the proposed close relationships between the short-headed and shorthorned types were later confirmed by molecular evidence (see below). Further, Keller did not believe in the existence of an African aurochs.
The resemblance of early African crania to those of modern European breeds noted by Dürst [28] was in 1926 also observed by Adametz [30], who compared crania of Apis bulls from the Egyptian culture with those of modern cattle. Adametz [30] applied the term Bos primigenius var. Hahni Hilzheimer to presumed Egyptian wild cattle, which he considered to be the ancestor not only of northern, eastern and southern African cattle, but also of several European breeds: Andalusian cattle, the Salers from Auvergne, other South-French breeds, Scottish Highland, the British Devon, Longhorn, Hereford and Welsh Black and short-headed Walliser type cattle (Hérens, Tux-Zillertaler, Pustertaler and Pinzgauer). Like Keller [29] and Duerst [32], Adametz [30] recognized the akeratos [33] as main cattle type and included within this group the shorthorned specimens from the polled northern Swedish Fjell (mountain) breed. Further, he believed that brachyceros cattle were descended from a wild Bos europaeus (brachyceros) closely related to Bos primigenius. He stated that this was the most widely accepted classification among livestock scientists.
In 1926 Dürst [28] differentiated several different cranial types ([15], Figure 3). He pointed out that variation in the region of the poll (Processus cornu ossis frontalis/Torus frontalis) is determined by the horn. Long and heavy horns result in a stretched, flat line between the horns; light weight horns result in a vault. This is more pronounced if horns are lighter and becomes a bump in polled cattle.
Holecek Holleschowitz [31] accepted in 1939 the same five basic types of European cattle as Adametz (Table 1), but without the macroceros (Table S4). Following the example of French authors (see below) he linked cranial types of cattle to ethnic origin.
The most recent cranial classifications emphasized a dichotomy of two main stem forms (B.t. primigenius and B.t. brachyceros/longifrons) with several crossbred intermediate forms ([37], J.W. Amschler cited by [38]; Table S5). An important difference to previous classifications is that the (implausible) idea that lowland dairy cattle belong to the primigenius group was abandoned.
Zeuner [21] proposed in 1963 that in several modern breeds the primigenius or longifrons type was relatively well preserved, but that most breeds had become mixed types. However, he chose other prototype breeds [38]: Brown Mountain, Jersey, and Shorthorns showing the longifrons type and Hungarian-Podolian steppe cattle, Romagnola, Scottish Highland and Spanish fighting bulls of the primigenius type. In Black-Pied Lowland cattle he found the full range from primigenius type to longifrons type.
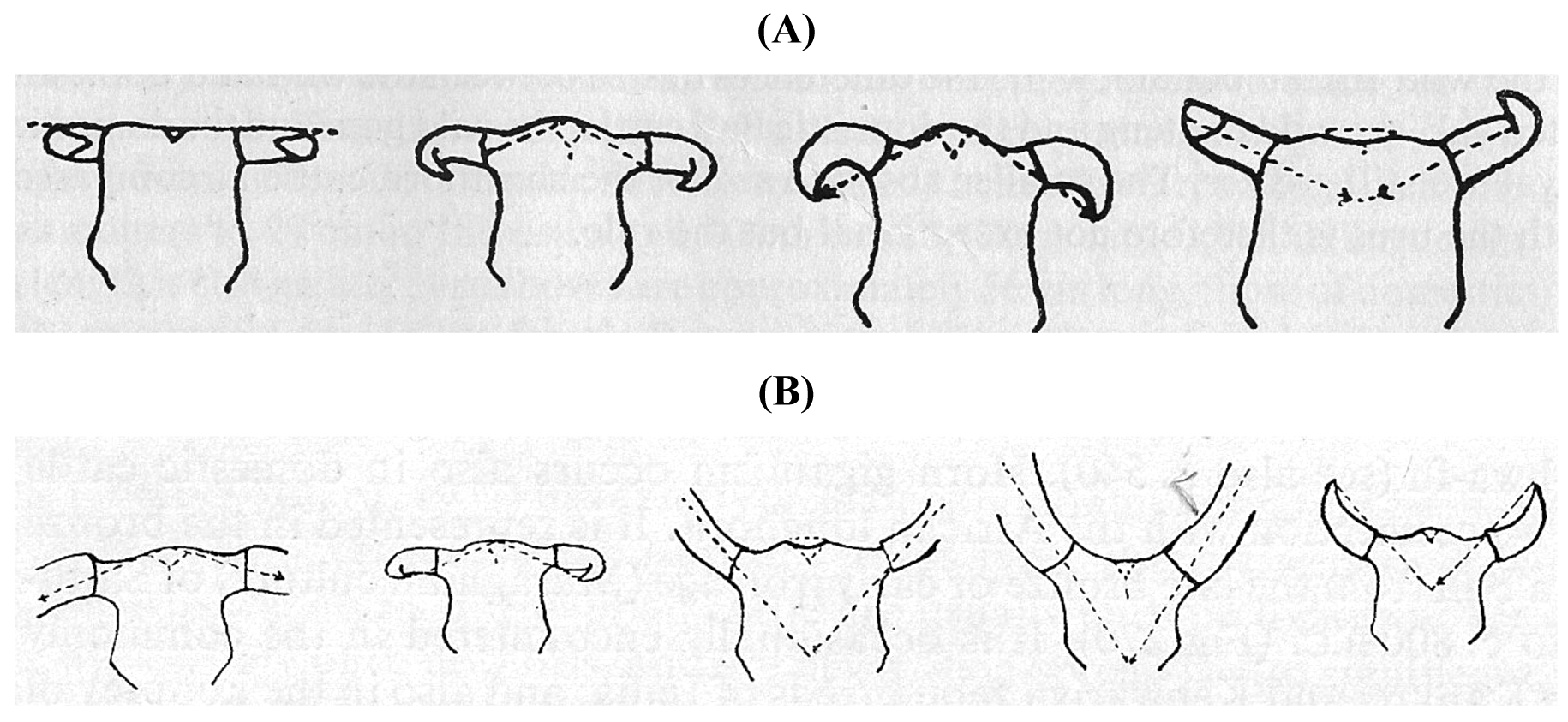
A different line of thought was developed in France. Sanson in 1884 [39] and Diffloth in 1914 [40] classified cattle according to their cranium, the form of the poll and horn implant and the length and form of the horns. These cattle skull types (Figure 4) were linked to human skulls types: dolichocephalus (long-headed) people were accompanied by long-skulled cattle and brachycephalus (short-headed) people by short-skulled cattle. Both the long- and short-headed ethnic groups were subdivided into six tribes that belonged to a certain region.
Thus Sanson [39] recognized 12 geographical types of cattle, the dolichocéphale types B.t. batavicus (Dutch), germanicus, hibernicus (Irish), britanicus, alpinus and aquitanicus and the brachycéphaletypes B.t. asiaticus, ibericus, ligeriensis (Ligurian), arvernensis (Auvergnat), jurassicus and caledoniensis (Scottish) (Table S6). Diffloth [40] replaced the liguriensis with the cattle from le bassin de la Loire (Table S7).
Also McKenny Hughes (1896, [41]), Kaltenegger (1904, [42]) and Wilson ([43], 1909) linked the cattle cranium types to ethnic origin. Kaltenegger [42] replaced the term brachycephalus (short-headed) by latifrons (broad-headed), frontosus by grandifrons (large-headed) and primigenius by planifrons (flat-fore-headed) but kept the term longifrons for long-headed cattle. By referring to the form of the crania only, Kaltenegger tried to maintain a consistent nomenclature (Table S8). Wilson [43] only recognized the primigenius and longifrons as basic types, but also considered coat colors (see below).
Dechambre (1913, [44]) combined the ethnic origin hypothesis from Sanson [39] with a classification proposed by Baron [45], the so-called coordonées baroniennes. In this system cattle breeds were arranged according to three main criteria: morphology (body profile, proportions, size), color (coat, muzzle, mucosa), and production type (Table S9). Dechambre [44] recognized three frontline silhouettes of the skull; each of these having three different sizes of horn, which were split into medium long and long horns and then divided into three types of bending (Table S10). This classification was adopted by the Larousse encyclopedia ([46], Table S11).
5.3. Coat Color
Coat color and pattern are the most obvious characteristic of cattle, at least for non-experts. Coat characteristics were also considered to indicate genetic purity and are relevant for the ‘branding’ of a breed. For instance, different color patterns of early 20th century cattle in the Netherlands were instrumental in the formation of Dutch breeds. The important role of color and pattern is reflected in several breed names and provides an easy key for classification. This was adopted particularly by British scientists, who largely ignored the German cranium theories. Probably inspired by their island status, they emphasized supposed contributions of various immigrant peoples to their cattle stock as a key for classification. McKenny Hughes [41], Kaltenegger [42] and Wilson [43] had strong, albeit unfounded ideas on the relation between the coat color of cattle and different ethnic groups that successively entered the isles. Celtic cattle were supposed to have been black, the Roman white, the Anglo-Saxon red and the Scandinavian light dun (brownish grey), while the broken colors were thought to originate from Dutch imports during the 17th and 18th century [43].
Kaltenegger [42] as well as Müller in 1957 [47] linked coat color of Austrian breeds to immigrations of ethnic groups with cattle of a specific type (Table S12).
Dechambre [44], who based his classification on the profile of the head and type of horns (see above) used coat color as a secondary criterion, specifying many types of color, patterns and marks as well as the different pigmentations of the muzzle and extremities.
So far classifications tended to neglect the Iberian breeds. Most German, French and British authors differentiated Andalusian and north-western blond-brown cattle types, but only described a few breeds from these regions. Duerst [32] classified the Barrosa, Minhota, Alentejana and Brava as African longhorned breeds in Europe. In several post-war publications Spanish and Portuguese authors recapitulated the 19th century classifications according to skull and presumed origin. Again a descent from a wide variety of hypothetical aurochs was proposed with a liberal use of Latin names. In 1907 Mirando do Vale [48] added more ethnic types, ‘troncos’, to the list of Sanson [39], among which were B.t. aquitanicus, B.t. ibéricus and B.t. atlanticus.
Aparicio [49] in 1960 designed a phylogenetic tree for a number of Spanish breeds in which each cluster of breeds was supposed to originate from a hypothetical aurochs variant, such as B.t. ibericus, B.t. desertorum hispanico, B. braquiceros Europeo and B. braquiceros Africano. A more modern classification in Sanchez-Belda in 1981 and 1984 [50,51] combined skull, coat color and region and recognized four branches of Iberian cattle, one of which is supposed to be related to North-African Atlas cattle (Table S13). All this did not result in a generally accepted classification or an agreement about the aurochs types to which the breed clusters were linked. Although none of the theories is consistent with molecular evidence, a catalogue of the recognized indigenous Spanish domestic breeds of 2008 [52] still mentions many of the hypothetical aurochs and derived bovid forms as the forebears of the color branches and even of certain breeds.
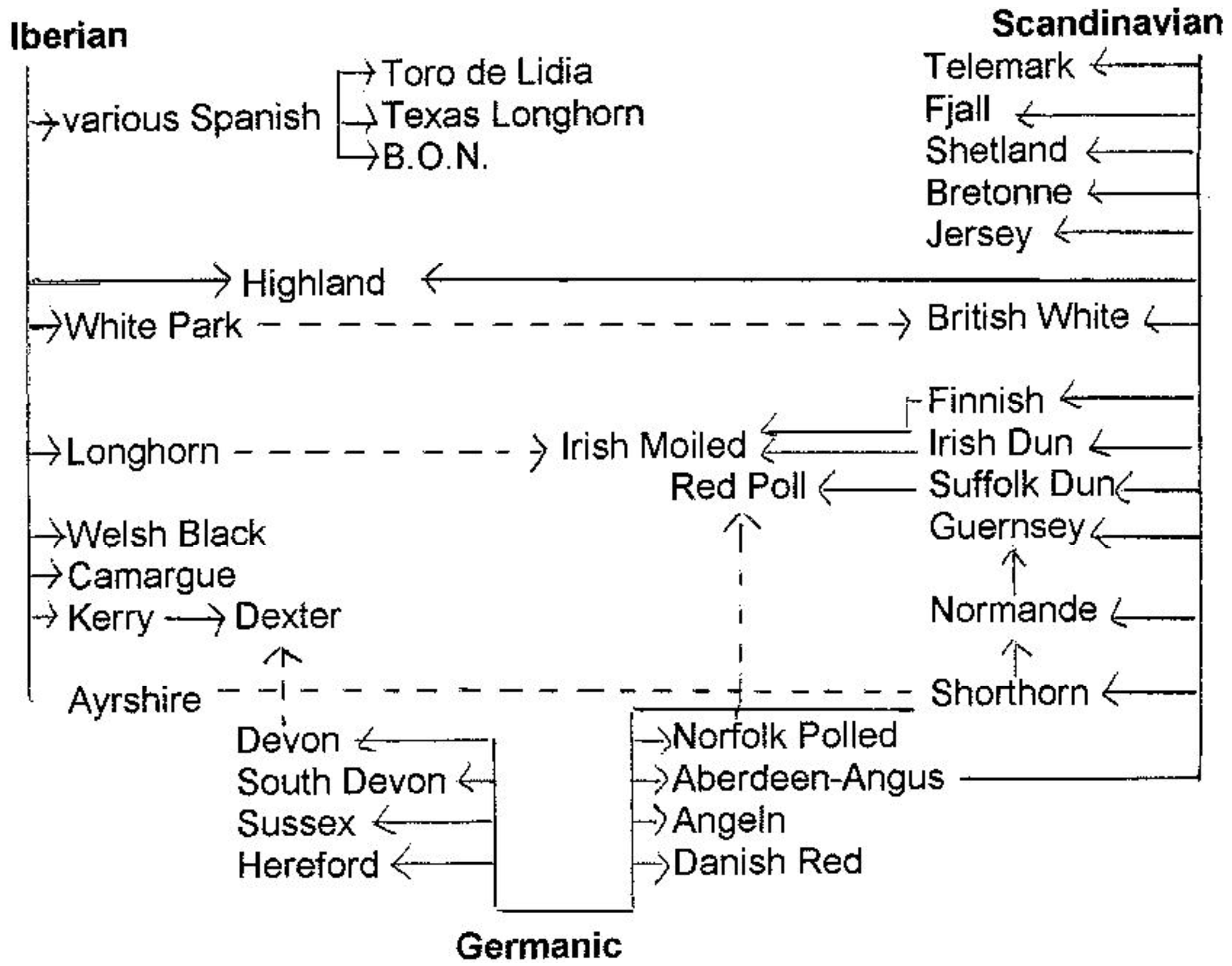
Coat color was also important in the first classification of European cattle in 1977 by Alderson [53]. He followed the British tradition (see above) of linking the classification to prehistoric and historic immigration of people and their cattle. His chart (Figure 5) shows three branches: Iberian, Scandinavian and Germanic. However, DNA analysis did not confirm an Iberian-British connection [54,55]. Furthermore, a Scandinavian influence on British breeds would have implied that Scandinavian immigrants imported substantial numbers of their cattle into countries with a long tradition of cattle husbandry. In 1992 Alderson [56] proposed other historical connections on the basis of an integrative classification (see 6.2.1).
- -
The Original Black-Pied group (1.2) contains the German, Estonian and Lithuanian Black-Pied breeds, all descending from Dutch-Friesians, but also the Italian Aosta Black-Pied and Bretonne Pie-Noir, which have no other link to the Friesian type of cattle than their color pattern.
- -
There is also no resemblance, even in coat color, of Faeroes cattle with the Spanish semi-feral brown mountain breed Albera and the crossbred population Marismeña (former Mostrenca) or with the crossbred Corsican island cattle and the French commercial breed line INRA95.
- -
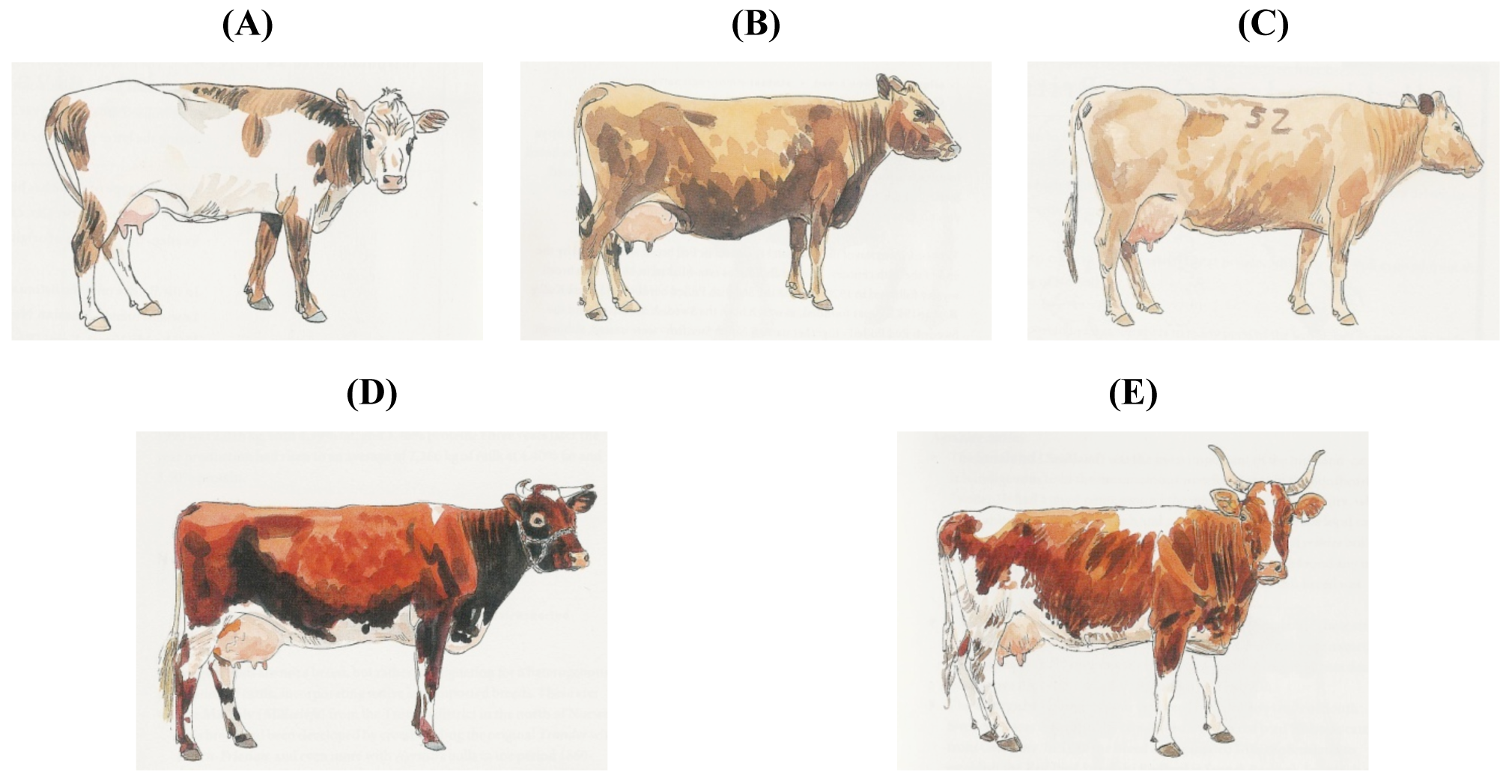
The Austrian Tux-Zillertaler is considered similar to the North Finncattle, Norwegian Black-sided Trondheim and Nordland, the Spanish Black Berrenda and Dagestan/Georgian Mountain cattle. However, apart from sharing a white stripe on the back, there is no other similarity (Figure 6) or historical relationship.
A few breeds do not even have the color of the group into which they are included. Subgroup 4.4., Scandinavian Red, includes the multicolored Icelandic cattle, the yellow-brown West Finncattle and Estonian Native, the Norwegian Red (consisting of red-and-whites and black-and-whites) and the red-pied Swedish (Figure 7).
- -
Breeds of the Iberian Red groups (4.6) are as brown as the Iberian Brown cattle (5.4) group.
- -
In subgroup 2.2, Aberdeen-Angus and German Angus are either black or red, while the Australian Murray Grey is dun (brownish grey).
Phenotypic classification makes more sense with transboundary breeds of recent common origin: Dutch-Friesian Black-Pied is almost indistinguishable from Sortbroget Dansk Malkekvaeg [Jutland Black-Pied], Nizinna-Czerno-Biala [Polish Black-and-White], Prim'Holstein and Pie-Noire-Holstein as well as most other European Dutch-Friesian/Holstein-like breeds. However, other black-pied breeds have independent histories: the French Bretonne Pie Noir [Breton Black-Pied], the Italian Valdostana Pezzata Nera [Aosta Black-Pied], the Russian Kholmogory, the Syrian Jaulan, the Indian Ponwar and Deoni, the Baoulé of West-Africa, as well as subtypes of Fulani, the (multi colored) Nguni and several other African breeds. Another popular transboundary breed is the Swiss Simmentaler, which in other countries is known as Fleckvieh, Čescky strakatý, Simentalska or Sychevka.
Bougler [58] presented in 1998 a classification of French cattle with coat color as the most important criterion (Table S15), which in 2009 was still cited and considered assez consensuelle [59].
5.4. Geographic Origin
Fitzinger [60] proposed in 1860 that that there were at least seven geographic forms of domestic cattle. Besides the Indian zebu and African humped cattle he recognized Alpine, valley, polder, steppe and Scotch types. Their Latin names (Bos alpium, scoticus, friburgensis etc.) even suggest separate species status and were cited in the influential standard work on zoology of Brehm, which appeared in several editions from 1860 to 1925.
We have already mentioned the geographical subdivision of two cranial basic types described by Sanson [39] and Diffloth [40]. Ramm (1901, [61]) described a geographical classification according to both country or region and altitude (Table S16), which resulted in a practical inventory rather than a zoological classification. A similar geographic categorization was published in 1920 by the Belgian Zwaenepoel [62], who considered the division into mountain, lowland and in-between breeds dating from 1804 [63] as the most simple and practical one (Table S17).
Hengeveld [64] in 1865 classified the Dutch cattle; first according to soil, then per province. Thus he recognized cattle varieties from (1) clay and sandy clay, (2) peat-soil and cultivated sandy soil, and (3) poor sand soil and heather (moor). We note that only in 1906 three different strains of Dutch cattle were recognized [65], which in 1965 were considered to be separate breeds.
The need for developing agriculture after World War I inspired several German writers to classify according to region in combination with purpose ([66,67], Table S18). Thus breeds were divided into high productive dairy, beef and dual-purpose types, lowland or highland, or classed as low productive triple-purpose land cattle types. In the decades after WW II breeds that were small in number or regarded as unproductive were amalgamated, most notably in France, Germany, Austria and Italy. At that time interest in local breeds was at its lowest point.
Also the classification from 1966 of French breeds ([38], Table S19) is strictly geographical. The European breeds are described by country, disregarding their origin (local or imported).
- -
Scandinavian and North-European group
- -
United Kingdom and Ireland
- -
North Sea and Baltic Littoral
- -
Western Europe
- -
Alpine Europe
- -
The Iberia Peninsula and Italy
- -
The Balkans and Turkey
- -
U.S.S.R.
5.5. Cattle outside Europe
5.5.1. Africa
The first classification of African humped cattle was proposed by Epstein (1933, [68]) and developed further by Curson and Epstein (1934, [69]). This classification has been generally accepted. Humped cattle were classed into true zebus of Asian origin and crossbred pseudo-zebus or sanga. This use of the term sanga was introduced by Keller [29] and came from designation Bos Zebu africanus Sanga (1860) for the Galla breed [60]. True zebus were sub-divided into the lateral-horned and the short-horned. Epstein [68] also quoted Bisschop (1937, [70]), for whom the anatomical structure and situation of the hump provides one of the principal clues by which the parentage of crossed types can be traced. Curson and Thornton [69] divided taurine cattle into Longhorns, (traced to the Hamitic Longhorn) and Shorthorns, which were thought to have entered through Egypt from Southwest Asia [71]. Although these views would change, this division of taurine cattle was generally accepted.
Doutresoulle [72] described in 1947 the breeds of the French territories south of the Sahara. He divided the region into climate zones and classed cattle into two main groups: taurine breeds (les Taurins) and the zebus (le Zébu), the latter all intermixed with taurine cattle and divided into three main types (Tables 2, S20). Mason [71] classified the breeds of West-Africa, covering the same area but with a more refined classification (Tables 2, S21). Joshi et al. [73] inventoried African types and breeds of the whole continent, using region and morphology as first and second criterion, respectively, for classification (Tables 2, S22). According to Joshi et al. [73], the East-African cattle are a heterogeneous population, composed of groups without clear demarcation. The Africander is a clear separate type, while the Madagascar zebu has a separate location. A publication by the British Colonial Office in 1957 also classified the cattle of ‘British dependant Territories’ according to geography followed by morphology. The zebus of East, Central and South Africa are divided into (a) the chest-humped or thoracic humped Indo-Pakistani or true zebus, and (b) the neck-humped or cervico-thoracic humped African zebus. The sanga is classified as a West-African type (as by Doutresoulle [72]), and indicated without a cervico-thoracic or thoracic placed hump (Table S23).
In 1960 Mason and Maule [74] refined the classification of West-African and East- and South-African humped cattle respectively. They emphasized the form and place of the hump and the horns. Rege and Tawah (1999, [75]) listed all recognized African cattle breeds and refined the previous classifications, also describing for the first time Ethiopian and Kenyan breeds and introducing the term zenga for zebu-sanga intermediates.
5.5.2. India-Pakistan
Olver (1938, [76]) related the different types of zebus on the Indian subcontinent to the migration of people into India in prehistoric times, as along the various migration routes characteristic zebu types are to be found. Some of these must have been in existence prior to these invasions. Thus he distinguished four types, consisting of different breeds and varieties, and one separate breed:
Large white cattle of the north.
The distinct Mysore type of the south.
The ‘highly peculiar’ Gir of Kathiawar and the west of India.
Small black, red or dun cattle found all over India, mainly in hilly tracts and forest areas.
The Dhanni breed of the Punjab.
Joshi and Phillips [77] based their classification of 1953 on these types and developed a scheme of six groups (Table S24):
Lyre-horned; wide forehead with flat or dished profile; deep body; grey color; powerful animals.
Shorthorned; long, coffin-shaped skulls, slightly convex profile; white or grey color; the best dairy cattle.
Curled, often lateral horns; ponderous build, loose skin; red or red spotted
Mysore cattle: long, pointed horns, rising close together; prominent forehead; poor milkers.
A heterogeneous mixture found particularly in rugged mountainous areas of India and Pakistan.
The Dhanni breed from Pakistan.
5.5.3. China
Epstein [23] published in 1969 the first classification of Chinese cattle breeds in a western language, describing yak, water buffalo and the several breeds of ‘yellow cattle’ (Huang Niu for all forms of taurine or zebu cattle) as the most widely distributed bovids. Yellow cattle have the highest concentration in Inner Mongolia and the north-east. The cattle from northern, central and southern China differ mainly in body size, presence or absence of a hump, and, where a hump is present, in its size and position (see Table S25).
In 1986 Cheng [78] divided the ‘Bovine Breeds’ of China into Yellow Cattle, Developed breeds and Introduced breeds, as well as Yak and Buffalo. Indigenous (Yellow) breeds were classified according to regions and climatic zones:
Humpless:
highland cattle,
steppe cattle,
Manchuria cattle;
Central Chinese Yellow in a region of moderate climate;
Southern Chinese zebu in the sub-tropics and tropics.
5.5.4. Tropical and Subtropical Cattle
Classifications of tropical and subtropical cattle include in addition to African and Asian breeds also breeds from the Americas and Oceania. Payne (1970) and Payne and Hodges [79] classified the cattle of the tropics and subtropics according to continent and then according to region. Within a given region, the cattle are divided into (1) humpless, (2) humped, (3) crossbreds (stabilized indigenous, intermediate and recent) and (4) of Bibovine origin (gaur-gayal, banteng-bali cattle). Payne and Hodges [79] subdivided the humped cattle of the Indian subcontinent according to purpose, and the West-African humped cattle according to length and form of horns. The crossbreds were subdivided into old types, types which are still in progress of formation and recently formed. However West-African crossbreds are sub-divided on the basis of their origin.
Maule [80] constructed a different classification with five groups (Table S26): zebu, sanga, humpless, humped × humpless and Bibovine cattle. A subdivision into subgroups indicated the locality: (Indo-Pakistan, African, Brazilian, Middle and Far East, etc.) of a regional type or breed (Brahman, South-African Longhorned, Humpless Cattle of West and North Africa, etc.).
6. Modern Classifications
6.1. Biochemical Markers
Scientific progress after 1970 allowed a new approach to the classification of cattle: the comparison of molecular markers such as blood groups and other biochemical polymorphisms. Using data on 10 polymorphic proteins Baker and Manwell (1980) [13] compared allele frequencies in 196 breeds and proposed 10 well-defined groups of cattle breeds (Table S27), stating “… breed groups …. are alluded to frequently in both historical and modern writings on cattle. The groups usually infer relationship; but, in the absence of well-documented historical information, the breed groups largely depend on morphology or geography. The chemical data support the morphological and geographical division of cattle into major breed groups…. The coherence within the groups and the differences between groups are often impressive. …. In some cases paradoxical distribution of rare genetic variants can be explained by more detailed inspection of breed history”.
The names of the seven European breed groups were clearly inspired by the German cranial classification and indicate a correlation with previous classification criteria: North-Scandinavian (geographic region); Pied Lowland (color pattern and altitude); European Red brachyceros (continental, color and type of origin); Channel Island brachyceros (geographic region and type of origin); Upland brachyceros (altitude and type of origin); Primigenius-brachyceros Mixed (mix of presumed original types, although more likely a rest group of related or unrelated breeds); and Primigenius (aurochs, original type).
They left the question open of whether the Red Flemish belongs to the Pied Lowland from the same region or to the European Red brachyceros. In the 19th century this breed was spread over a much wider region than today. In the Ardennes they were connected to red cattle from Germany. Currently, remnants are confined to the west of Belgium (West Flemish Red) and northwestern France (Red Flemish) and have been influenced by the pied cattle in the same region. All these breeds were also influenced by imported Durham, Dutch-Friesian and later MRY sires. The Baltic Red breeds, such as the Latvian Brown, were strongly influenced by Angler and Danish Red, but not by the French or Belgian Red breeds. So the breed's history argues against a grouping of the red dairy cattle from Belgium and the Baltic coast. Baker and Manwell [13] further classified within the European group breeds from other continents with a recent history of crossbreeding: the Asian Ala-tau, several American Criollo breeds, Mexican Fighting cattle, Texas Longhorns and the Cuban Tinima breed.
Two articles applied the biochemical approach to Iberian cattle. Vallejo et al. in 1990 [11] typed 10 genetic blood markers in 13 native Spanish breeds, while Fernández et al. (1998, [9]) analyzed 11 blood proteins in 10 breeds from Galicia and northern Portugal. A number of breeds are shared by both studies, but with different outcomes. (see Figures S1). Vallejo et al. [11] indicate that quantification is difficult because of the short evolutionary distances (Table S28). Although biochemical comparison provides evidence for a number of close relationships between breeds, their interpretation in prehistoric terms lacks scientific support.
Using 13 biochemical polymorphisms Grosclaude et al. [81] classified eighteen French breeds into three regional groups plus the Normande as a separate breed (Table S29). This classification is different from the coat-color based classification of Bougler [58] or the geographic classification of Denis and Avon [82] (see above). The biochemical classification from 1990 was in 2010 adapted by Gautier et al. [83], who recognize both the Normande and the Bretonne Pie-Noir and Parthenaise as separate breeds next to three previously recognized groups (Table S30).
6.2. Integrative Classifications
6.2.1. Alderson (1992)
In 1992 Alderson [56] integrated the color-based classification with archaeological, socio-historical, and morphological as well as biochemical evidence (Table S31). He included only (supposedly) pure representatives for categorizing types and breeds of cattle in Europe, thus excluding Rubia Gallega as it was influenced by the Shorthorn and South Devon. This rule was not applied rigidly however, as, the German Yellow (Gelbvieh), the French Blonde d′Aquitaine and the Portuguese Minhota, recent breeds of mixed origin, were still included in his Central Europe Yellow-Brown group. Minhota is indeed related, if not identical, to German Yellow, because of the frequent use of German sires in Portugal [2,12].
6.2.2. Denis (2010)
In 2010 Denis and Avon [82] amended an earlier classification of French cattle which was clearly inspired by the classifications of Sanson [39] and Diffloth [40], but combined geography, morphology and origin. Denis and Avon [82] acknowledged the new insights offered by molecular-genetic comparison of breeds.
6.2.3. Felius (1995)
Felius [2] developed in 1995 a comprehensive classification of bovine domestic breeds, varieties as well as wild species and their hybrid forms (Table S33A, Figure 8). This classification is based on morphological, geographical and historical data ([15,23,74,84,85]. It also builds on the classifications developed for Indo-Pakistani zebu and African cattle zebu cattle [73,77]. After a previous classification of 470 breeds into 16 groups [86], the classification from 1995 puts more emphasis on geographical location and covers 700 breeds. It is supported by pictures, which for all breeds are on the same scale and focuses on visible external differences and similarities. Water color paintings instead of photographs enable the use of a wide range of sources and the maintenance of a uniform standard of illustrations for all breeds throughout the book. Table S33B presents a slightly revised classification.
Of the three criteria for classification, geography is proposed to be the most important. The breeds have been arranged first according to continental origin, which is plausible because cattle from different continents are likely to have developed relatively independently (isolation by distance). Exceptions are made for breeds near the continental boundaries. For instance Podolian steppe cattle are found in south-eastern Europe and in the Asian part of Turkey, while. Egyptian cattle seem to form a transitional type between the breeds of North Africa and Mediterranean Asia.
Next, breeds of each continent are classified on the basis of a subdivision of the continent into regions with different climates, altitudes and/or agricultural systems. For instance, the West-European Lowlands, the Central European Highlands, the Iberian Peninsula and the Balkan all harbor different types of cattle (Figure 8). As appropriate, regions were subdivided, but at this level history and morphology are becoming more important. All the groups and subgroups are arranged in a northwest-to-southeast order (Figure 8).
Within geographical groups, breeds were subdivided according to the breed history. Breeds are indicated to be old (local, authentic), modern or recently formed. The breed history often indicates a common origin of a group of breeds, which is a most evident criterion for classification. If the breed history involves crossbreeding to sires from other regions to the point that the breed characteristics reflect the paternal origin, the historic criterion overrides the geographical classification. For instance the Ayrshire, which is of mixed origin, is classified with the Scandinavian breeds whose development it has influenced. However, the Maine-Anjou, which essentially has become a Shorthorn type, is classified with the other breeds of Bretagne and Normandy as it was developed on the now extinct local Mancelle breed. Further, Portuguese Minhota, which was heavily influenced by the German Yellow is still classified in the group of northwest Iberian blond breeds as it was founded on the Galician Blond.
For American and Australian import breeds that have well documented histories, geography and history are not considered and are replaced by production traits as classification criteria. However, in the subgroups, the country or region of origin as well as the period in which they were imported are also relevant for classification.
For the final subdivision, morphological criteria are taken into account. This recognizes that animals from most breeds can be identified by their appearance, which is also specified in the breed standards. If two or more recognizably distinct breed types are found within one region, separate groups or subgroups have been defined. However, only a few breeds are so unique in their morphology that they stand completely apart, since genetic exchange between neighboring breeds makes differences often gradual. In a number of cases, the last representative from one group or subgroup may merge with the first of the next group.
In spite of its systematic approach, the classification of Felius [2] also needs exceptions in order to cover all breeds. For instance, the Danish Forest breed is a young synthetic breed, an amalgamation of 12 breeds from all over Europe; it is not specific in type, and fits the Northwest European group only because of its location. The Ukrainian Beef and Askian Meat breeds are Eastern European, but originate from Central European types of cattle.
6.2.4. DNA-Based Classification
The rapid development of DNA technology has had its impact also on the analysis of cattle breeds, which, as other livestock breeds, are now compared at the DNA level via several types of genetic markers. Mitochondrial and Y-chromosomal DNA variants are markers for the female and male lineage, respectively. Autosomal DNA markers as microsatellites and single-nucleotide polymorphisms (SNP) indicate the genetic similarity of animals or breeds [12].
These studies have revealed the complexity of the domestication process, migration routes and relationships of modern cattle breeds [87-90]. A most important finding was the separate domestication of taurine and zebu cattle in Southwest Asia and the Indus Valley, respectively [1,12,91,92]. This confirmed, after 135 years, the theory of Rütimeyer [16], although his ideas on where and when the two species had evolved were untenable.
Molecular studies also demonstrated that the Sanga did not develop in Northeast Africa, as a Y-chromosomal survey showed that zebu bulls were spread gradually and changed original African taurine cattle into humped cattle on their way south. MtDNA haplotypes of African origin have been found in Iberian breeds, which confirmed an African-Iberian connection as already proposed by Dürst [28] and Miranda do Vale [48]. However, it was also shown that British breeds do not have their origin in Iberia, as was proposed by several British authors. Similarly, in several publications (e.g., [93]) the Italian Piemontese breed is presented as a mix of local aurochs × Indo-Pakistani zebu, which was supposed to have entered the region long before domestication. Molecular genetic analysis now confirms the 19-century records of a recent origin of the Piemontese breed as a mix of several taurine breeds, discarding a link with the aurochs and zebu, one of the several urban legends on the history of cattle.
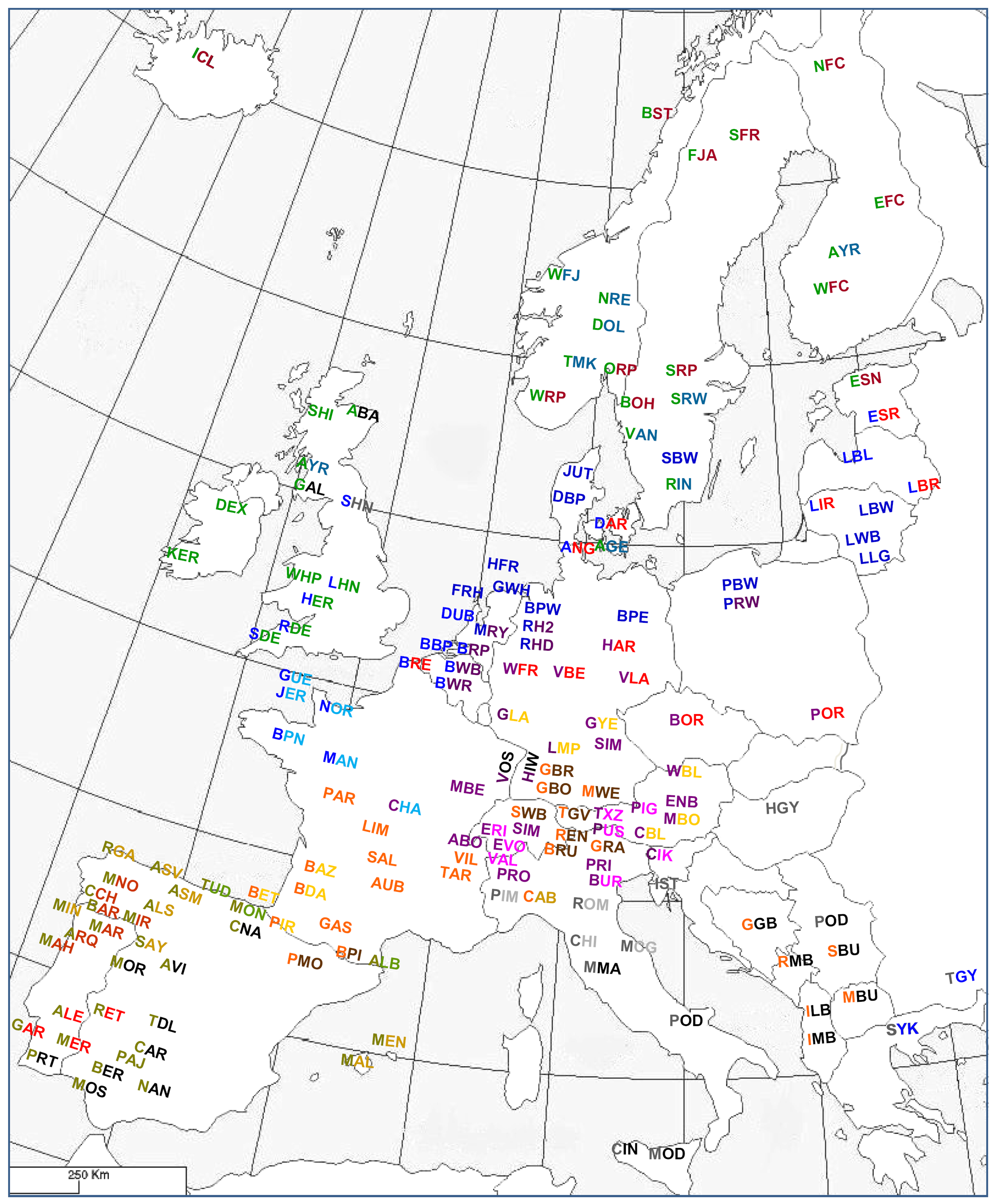
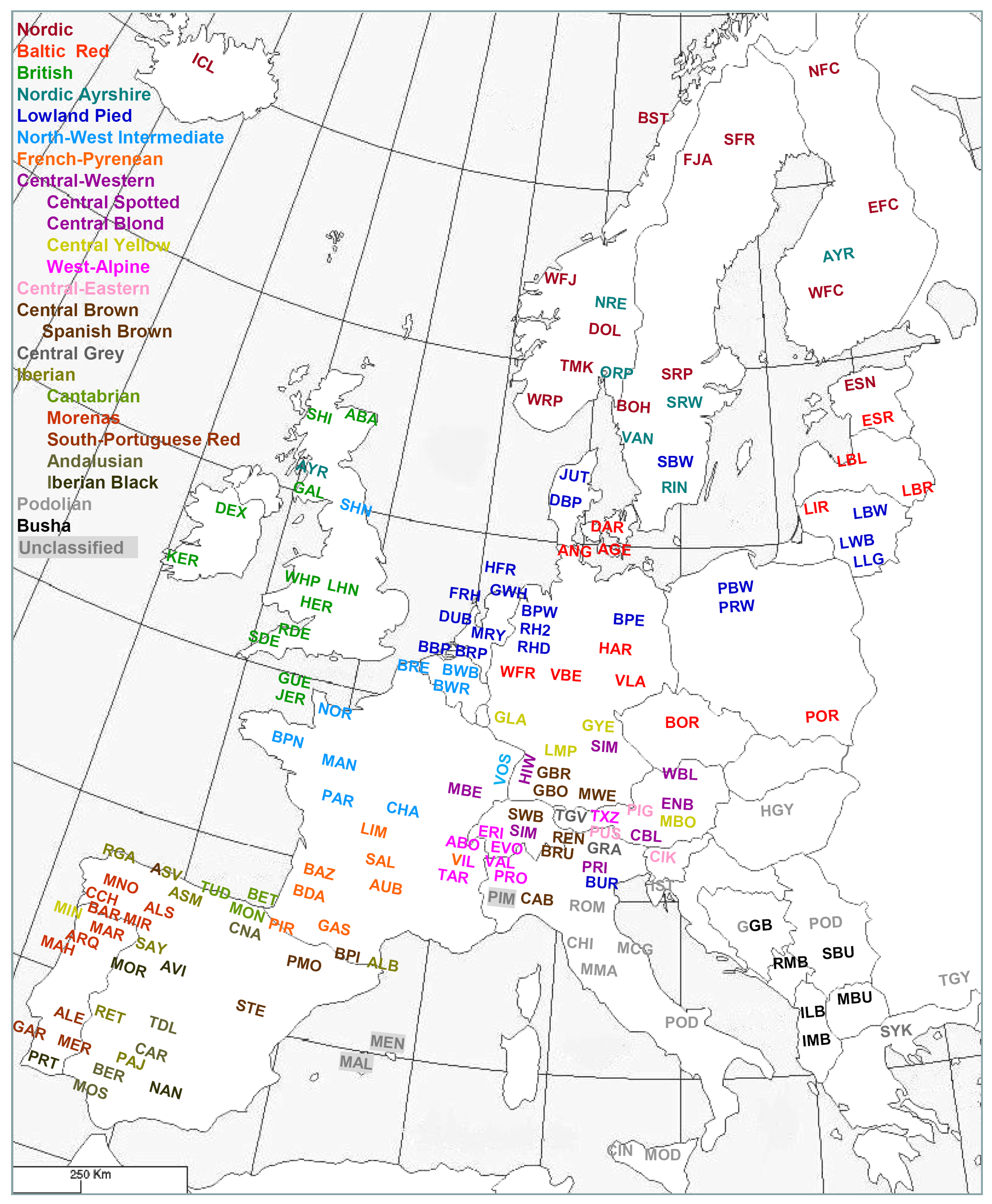
A collective effort of several European laboratories supported by the European Commission led to a compilation of a microsatellite data set of all major and several local cattle breeds (Table S34) [54,55,94,95]. Analysis of the data with phylogenetic networks (Figure S2) in combination with model-based clustering [96] indicated four major groups of breeds, Northern, Central, Iberian and Podolian cattle, respectively, with the Balkan and Anatolian taurine cattle representing the less developed ancestor populations. A further subdivision yielded 16 geographical groups of genetically related breeds and a further differentiation of the Central-Western and Iberian breed clusters (Figures 9, S2; Table S35). The resulting clusters of genetically related breeds are consistent with AFLP [95] and 50K SNP analysis [83,97]. The regional Iberian subclusters (Catabrian, Morenas, South-Portuguese Red, Iberian Black and Andalusian) are consistent with previous analyses [98,99] and partially with the morphological classifications (see above).
In view of previous classifications, the most unexpected result was a consistent relationship of South-French beef breeds with the brown or spotted Alpine dairy breed clusters, which was also clearly supported by SNP genotyping [83]. This has been explained by repopulation of South France after the Gallic conquest or during the Middle Ages by Alpine cattle [55], but is not consistent with the proposed different migration routes for Alpine and South-French cattle, respectively [83].
Meta-analysis of several microsatellite datasets allowed an assignment of more breeds to the clusters and an extensive coverage of European cattle (Figure 9, European Cattle Genetic Diversity Consortium, unpublished results).
6.2.5. Comparison of Classifications
Figure 10 compares the biochemical [13], the integrative [2] and the molecular-genetic classification (Figure 9; Table S34). Most genetic breed clusters are within a single category of the other classifications, implying that these categories correspond to genetic realities. The Lowland pied cattle in the biochemical Red brachyceros group refer to the Red Flemish, for which the biochemical evidence was not conclusive (see above). In the classification of Felius [2] the Baltic Red cattle is divided between two groups, reflecting that the German Highland Red cattle descend from central European cattle but have been crossbred to Baltic Red. In both the biochemical [13] and integrative [2] classifications, the well diverged British breeds are divided between different groups.
7. Discussion
Classification of cattle is potentially most useful, but not straightforward. The origin of many breeds is lost in history and only the most recent period of systematic breeding has been documented. Defining a breed is partially arbitrary, because of gradual differences between breeds, crossbreeding, multiple origins, development of expatriate breeds and changing breeding objectives. Newly formed breeds are often denoted as “man-made” or “synthetic”, but most of the older breeds originated in the same way.
In the course of time cattle breeds were classified via different approaches, which also reflected the state of the science of the era in which they were developed. A list of all scientists who proposed a classification is provided in the Supplemental Information. The first classification on the basis of skull and horns, the several attempts to link the different types of cattle with different types of aurochs and the liberal use of Latin denotations (see the list in the Supplemental Information) were inspired by the strictly hierarchical Linnaean classification.
The tendency of 19th and early 20th century scientists to summarize a complex genetic reality in simplifying schemes that were more based on personal ideas than on scientific support would not have been accepted in the more rigorous scientific practice of today. This applies especially to the theories of about 100 years ago that link cattle types and coat colors with human migrations and ethnic origins. Furthermore, the proposed classifications focused on national breeds with apparently little communication between the German, French and English.schools. An overall preoccupation of most 19th century scientists with European cattle may reflect a more general tendency of the western society of that time towards eurocentrism.
Although not universally accepted, the cranial typing from the German school persisted until the mid-20th century. Since Duerst [32] the form and length of the horns was more important for classification than the shape of the cranium. Accordingly, the term primigenius became used for all longhorned cattle breeds, and brachyceros for all shorthorned cattle breeds, irrespective of their origin or relationships. Early ideas of an independent domestication of the bracyceros, still mentioned in 2000 ([100], are no longer followed [101] and the term macroceros from the German school for long-horned African and Iberian cattle did not find wide recognition. In time also the names frontosus, brachycephalus and akeratos became less popular as these terms can be used for non-related breeds from different regions.
We now also know that a common coat color does not imply a recent common origin. For example, Italian white breeds are claimed to have been imported into Britain during the Roman occupation and to have been the ancestors of the White Park and Chillingham. However, the colored ears of the British cattle show that these breeds have the ‘color pointed’ pattern: a color sided pattern form with only a few colored spots.
A systematic combination of geography, history and morphology [2] appears more plausible and to give appropriate emphasis on geographical origin as primary criterion of classification. Biochemical clustering was the start of a new scientific approach and also generally followed a mainly geographic division. Classifying on the basis of geography is supported by molecular analysis which shows that geographical origin is the most important determinant of breed relationships [12,54,55,97] This is of fundamental interest and is also justified by the notion that most breed names refer to geographical origin.
These classifications allow us to discard several urban legends and unfounded theories on the origin of breeds, but also confirm the separate positions of taurine and zebu cattle observed in the 19th century. The genetic subdivision into Northern, Central and Mediterranean cattle as main groups is also apparent from both other classifications, although only the genetic classification assigns a separate position to the primitive Balkan and Anatolian cattle. One group consistently recognized by all classifications is the Podolian, or Grey steppe cattle. The productive dairy breeds from the Northwest European lowlands are noted as a separate group by most, but not all classifications. Because of a large phenotypic variation in British and Iberian breeds, these are most often dispersed over different groups. Remarkably, relatively recent classifications [56,57] still combined cattle with different histories in one group on the basis of a few visible traits.
The British, Iberian, Nordic and the combined Central-European breed clusters identified by the genetic analysis each comprise breeds that are phenotypically different, yet are genetically related. This is explained by their common origin and/or gene flow between neighboring populations and makes geographical proximity the most reliable guide for classification. Other breed clusters, such as the Lowland Pied, Baltic Red, Nordic Ayrshire, West-Central, Central Brown and probably also the Podolian cattle correspond to successful breed types that expanded by migration and/or crossbreeding. Particularly the Lowland black-pied, Ayrshires and Central Brown now occur in European regions far from their region of origin. However, the contrast of Northern cattle, predominantly carrying the Y1-type Y-chromosomes, and the central and southern European cattle, mainly carrying Y2 Y-chromosomes, has been retained and has apparently an old origin [55].
We expect that new genome-wide approaches, such as high-density SNP genotyping and whole-genome sequencing, will further refine the classification with a more detailed reconstruction of the demographic history of the cattle breeds, a finer resolution of paternal lineages and a better insight into the emergence and spreading of functional gene variants [102].
Another lesson already learned by analyzing DNA is that most breeds carry most of the genetic diversity of the whole species and that differences between breeds are relatively small. This complicates the assessment of the conservation value of breeds on the basis of molecular data. In addition, the current molecular diversity data sets do not indicate the phenotypic uniqueness of a breed, which may be also be a consideration for conservation. In practice, the perceived value of a breed mainly depends on its role in local tradition and history—the breed as social concept—even for breeds that have only emerged hundred years ago or later.
Yet we propose that classifications may prove to be useful for management of the genetic diversity of cattle. First, breeds that occupy a separate position in the classification are more likely to possess unique features than breeds that have many close relatives. Second, if crossbreeding for a breed is considered, either because of inbreeding or because of upgrading, a classification may show which breeds are related and would thus maintain as far as possible the genetic identity of the breed. We conclude that insight into the classification of cattle is not only of scientific interest, but is also relevant for genetic management and conservation.
Supplementary Material
diversity-03-00660-s001.doc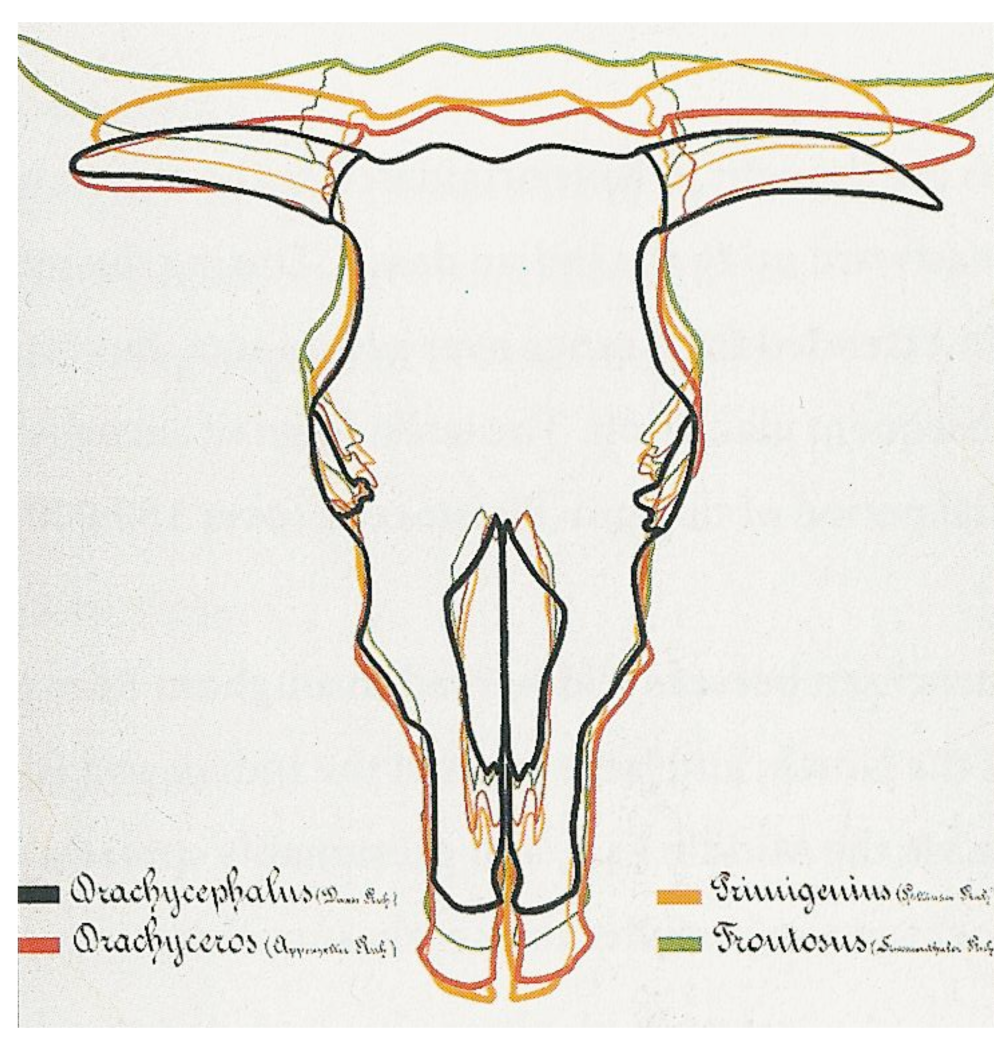



| (A) | |||
|---|---|---|---|
| German name | Latin name | Description | Typical breed |
| Primigeniusrind | Bos taurus primigenius | aurochs type | Podolian Grey Steppe cattle, lowland dairy breeds, Galloway |
| Langstirnrind | Bos taurus brachyceros | shorthorned | Grey and brown mountain |
| Bos taurus longifrons | breeds | ||
| Grossstirnrind | Bos taurus frontosus | broad-headed | Simmental |
| Kurzkopfrind | Bos taurus brachycephalus | short-headed | Hérens, Tuxer |
| none | crossbred land cattle | Pinzgauer, Mariahofer | |
| (B) | |||
| Latin name | Description | Typical breed | |
| Bos taurus akeratos | hornless | All polled cattle | |
| Bos taurus macroceros | longhorned | African zebu, sanga breeds Iberian Barrosa, Minhota, Alentejana, Brava | |
| Doutresoulle, 1947 [72] | Mason, 1951 [71] | Joshi et al. 1957 [73] |
|---|---|---|
| North-African and Egyptian humpless and vestigially humped (Egyptian, Libyan, Brown Atlas) | ||
| 1. Taurine | 1. Humpless
| Humpless with bulbous horns (Lake Chad cattle) |
| Humpless, straight-backed West- African (N′Dama, West-African Shorthorn) | ||
2. Zebu
| 2. Humped Cattle (zebus)
| Sub Sahara (Indo-Pakistani type) zebus |
| 1. Medium and shorthorned (North Sudan, 5 tribal strains) | ||
| 2. Lyre- and longhorned (Fulani, M′Bororo) | ||
| Central and southern African Sanga (9 tribal named types) | ||
| East-African cattle, predominantly zebu (9 tribal named types) | ||
| Africander, Madagascar zebu | ||
Acknowledgments
This work has been partially supported by the European Commission (projects ResGen 09-118 and GlobalDiv Agri Gen Res 067).
References
- Ajmone-Marsan, P.; Garcia, J.F.; Lenstra, J.A. On the origin of cattle: How aurochs became cattle and colonized the world. Evol. Anthropol. 2010, 19, 148–157. [Google Scholar]
- Felius, M. Cattle Breeds, an Encyclopedia; Misset Uitgeverij: Doetinchem, The Netherlands, 1995. [Google Scholar]
- Aristoteles. Historia Animalium; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Linnaeus. Caroli Linnaei Systema Naturae, Sive Regna Tria Naturae Systematice Proposita Per Classes, Ordines, Genera Et Species; Lugduni Batavorum: Hollandia, Indonesia, 1735. [Google Scholar]
- Knowles, H. The New English Bible; Oxford University Press: Oxford, UK; Cambridge University Press: Cambridge, UK, 1970. [Google Scholar]
- De Buffon, G.L.L. Histoire Naturelle Générale Et Particulière; F. Dufart: Paris, France, 1749. [Google Scholar]
- Lamarck, J.B. Philosophie Zoologique; Flamarrion: Paris, France, 1809. [Google Scholar]
- Magerstedt, A.E. Die Viehzucht Der Römer; Sondershausen: Walluf, Germany, 1859. [Google Scholar]
- Fernández, A.; Viana, J.L.; Iglesias, A.; Sánchez, L. Genetic variability and phylogenetic relationships between ten native cattle breeds from galicia and the north of portugal. Arch. Zootec. 1998, 47, 63–79. [Google Scholar]
- Cuiá De Campo De Las Razas Autóctonas Españolas; Gobierno de Espana, Ministerio de Medio Ambiente y Medio Rural y Marino, Centro de Publicaciones: Madrid, Spain, 2009.
- Vallejo, M.; Iglesias, A.; Sánchez Garcia, L.; Conzález, P.; Tuñon, M.J. Variabilidad genetica y relaciones filogeneticas de trece razas bovinas autoctonas españolas. Arch. Zootec. 1990, 39, 197–208. [Google Scholar]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groeneveld, E.; et al. Genetic diversity in farm animals: A review. Anim. Genet. 2010, 41, 6–31. [Google Scholar]
- Baker, C.M.; Manwell, C. Chemical classification of cattle. 1. Breed groups. Anim. Blood Groups Biochem. Genet. 1980, 11, 127–150. [Google Scholar]
- Youatt, W. Cattle, Their Breeds, Management and Diseases; Baldwin and Cradock: London, UK, 1834. [Google Scholar]
- Epstein, H.; Mason, I.L. The Origin of the Domestic Animals of Africa; African Publishing Corporation: New York, NY, USA; London, UK; Munich, Germany, 1971. [Google Scholar]
- Rütimeyer, L. Versuch Einer Natürlichen Geschichte Des Rindes, in Seinen Beziehungen Zu Den Wiederkauern Im Allgemeinen; Druck von Zürcher und Furrer: Zürich, Switherland, 1867. [Google Scholar]
- Bojanus, L.H. De Uro Nostrate Eiusque Sceleto Commentatio; Nova Acta Phys-Med Acad. Cesareae Leopoldino-Caroline Nat. Curiosum; 1827; Volume 32, pp. 1–184. [Google Scholar]
- Falconer, H. Paleontological Memoirs and Notes of the Late Hugh Falconer I.; Robert Hardwicke: London, UK, 1868. [Google Scholar]
- Adametz, L. Studien über bos (brachyceros) europaeus, die wilde stamform der brachycerosrassen der europäischen hausrindes. J. Landwirtsch. 1898, 48, 17–31. [Google Scholar]
- Leithner, O.F. Der Ur. Bericht Int. Gesellsch. Erhaltung Wisents; 1927; Volume 2, p. 140. [Google Scholar]
- Zeuner, F.E. A History of Domesticated Animals; Harper & Row: New York, NY, USA, 1963. [Google Scholar]
- Nilsson, S. On the extinct and existing bovine animals of scandinavia. Ann. Mag. Nat. Hist. 1849, 4, 415–424. [Google Scholar]
- Epstein, H. Cattle. In Domestic Animals of China; Epstein, H., Ed.; Commonwealth Agricultural Bureaux: Farnham Royal, Buckinghamshire, UK, 1969; pp. 1–19. [Google Scholar]
- Clason, A.T. Groningen, The Netherland, 1980; Personal Communication. [Google Scholar]
- Von Nathusius, H.E. Vorträge Über Viehzucht Und Rassenkenntnis; Wiegandt Hempel: Berlin, Germany, 1872. [Google Scholar]
- Wilckens, M. Die Rinderrassen Mittel-Europas; Wilhelm Braumüller: Wien, Austria, 1876. [Google Scholar]
- Werner, A.H. Die Rinderzucht; Paul Parey: Berlin, Germany, 1912. [Google Scholar]
- Dürst, J.U. Die Rinder Von Babylonien, Assyrien Und Ägypten Und Ihr Zusammanhang Mit Den Rindern Der Alten Welt; Georg Reimer: Berlin, Germany, 1899. [Google Scholar]
- Keller, C. Naturgeschichte Der Haustiere; Paul Parey: Berlin, Germany, 1905. [Google Scholar]
- Adametz, L. Lehrbuch Der Allgemeinen Tierzucht; Springer: Vienna, Austria, 1926. [Google Scholar]
- Holecek Holleschowitz, C. Angewandte Tierzucht Auf Rassenbiologischer Grundlage; Julius Springer: Vienna, Austria, 1939. [Google Scholar]
- Duerst, J.U. Grundlagen Der Rinderzucht; Springer: Berlin, Germany, 1931. [Google Scholar]
- Arenander, E.O. Studien Über Das Ungehörnte Rindvieh Im Nördlichen Europa. Inaugural-Dissertation, J. Pässler, Halle, Germany, 1898.
- Auld, R.C.M. Polled and horned cattle. J. Hered. 1927, 18, 309–321. [Google Scholar]
- Hilzheimer, M. Naturalische Rassengeschichte Der Haussäugetiere; Walter de Gruyter & Co.: Berlin, Germany, 1926. [Google Scholar]
- Duerst, J.U. Grundlagen Der Rinderzucht; Julius Springer: Berlin, Germany, 1931. [Google Scholar]
- Haring, F.; Hammond, J.; Johannson, I. Handbuch Der Tierzüchtung; Paul Parey: Hamburg-Berlin, Germany, 1961. [Google Scholar]
- French, M.H.; Johansson, I.; Joshi, N.R.; McLaughlin, R.A. European Breeds of Cattle; FAO: Rome, Italy, 1966. [Google Scholar]
- Sanson, A. Traité De Zootechnie Tôme 4: Bovidés Taurins Et Bubalins, 3rd ed.; Agricole de las Maison Rustique: Paris, France, 1884. [Google Scholar]
- Diffloth, P. Races Bovines; J.B. Bailliéres & Fils: Paris, France, 1914; pp. 43–44. [Google Scholar]
- McKenny Hughes, T. On the more important breeds of cattle which have been recognised in the British Isles in successive periods and their relation to other archaeological and historical discoveries. Archaeologia 1896, 55, 125–158. [Google Scholar]
- Kaltenegger, F. Prähistorische Und Frühgeschichtlige Periode, I Gruppierung; Wilhelm Frick: Vienna, Austria, 1904. [Google Scholar]
- Wilson, J. The Evolution of British Cattle and the Fashioning of Breeds; Vinton & Company: Dublin, Ireland, 1909. [Google Scholar]
- Dechambre, P. Les Bovins; Ch. Amat. Éditeur: Paris, France, 1913; p. 26. [Google Scholar]
- Baron, R. Système Coordonnées Baroniennes; Libraire Agricole de la Maison Rustique et Libraire des Sciences Agricole: Paris, France, 1928. [Google Scholar]
- Chacrin, E.; Dumont, R. Bovins (Races); Librairie Larousse: Paris, France, 1921. [Google Scholar]
- Müller, W. Die Rinderzucht in Österreich; Carl Gerolds Sohn: Vienna, Austria, 1957. [Google Scholar]
- Miranda do Vale, J. Bovídeos Portuguezes; La Bécarre: Lissabon, Portugal, 1907. [Google Scholar]
- Aparicio, G. Zootecnia Especial, 4th ed.; Imprenta Moderna: Córdoba, Spain, 1960. [Google Scholar]
- Sanchez Belda, A. Razas Bovinas Españoles; Minosterio de Agricultura Pesca y Alimentación: Madrid, Spain, 1984. [Google Scholar]
- Sanchez Belda, A. Especie Bovina, 2nd ed.; Ministrerio de Agricultura y Pesca: Madrid, Spain, 1981. [Google Scholar]
- Autochthonous Livestock in Spain; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2008; p. 64.
- Alderson, L. Breed origins. Ark 1977, 4, 37–38. [Google Scholar]
- Laloë, D.; Moazami-Goudarzi, K.; Lenstra, J.A.; Ajmone Marsan, P.; Azor, P.; Rodero, E.; Baumung, R.; Bradley, D.G.; Bruford, W.; Canon, J.; et al. Spatial trends of domestic ruminants in Europe. Diversity 2010, 2, 932–945. [Google Scholar]
- Edwards, C.J.; Ginja, C.; Kantanen, J.; Pérez-Pardal, L.; Tresset, A.; Stock, F.; Gama, L.T.; Penedo, M.C.T.; Bradley, D.G.; Lenstra, J.A.; et al. Dual origins of dairy cattle farming—Evidence from a comprehensive survey of European Y-chromosomal variation. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Alderson, L. The Categorisation of types and breeds of cattle in Europe. Arch. Zootec. 1992, 41, 325–334. [Google Scholar]
- Simon, D.L.; Buchenauer, D. Genetic Diversity of European Livestock Breeds; Wageningen Pers: Wageningen, The Netherlands, 1993. [Google Scholar]
- Bougler, J. Introduction. In Races Bovines Françaises; Babo, D., Ed.; Éditions France Agricole: Paris, France, 1998; p. 20. [Google Scholar]
- Denis, B. Introduction. In Races Bovines; Patin, S., Dervillé, M., Avon, L., Eds.; Éditions France Agricole: Paris, France, 2009; p. 7. [Google Scholar]
- Fitzinger, L.J. Wissenschaftlich-Populäre Naturgeschichte Der Haussäugetieren; K.K. Hof-und Staatsdruckerei: Vienna, Austria, 1860. [Google Scholar]
- Ramm, E. Die Arten Und Rassen Des Rindes; Ulmer: Stuttgart, Germany, 1901. [Google Scholar]
- Zwaenepoel, H. Les Bovins; G. Bothy: Brussels, Belgium, 1920. [Google Scholar]
- Thaer, D.U. Einleitung Zur Kenntniss Der Englischen Landwirtschaft Und Ihrer Neueren Practischen Und Theoretischen Fortschritt in Rücksicht Auf Werdvollkommnung Deutscher Landwirtschaft Für Denkende Landwirte Und Kameralisten; Gebrudern Hahn: Hannover, Germany, 1804. [Google Scholar]
- Hengeveld, G.J. Het Rundvee, Zijne Verschillende Soorten, Rassen En Veredeling; De Erven Loosjes: Haarlem, The Netherlands, 1865. [Google Scholar]
- Kroes, H.A. Huisdierenteelt. Deel III; Van der Kamp: Groningen, The Netherlands, 1921; p. 100. [Google Scholar]
- Kronacher, C. Der Artbegriff Und Die Wege Der Artbildung, Die Rassen; Paul Parey: Berlin, Germany, 1921. [Google Scholar]
- Hansen, J. Die Arten Des Rindes; Paul Parey: Berlin, Germany, 1927. [Google Scholar]
- Epstein, H. Descent and origin of the afrikaner cattle. J. Hered. 1933, 24, 449–462. [Google Scholar]
- Curson, H.H.; Epstein, H. A comparison of Hamitic longhorn, West African shorthorn and Africander cattle, particularly in regard to the skull. Onderstepoort J. Vet. Sci. Anim. Ind. 1934, 3, 487–495. [Google Scholar]
- Bisschop, J.H.R. Parent stock and derived types of African cattle with particular reference to the importance of conformation characteristics in the study of their origin. South. Afr. J. Sci. 1937, 33, 852–870. [Google Scholar]
- Mason, I.L. The Classification of West African Livestock; Commonwealth Agricultural Bureaux: Farnham Royal, Buckinghamshire, UK, 1951; pp. 7–8. [Google Scholar]
- Doutresoulle, G. L′Élevage En Afrique Occidentale Française; Éditions Larose: Paris, France, 1947. [Google Scholar]
- Joshi, N.R.; McLaughlin, E.A.M.; Phillips, R. Types and Breeds of African Cattle; FAO Agricultural Study No. 37; FAO: Rome, Italy, 1957; p. 7. [Google Scholar]
- Mason, I.L.; Maule, J.P. The Indigenous Livestock of Eastern and Southern Africa; Commonwealth Agricultural Bureaux: Farnham Royal, Buckinghamshire, UK, 1960; p. 31. [Google Scholar]
- Rege, J.E.O.; Tawah, C.L. The state of African cattle genetic resources II, geographical distribution, characteristics and uses of present-day breeds and strains. Anim. Genet. Resour. Inf. 1999, 26, 1–25. [Google Scholar]
- Olver, A. A brief survey of some of the important breeds of cattle in India. Imper. Council Agric. Res. 1938, 17, 1–8. [Google Scholar]
- Joshi, N.R.; Phillips, R. Zebu Cattle of India and Pakistan; FAO Agriculture Studies No. 19; FAO: Rome, Italy, 1953; p. 22. [Google Scholar]
- Cheng, P. Bovine Breeds of China; Shanghai Scientific & Technical Publishers: Shanghai, China, 1986; p. 2. [Google Scholar]
- Payne, W.J.A.; Hodges, J. Classification systems. In Tropical Cattle, Origins, Breeds and Breeding Policies; Hodges, J., Payne, W.J.A., Eds.; Blackwell Science LTD: Oxford, UK, 1997; pp. 89–94. [Google Scholar]
- Maule, J.P. The Cattle of the Tropics; Redwood Press: Melksham, Wiltshire, UK, 1990; p. 22. [Google Scholar]
- Grosclaude, F.; Aupetit, R.Y.; Lefebvre, J.; Meriaux, J.C. Essai d'Analyse des relations genetiques entre les races bovines francaises a l'Aide du polymorphisme biochimique. Genet. Sel. Evol. 1990, 22, 317–338. [Google Scholar]
- Denis, B.; Avon, L. Races Bovines, Histoire, Aptitudes, Situation Actuelle; Éditions Castor & Pollux: Chaumont, France, 2010; p. 324. [Google Scholar]
- Gautier, M.; Laloë, D.; Moazami-Goudarzi, K. Insights into the genetic history of French cattle from dense SNP data on 47 worldwide breeds. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Hodges, J.; Payne, W.J.A. Tropical Cattle: Origin, Breeding and Breeding Policies; Iowa State Press: Davenport, IA, USA, 1997. [Google Scholar]
- Maule, J.P. Balerno, Scotland, UK, 1992; Personal Communication. [Google Scholar]
- Felius, M. Genus Bos: Cattle Breeds of the World. MSD-AGVET; Merck and Co.: Rahway, NJ, USA, 1985. [Google Scholar]
- Hanotte, O.; Bradley, D.G.; Ochieng, J.W.; Verjee, Y.; Hill, E.W.; Rege, J.E.O. African pastoralism: Genetic imprints of origins and migrations. Science 2002, 296, 336–339. [Google Scholar]
- Bruford, M.; Bradley, D.; Luikart, G. DNA Markers reveal the complexity of livestock domestication. Nat. Rev. Genet. 2003, 4, 900–910. [Google Scholar]
- Cymbron, T.; Freeman, A.; Malheiro, M.I.; Vigne, J.D.; Bradley, D. Microsatellite diversity suggests different histories for mediterranean and Northern European cattle populations. Proc. R. Soc. B Biol. Sci. 2005, 272, 1837–1843. [Google Scholar]
- Freeman, A.R.; Bradley, D.G.; Nagda, S.; Gibson, J.P.; Hanotte, O. Combination of multiple microsatellite data sets to investigate genetic diversity and admixture of domestic cattle. Anim. Genet. 2006, 37, 1–9. [Google Scholar]
- Bradley, D.G.; MacHugh, D.E.; Cunningham, P.; Loftus, R.T. Mitochondrial diversity and the origins of African and European cattle. Proc. Natl. Acad. Sci. USA 1996, 93, 5131–5135. [Google Scholar]
- Troy, C.S.; MacHugh, D.E.; Bailey, J.F.; Magee, D.A.; Loftus, R.T.; Cunningham, P.; Chamberlain, A.T.; Sykes, B.C.; Bradley, D.G. Genetic evidence for near-eastern origins of European cattle. Nature 2001, 410, 1088–1091. [Google Scholar]
- Rath, S. The Complete Cow; Voyageur Press: Stillwater, OK, USA, 1998. [Google Scholar]
- European Cattle Genetic Diversity Consortium. Marker-assisted conservation of European cattle breeds: An evaluation. Anim. Genet. 2006, 37, 475–481. [Google Scholar]
- Negrini, R.; Nijman, I.J.; Milanesi, E.; Moazami-Goudarzi, K.; Williams, J.L.; Erhardt, G.; Dunner, S.; Rodellar, C.; Valentini, A.; Bradley, D.G.; et al. Differentiation of European cattle by AFLP fingerprinting. Anim. Genet. 2007, 38, 60–66. [Google Scholar]
- Lenstra, J.A. What's in a breed. Criteria for conservation. Proceeding of Genetic diversity, selection and adaptation in wildlife and livestock—Molecular approaches, Salzburg, Austria; 2008. Available online: http://www.nas.boku.ac.at/fileadmin/_/H93/H932-NUWI/Kurse-Workshops-Tagungen/ESF-Workshop/Session1/Lenstra.pdf (accessed on 30 September 2011). [Google Scholar]
- Decker, J.E.; Pires, J.C.; Conant, G.C.; McKay, S.D.; Heaton, M.P.; Chen, K.; Cooper, A.; Vilkki, J.; Seabury, C.M.; Caetano, A.R.; et al. Resolving the evolution of extant and extinct ruminants with high-throughput phylogenomics. Proc. Natl. Acad. Sci. USA 2009, 106, 18644–18649. [Google Scholar]
- Martin-Burriel, I.; Rodellar, C.; Lenstra, J.A.; Sanz, A.; Cons, C.; Osta, R.; Reta, M.; De Arguello, S.; Sanz, A.; Zaragoza, P. Genetic diversity and relationships of endangered Spanish cattle breeds. J. Hered. 2007, 98, 687–691. [Google Scholar]
- Martin-Burriel, I.; Rodellar, C.; Canon, J.; Cortes, O.; Dunner, S.; Landi, V.; Martinez-Martinez, A.; Gama, L.T.; Ginja, C.; Penedo, M.C.; et al. Genetic diversity, structure, and breed relationships in Iberian cattle. J. Anim. Sci. 2011, 89, 893–906. [Google Scholar]
- Mommens, G. Boviene Microsatallieten Als DNA-Merkers; Een Studie Naar De Genetische Diversiteit in Runderpopulaties; Universiteit Gent: Gent, Belgium, 2000. [Google Scholar]
- Payne, W.J.A. Domestication: A step forward in civilization. In Cattle Genetic Resources; Hickman, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 51–72. [Google Scholar]
- Lenstra, J.A.; Groeneveld, L.F.; Eding, H.; Kantanen, J.; Williams, J.L.; Taberlet, P.; Nicolazzi, E.L.; Sölkner, J.; Simianer, H.; Ciani, E.; et al. Molecular tools and analytical approaches for the characterization of farm animal diversity. Anim. Genet. 2011. in press. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Felius, M.; Koolmees, P.A.; Theunissen, B.; European Cattle Genetic Diversity Consortium; Lenstra, J.A. On the Breeds of Cattle—Historic and Current Classifications. Diversity 2011, 3, 660-692. https://doi.org/10.3390/d3040660
Felius M, Koolmees PA, Theunissen B, European Cattle Genetic Diversity Consortium, Lenstra JA. On the Breeds of Cattle—Historic and Current Classifications. Diversity. 2011; 3(4):660-692. https://doi.org/10.3390/d3040660
Chicago/Turabian StyleFelius, Marleen, Peter A. Koolmees, Bert Theunissen, European Cattle Genetic Diversity Consortium, and Johannes A. Lenstra. 2011. "On the Breeds of Cattle—Historic and Current Classifications" Diversity 3, no. 4: 660-692. https://doi.org/10.3390/d3040660
APA StyleFelius, M., Koolmees, P. A., Theunissen, B., European Cattle Genetic Diversity Consortium, & Lenstra, J. A. (2011). On the Breeds of Cattle—Historic and Current Classifications. Diversity, 3(4), 660-692. https://doi.org/10.3390/d3040660




