Influence of Habitat on the Impact of Non-Native Fishes on Native Ichthyofauna in a Group of Lakes of the Lower Doce River, Espírito Santo, Southeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
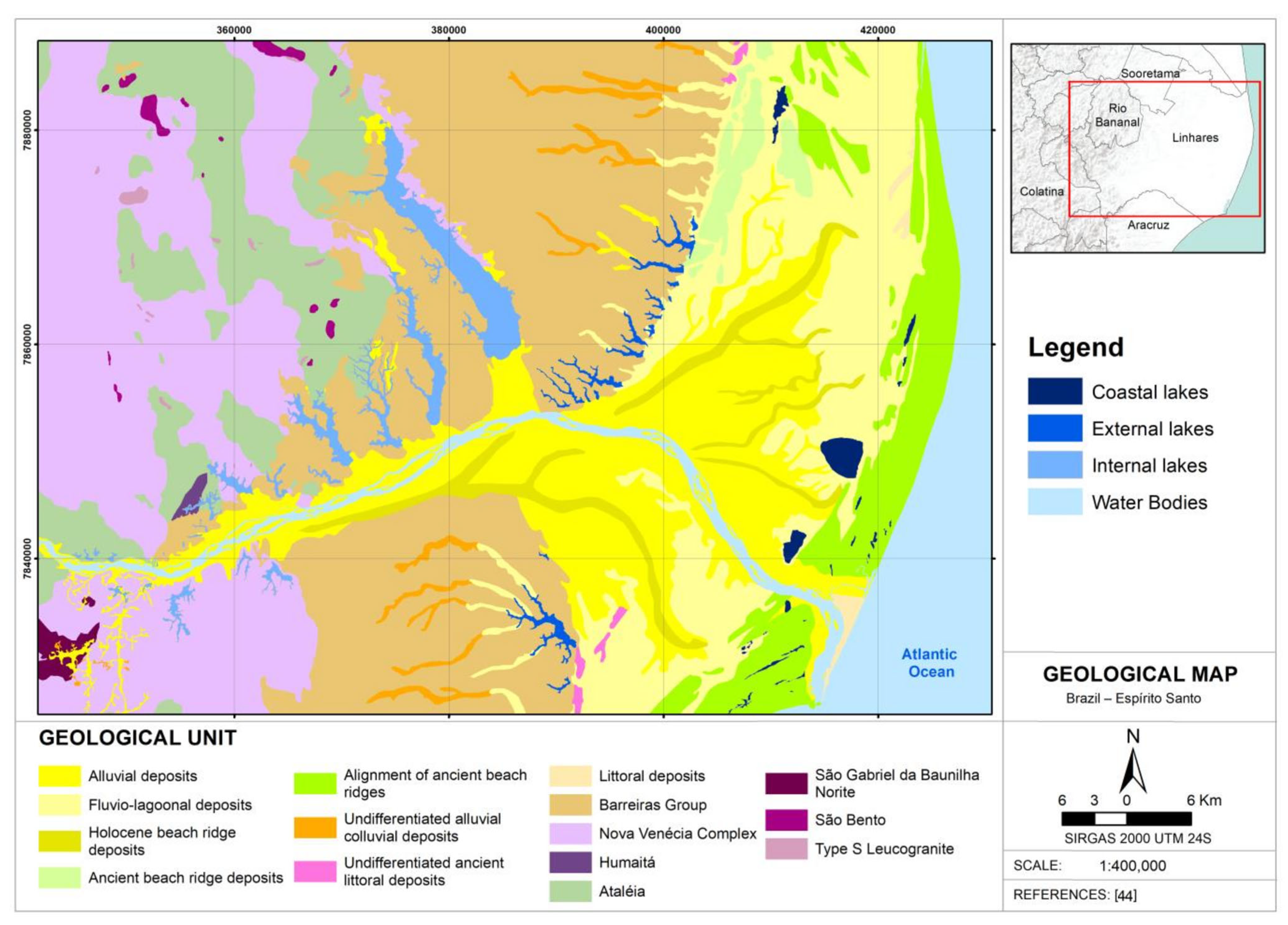
2.1. Study Area
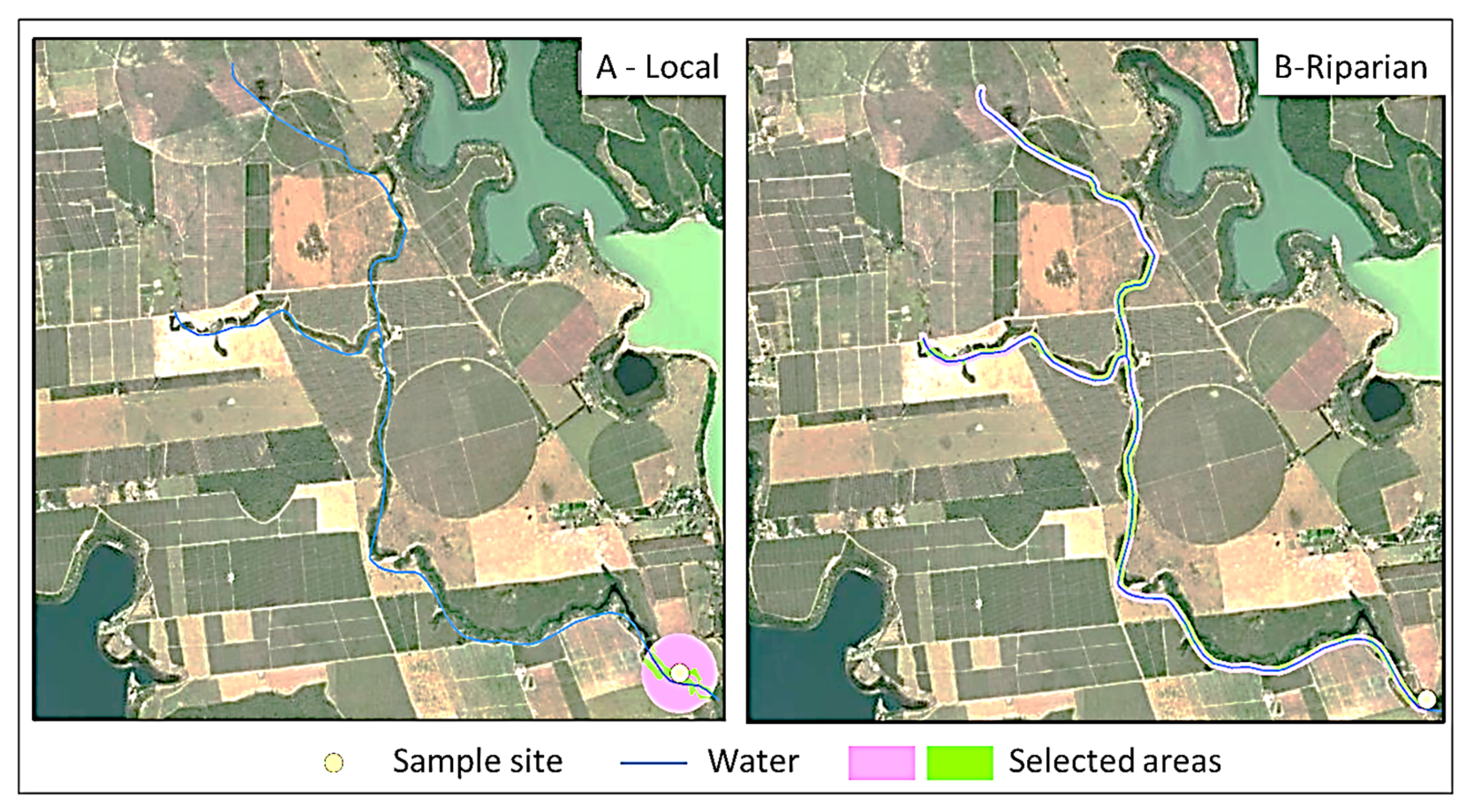
2.2. Sample Design
2.3. Data Collection
2.3.1. Fishes
2.3.2. Structural Data
2.3.3. Physicochemical Parameters
2.3.4. Native Vegetation in the Landscape
2.4. Data Analysis
2.4.1. Comparisons Between the Ichthyofauna of Lakes and Streams
2.4.2. Distribution of Non-Native Ichthyofauna Across the Internal Lake Complex
2.4.3. Influence of Non-Native Ichthyofauna on Native Fish Communities
2.4.4. Influence of Habitat on the Fish Community in Streams
3. Results
3.1. Overall Results
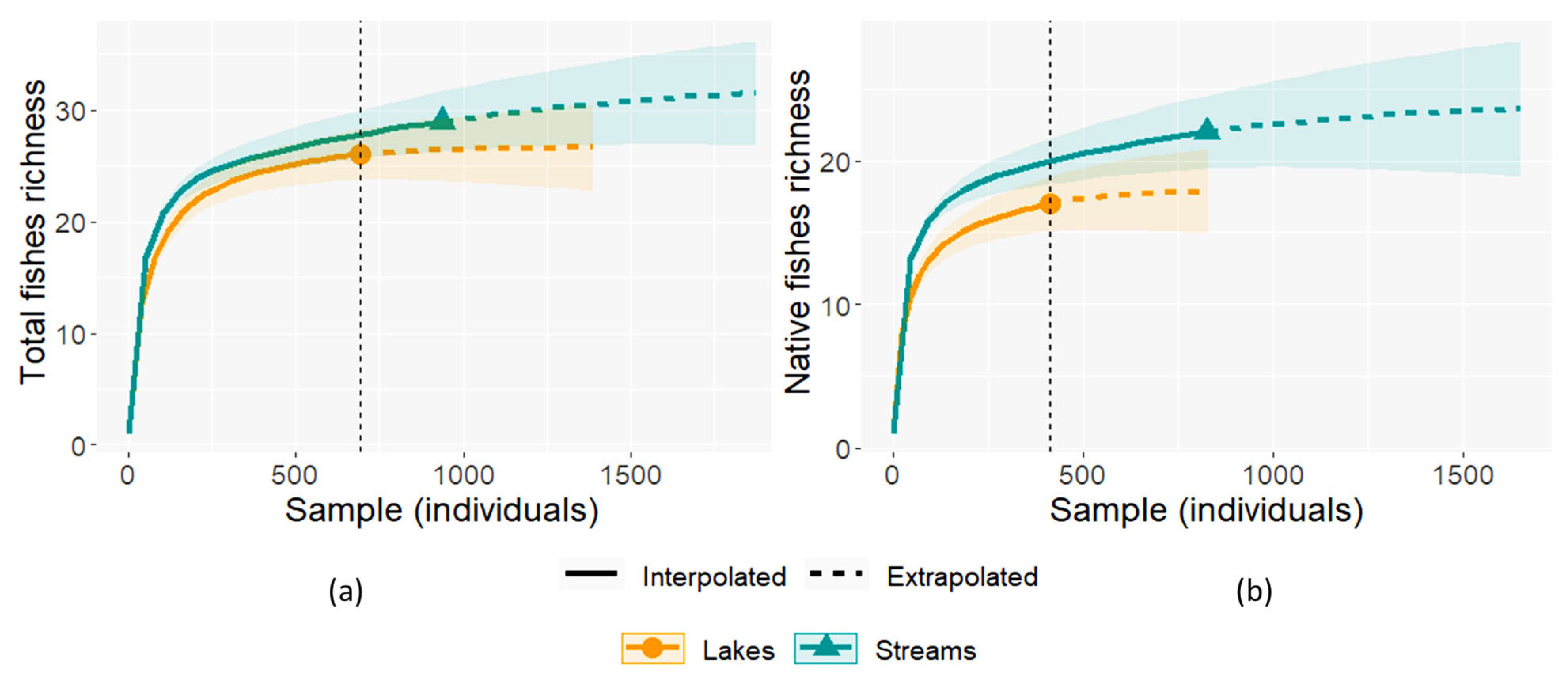
3.2. Comparisons Between the Ichthyofauna of Lakes and Streams
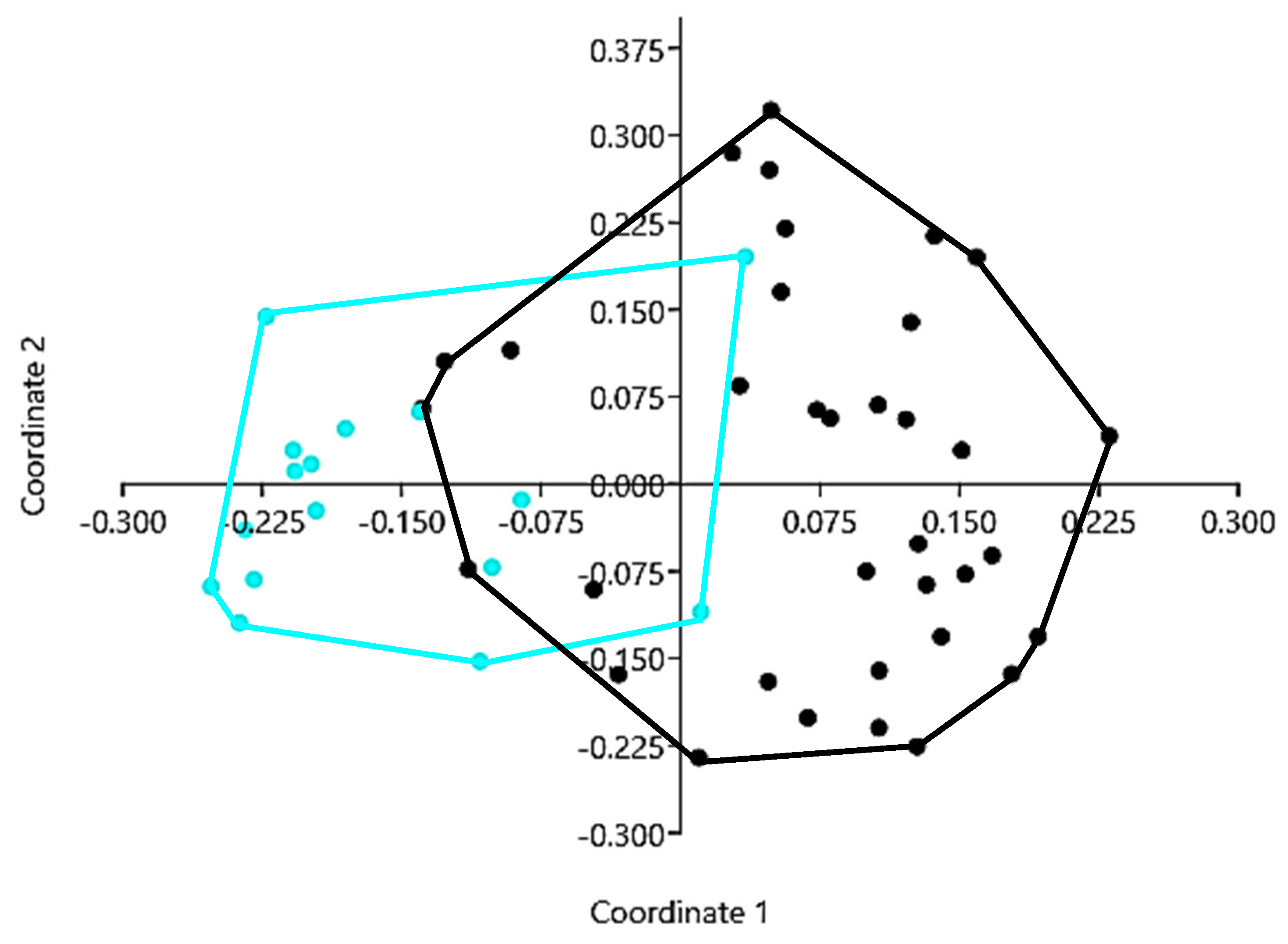
3.3. Distribution of Non-Native Ichthyofauna Across the Internal Lake Complex
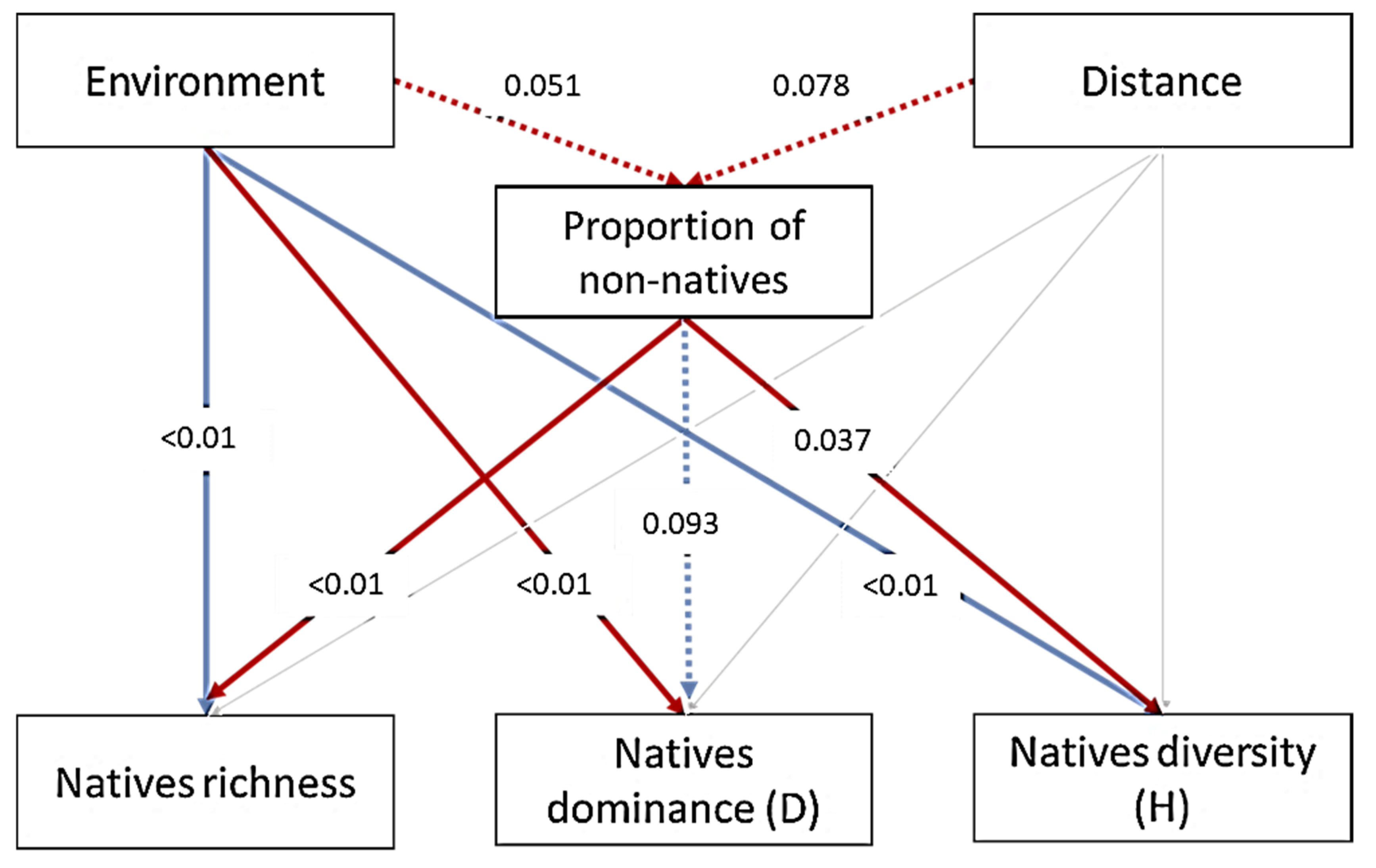
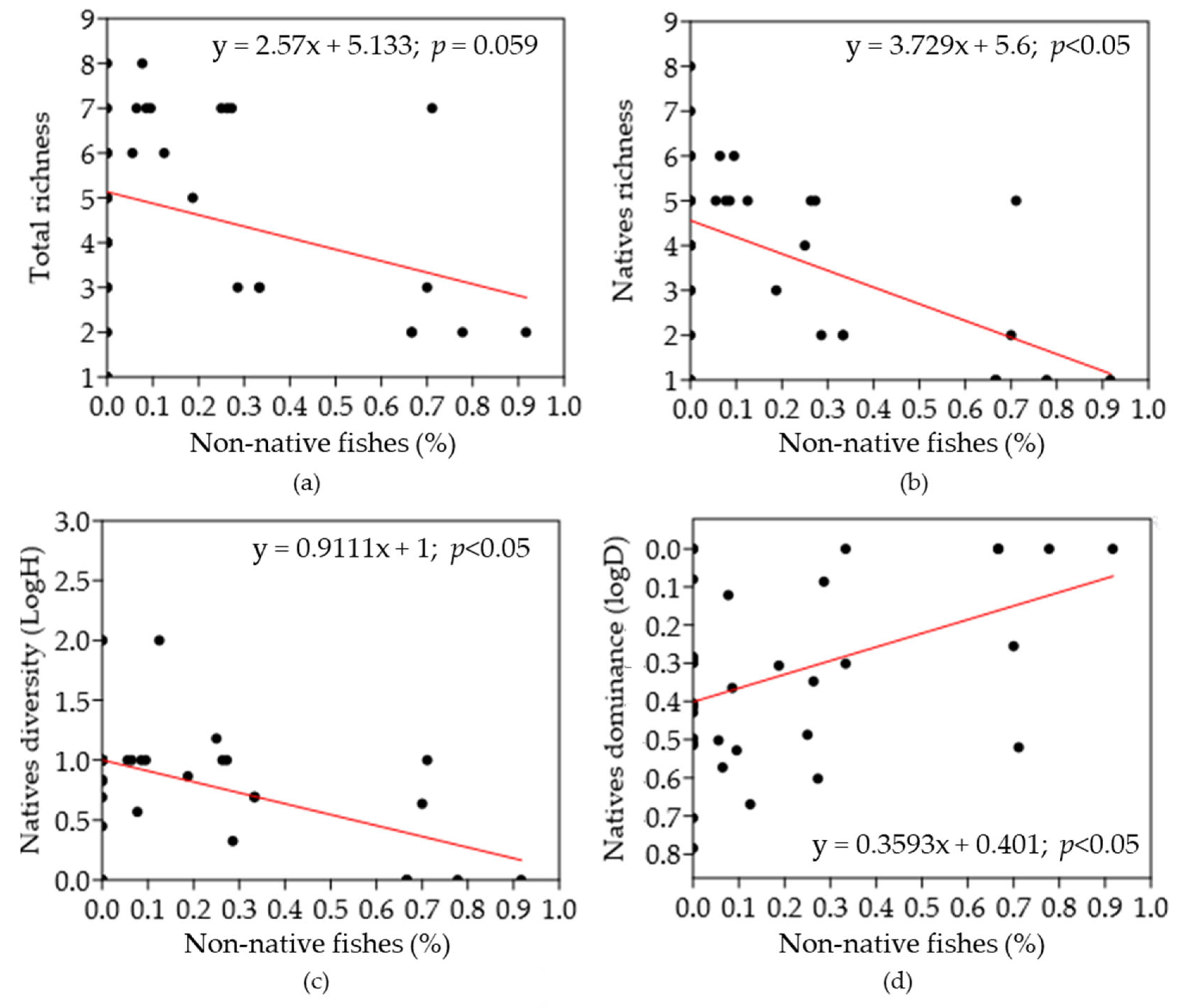
3.4. Influence of Non-Native Ichthyofauna on Native Fish Communities
3.5. Influence of Habitat on the Fish Community in Streams
4. Discussion
4.1. Comparisons Between the Ichthyofauna of Lakes and Streams
4.2. Distribution of Non-Native Ichthyofauna Across the Internal Lake Complex
4.3. Influence of Non-Native Ichthyofauna on Native Fish Communities
4.4. Influence of Habitat on the Fish Community in Streams
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Taxa | Origin | Total Individuals | Individuals on Lakes | Individuals on Streams |
|---|---|---|---|---|
| Atheriniformes | ||||
| Atherinopsidae | ||||
| Atherinella brasiliensis | Native | 13 | 13 | 0 |
| Characiformes | ||||
| Anostomidae | ||||
| Hypomasticus copelandii | Native | 19 | 19 | 0 |
| Characidae | ||||
| Astyanax lacustris | Native | 183 | 107 | 76 |
| Astyanax sp. | Native | 129 | 0 | 129 |
| Psalidodon rivularis | Native | 75 | 0 | 75 |
| Hyphessobrycon eques | Non-native | 19 | 0 | 19 |
| Hyphessobrycon luetkenii | Native | 22 | 0 | 22 |
| Knodus moenkhausi | Native | 132 | 0 | 132 |
| Moenkhausia vittata | Native | 1 | 0 | 1 |
| Oligosarcos acutirostris | Native | 7 | 2 | 5 |
| Curimatidae | ||||
| Cyphocharax gilbert | Native | 1 | 0 | 1 |
| Erythrinidae | ||||
| Hoplias malabaricus | Native | 42 | 25 | 17 |
| Prochilidontidae | ||||
| Prochilodus lineatus | Non-native | 10 | 10 | 0 |
| Serrasalmidae | ||||
| Metynnis lippincottianus | Non-native | 145 | 109 | 36 |
| Pygocentrus nattereri | Non-native | 120 | 107 | 13 |
| Pygocentrus piraya | Non-native | 12 | 12 | 0 |
| Cichliformes | ||||
| Cichlidade | ||||
| Astronotus ocellatus | Non-native | 4 | 4 | 0 |
| Australoheros capixaba | Native | 42 | 6 | 36 |
| Cichla kelberi | Non-native | 24 | 17 | 7 |
| Coptodon rendalli | Non-native | 17 | 5 | 12 |
| Crenicichla lacustris | Native | 57 | 7 | 50 |
| Geophagus brasiliensis | Native | 107 | 47 | 60 |
| Oreochromis niloticus | Non-native | 2 | 2 | 0 |
| Clupeiformes | ||||
| Engraulidae | ||||
| Lycengraulis grossidens | Native | 3 | 3 | 0 |
| Cyprinodontiformes | ||||
| Poeciliidae | ||||
| Poecilia reticulata | Non-native | 24 | 0 | 24 |
| Poecilia vivipara | Native | 137 | 18 | 119 |
| Gymnotiformes | ||||
| Gymnotidae | ||||
| Gymnotus carapo | Native | 12 | 1 | 11 |
| Mugiliformes | ||||
| Mugilidae | ||||
| Mugil curema | Native | 11 | 0 | 11 |
| Perciformes | ||||
| Sciaenidae | ||||
| Pachyurus adspersus | Native | 7 | 7 | 0 |
| Siluriformes | ||||
| Auchenipteridae | ||||
| Trachelyopterus striatulus | Native | 10 | 8 | 2 |
| Callichthyidae | ||||
| Hoplosternum littorale | Native | 146 | 133 | 13 |
| Heptapteridae | ||||
| Pimelodella aff. vittata | Native | 1 | 0 | 1 |
| Rhamdia quelen | Native | 2 | 0 | 2 |
| Loricariidae | ||||
| Hypostomus affinis | Native | 13 | 0 | 13 |
| Loricariichthys castaneus | Native | 1 | 1 | 0 |
| Otothyris travassosi | Native | 12 | 0 | 12 |
| Pimelodidae | ||||
| Pimelodus maculatus | Non-native | 13 | 12 | 1 |
| Synbranchiformes | ||||
| Synbranchidae | ||||
| Synbranchus marmoratus | Native | 2 | 2 | 0 |
| RICHNESS | 38 | 25 | 28 |
Appendix B
| Sample Date | Local Id | Locality | Environment | Lat | Long | Specie | Collection Code | Voucher Number |
|---|---|---|---|---|---|---|---|---|
| 30 March 2022 | L. JUPARANÃ | Lagoa Juparanã | Lago | −1,923,160 | −4,016,285 | Anchoviella lepidentostole | CZNC | 4899 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Astronotus ocellatus | CZNC | 4930 |
| 13 April 2021 | ANG 1A | Córrego Timirim, proximo a cachoeira de Angeli | Córrego | −1,934,915 | −4,042,013 | Astyanax cf. intermedius | CZNC | 4845 |
| 13 April 2021 | ANG 1B | Afluente da Lagoa Terra Alta | Córrego | −1,936,425 | −4,041,918 | Astyanax cf. intermedius | CZNC | 4815 |
| 17 April 2021 | LIM 3A | Afluente da Lagoa do Limão | Córrego | −1,962,088 | −4,040,378 | Astyanax cf. intermedius | CZNC | 4842 |
| 7 April 2021 | NOV 1A | Lagoa Nova | Córrego | −1,941,836 | −4,015,389 | Astyanax cf. intermedius | CZNC | 4814 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Astyanax cf. intermedius | CZNC | 4890 |
| 11 April 2021 | SÃO 1A | Rio São José | Córrego | −1,915,960 | −4,021,523 | Astyanax cf. intermedius | CZNC | 4874 |
| 19 January 2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −4,022,9444 | Astyanax lacustris | MBML-PEIXES | 14,006 |
| 13 April 2021 | ANG 1A | Córrego Timirim, proximo a cachoeira de Angeli | Córrego | −1,934,915 | −4,042,013 | Astyanax lacutris | CZNC | 4848 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | −1,938,783 | −4,029,887 | Astyanax lacutris | CZNC | 4881 |
| 12 April 2021 | JES 1B | Afluente sem nome da Lagoa Juparanã | Córrego | −1,916,424 | −4,029,590 | Astyanax lacutris | CZNC | 4904 |
| 30 March 2022 | L. JUPARANÃ | Lagoa Juparanã | Lago | −1,923,160 | −4,016,285 | Astyanax lacutris | CZNC | 4897 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Astyanax lacutris | CZNC | 4956 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Astyanax lacutris | CZNC | 4929 |
| 1 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Astyanax lacutris | CZNC | 4961 |
| 15 April 2021 | LIM 1C | Afluente da Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Astyanax lacutris | CZNC | 4822 |
| 17 April 2021 | LIM 3A | Afluente da Lagoa do Limão | Córrego | −1,962,088 | −4,040,378 | Astyanax lacutris | CZNC | 4907 |
| 6 May 2021 | LSD 1A | Lagoa Terra Altinha | Córrego | −1,947,708 | −4,028,962 | Astyanax lacutris | CZNC | 4863 |
| 7 April 2021 | NOV 1A | Lagoa Nova | Córrego | −1,941,836 | −4,015,389 | Astyanax lacutris | CZNC | 4810 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Astyanax lacutris | CZNC | 4860 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Astyanax lacutris | CZNC | 4889 |
| 2 May 2021 | PAL 1C | Afluente da Lagoa Palminhas | Córrego | −1,943,500 | −4,016,622 | Astyanax lacutris | CZNC | 4832 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Astyanax lacutris | CZNC | 4936 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Astyanax lacutris | CZNC | 4851 |
| 2 May 2021 | RES 1B | Afluente da Lagoa Juparanã | Córrego | −1,936,511 | −4,011,470 | Astyanax lacutris | CZNC | 4886 |
| 11 April 2021 | SÃO 1A | Rio São José | Córrego | −1,915,960 | −4,021,523 | Astyanax lacutris | CZNC | 4872 |
| 11 April 2021 | SÃO 1B | Rio São José | Córrego | −1,914,903 | −4,022,318 | Astyanax lacutris | CZNC | 4877 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Astyanax sp. | CZNC | 4883 |
| 17 April 2021 | LIM 3A | Afluente da Lagoa do Limão | Córrego | −1,962,088 | −4,040,378 | Astyanax sp. | CZNC | 4906 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Astyanax sp. | CZNC | 4859 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Astyanax sp. | CZNC | 4937 |
| 19 January 2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −40,229,444 | Atherinella brasiliensis | MBML-PEIXES | 14,000 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Australoheros capixaba | CZNC | 4941 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Australoheros capixaba | CZNC | 4861 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Australoheros capixaba | CZNC | 4852 |
| 12 April 2021 | JES 1B | Afluente sem nome da Lagoa Juparanã | Córrego | −1,916,424 | −4,029,590 | Cichla kelberi | CZNC | 4903 |
| 30 March 2022 | L. JUPARANÃ | Lagoa Juparanã | Lago | −1,923,160 | −4,016,285 | Cichla kelberi | CZNC | 4898 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Cichla kelberi | CZNC | 4947 |
| 7 April 2021 | NOV 1A | Lagoa Nova | Córrego | −1,941,836 | −4,015,389 | Cichla kelberi | CZNC | 4812 |
| 11 April 2021 | SÃO 1A | Rio São José | Córrego | −1,915,960 | −4,021,523 | Cichla kelberi | CZNC | 4870 |
| 19 January 2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −40,229,444 | Cichla sp. | MBML-PEIXES | 14,004 |
| 12 April 2021 | SAO 1C | Rio São José | Córrego | −1,911,154 | −4,024,773 | Coptodon rendalli | CZNC | 4835 |
| 8 April 2021 | 3 MA 1B | Ribeirão das Palmas | Córrego | −1,943,544 | −4,017,800 | Crenicichla lacustris | CZNC | 4910 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Crenicichla lacustris | CZNC | 4950 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Crenicichla lacustris | CZNC | 4923 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Crenicichla lacustris | CZNC | 4946 |
| 27 October 2022 | L.TERRA ALTINHA | Lagoa Terra Altinha | Córrego | −1,944,994 | −4,028,975 | Crenicichla lacustris | CZNC | 4912 |
| 15 April 2021 | LIM 1C | Afluente da Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Crenicichla lacustris | CZNC | 4823 |
| 15 April 2021 | LIM 1C | Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Crenicichla lacustris | CZNC | 4830 |
| 6 May 2021 | LSD 1A | Lagoa Terra Altinha | Córrego | −1,947,708 | −4,028,962 | Crenicichla lacustris | CZNC | 4864 |
| 7 April 2021 | NOV 1A | Lagoa Nova | Córrego | −1,941,836 | −4,015,389 | Crenicichla lacustris | CZNC | 4811 |
| 12 June 2021 | PAL 1A | Ribeirão das Palmas | Córrego | −1,944,742 | −4,022,411 | Crenicichla lacustris | CZNC | 4817 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Crenicichla lacustris | CZNC | 4849 |
| 6 May 2021 | TAL 1B | Lagoa Terra Alta | Lago | −1,948,494 | −4,029,217 | Crenicichla lacustris | CZNC | 4902 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Cyphocharax gilbert | CZNC | 4885 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Cyphocharax gilbert | CZNC | 4858 |
| 13 April 2021 | ANG 1A | Córrego Timirim, proximo a cachoeira de Angeli | Córrego | −1,934,915 | −4,042,013 | Geophagus brasiliensis | CZNC | 4846 |
| 4 May 2021 | DES 1C | Lagoa Juparanã | Córrego | −1,936,319 | −4,011,679 | Geophagus brasiliensis | CZNC | 4828 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Geophagus brasiliensis | CZNC | 4880 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Geophagus brasiliensis | CZNC | 4918 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Geophagus brasiliensis | CZNC | 4945 |
| 1 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Geophagus brasiliensis | CZNC | 4962 |
| 6 May 2021 | LSD 1A | Lagoa Terra Altinha | Córrego | −1,947,708 | −4,028,962 | Geophagus brasiliensis | CZNC | 4866 |
| 10 April 2021 | OLEO 1B | Afluente da Lagoa do Batista | Córrego | −1,951,219 | −4,043,625 | Geophagus brasiliensis | CZNC | 4840 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Geophagus brasiliensis | CZNC | 4934 |
| 16 April 2021 | PAU 1A | Afluente da Lagoa Pau Grosso | Córrego | −1,948,928 | −4,034,423 | Geophagus brasiliensis | CZNC | 4820 |
| 19 January 2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −40,229,444 | Geophagus brasiliensis | MBML-PEIXES | 14,003 |
| 28 October 2022 | L. TERRA ALTA | Lagoa Terra Alta | Lago | −1,945,396 | −4,035,517 | Gymnotus carapo | CZNC | 4914 |
| 6 May 2021 | LSD 1A | Lagoa Terra Altinha | Córrego | −1,947,708 | −4,028,962 | Gymnotus carapo | CZNC | 4865 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Gymnotus carapo | CZNC | 4891 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Gymnotus carapo | CZNC | 4938 |
| 11 April 2021 | SÃO 1B | Rio São José | Córrego | −1,914,903 | −4,022,318 | Gymnotus carapo | CZNC | 4875 |
| 4 May 2021 | DES 1C | Lagoa Juparanã | Córrego | −1,936,319 | −4,011,679 | Hoplias malabaricus | CZNC | 4827 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Hoplias malabaricus | CZNC | 4878 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Hoplias malabaricus | CZNC | 4952 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Hoplias malabaricus | CZNC | 4921 |
| 1 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Hoplias malabaricus | CZNC | 4966 |
| 17 April 2021 | LIM 3A | Afluente da Lagoa do Limão | Córrego | −1,962,088 | −4,040,378 | Hoplias malabaricus | CZNC | 4844 |
| 10 April 2021 | OLEO 1B | Afluente da Lagoa do Batista | Córrego | −1,951,219 | −4,043,625 | Hoplias malabaricus | CZNC | 4837 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Hoplias malabaricus | CZNC | 4931 |
| 11 April 2021 | SÃO 1A | Rio São José | Córrego | −1,915,960 | −4,021,523 | Hoplias malabaricus | CZNC | 4869 |
| 30 March 2022 | L. JUPARANÃ | Lagoa Juparanã | Lago | −1,923,160 | −4,016,285 | Hoplosternum littorale | CZNC | 4896 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Hoplosternum littorale | CZNC | 4920 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Hoplosternum littorale | CZNC | 4926 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Hoplosternum littorale | CZNC | 4948 |
| 1 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Hoplosternum littorale | CZNC | 4967 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Hoplosternum littorale | CZNC | 4940 |
| 2 May 2021 | RES 1B | Afluente da Lagoa Juparanã | Córrego | −1,936,511 | −4,011,470 | Hoplosternum littorale | CZNC | 4888 |
| 11 April 2021 | SÃO 1A | Rio São José | Córrego | −1,915,960 | −4,021,523 | Hoplosternum littorale | CZNC | 4871 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Hyphessobrycon bifasciatus | CZNC | 4932 |
| 8 April 2021 | 3 MA 1B | Ribeirão das Palmas | Córrego | −1,943,544 | −4,017,800 | Hyphessobrycon eques | CZNC | 4909 |
| 27 October 2022 | L.TERRA ALTINHA | Lagoa Terra Altinha | Córrego | −1,944,994 | −4,028,975 | Hyphessobrycon eques | CZNC | 4911 |
| 15 April 2021 | LIM 1C | Afluente da Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Hyphessobrycon eques | CZNC | 4825 |
| 6 May 2021 | LSD 1A | Lagoa Terra Altinha | Córrego | −1,947,708 | −4,028,962 | Hyphessobrycon eques | CZNC | 4868 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Hyphessobrycon eques | CZNC | 4854 |
| 13 April 2021 | ANG 1B | Afluente da Lagoa Terra Alta | Córrego | −1,936,425 | −4,041,918 | Hypostomus affinis | CZNC | 4816 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Hypostomus affinis | CZNC | 4879 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Hypostomus affinis | CZNC | 4856 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Knodus aff. Moenkhausii | CZNC | 4884 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Knodus aff. Moenkhausii | CZNC | 4893 |
| 10 April 2021 | OLEO 1B | Afluente da Lagoa do Batista | Córrego | −1,951,219 | −4,043,625 | Knodus aff. Moenkhausii | CZNC | 4841 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Knodus aff. Moenkhausii | CZNC | 4935 |
| 19 December 2020 | PALMAS INTER 2/4 | Afluente da Lagoa Palmas | Córrego | −1,942,385 | −4,026,169 | Knodus aff. Moenkhausii | CZNC | 4829 |
| 12 April 2021 | SAO 1C | Rio São José | Córrego | −1,911,154 | −4,024,773 | Knodus aff. Moenkhausii | CZNC | 4834 |
| 28 October 2022 | L. TERRA ALTA | Lagoa Terra Alta | Lago | −1,945,396 | −4,035,517 | Loricariichthys castaneus | CZNC | 4915 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Lycengraulis grossidens | CZNC | 4958 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Metynnis lippincottianus | CZNC | 4955 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Metynnis lippincottianus | CZNC | 4917 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Metynnis lippincottianus | CZNC | 4927 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Metynnis lippincottianus | CZNC | 4943 |
| 1 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Metynnis lippincottianus | CZNC | 4963 |
| 16 April 2021 | PAU 1A | Afluente da Lagoa Pau Grosso | Córrego | −1,948,928 | −4,034,423 | Metynnis lippincottianus | CZNC | 4821 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Metynnis lippincottianus | CZNC | 4850 |
| 19 January 2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −40,229,444 | Metynnis maculatus | MBML-PEIXES | 14,002 |
| 15 April 2021 | LIM 1C | Afluente da Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Moenkhausia doceana | CZNC | 4826 |
| 11 April 2021 | SÃO 1B | Rio São José | Córrego | −1,914,903 | −4,022,318 | Oligosarcus acutirostris | CZNC | 4876 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Otothyris travassosi | CZNC | 4894 |
| 10 April 2021 | OLEO 1B | Afluente da Lagoa do Batista | Córrego | −1,951,219 | −4,043,625 | Otothyris travassosi | CZNC | 4838 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Pachyurus adspersus | CZNC | 4957 |
| 1 November 2022 | L. OLEO | Lagoa do Óleo | Lago | −1,945,711 | −4,025,622 | Pachyurus adspersus | CZNC | 4913 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Pimelodella cf. lateristriga | CZNC | 4862 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Pimelodus maculatus | CZNC | 4922 |
| 13 April 2021 | ANG 1A | Córrego Timirim, proximo a cachoeira de Angeli | Córrego | −1,934,915 | −4,042,013 | Poecilia vivipara | CZNC | 4847 |
| 20 December 2020 | INTERMED AB | Afluente da Lagoa Palmas | Córrego | ########### | −4.02989 × 1012 | Poecilia vivipara | CZNC | 4882 |
| 12 April 2021 | JES 1B | Afluente sem nome da Lagoa Juparanã | Córrego | −1,916,424 | −4,029,590 | Poecilia vivipara | CZNC | 4905 |
| 30 March 2022 | L. JUPARANÃ | Lagoa Juparanã | Lago | −1,923,160 | −4,016,285 | Poecilia vivipara | CZNC | 4900 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Poecilia vivipara | CZNC | 4942 |
| 1 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Poecilia vivipara | CZNC | 4959 |
| 15 April 2021 | LIM 1C | Afluente da Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Poecilia vivipara | CZNC | 4824 |
| 15 April 2021 | LIM 1C | Lagoa do Limão | Córrego | −1,958,801 | −4,037,719 | Poecilia vivipara | CZNC | 4831 |
| 17 April 2021 | LIM 3A | Afluente da Lagoa do Limão | Córrego | −1,962,088 | −4,040,378 | Poecilia vivipara | CZNC | 4843 |
| 17 April 2021 | LIM 3A | Afluente da Lagoa do Limão | Córrego | −1,962,088 | −4,040,378 | Poecilia vivipara | CZNC | 4908 |
| 6 May 2021 | LSD 1A | Lagoa Terra Altinha | Córrego | −1,947,708 | −4,028,962 | Poecilia vivipara | CZNC | 4867 |
| 7 April 2021 | NOV 1A | Lagoa Nova | Córrego | −1,941,836 | −4,015,389 | Poecilia vivipara | CZNC | 4813 |
| April 2021 | NOV 2B | Lagoa Nova | Córrego | −1,926,074 | −4,020,624 | Poecilia vivipara | CZNC | 4857 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Poecilia vivipara | CZNC | 4895 |
| 10 April 2021 | OLEO 1B | Afluente da Lagoa do Batista | Córrego | −1,951,219 | −4,043,625 | Poecilia vivipara | CZNC | 4839 |
| 12 June 2021 | PAL 1A | Ribeirão das Palmas | Córrego | −1,944,742 | −4,022,411 | Poecilia vivipara | CZNC | 4818 |
| 2 May 2021 | PAL 1C | Afluente da Lagoa Palminhas | Córrego | −1,943,500 | −4,016,622 | Poecilia vivipara | CZNC | 4833 |
| 20 December 2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Poecilia vivipara | CZNC | 4933 |
| 16 April 2021 | PAU 1A | Afluente da Lagoa Pau Grosso | Córrego | −1,948,928 | −4,034,423 | Poecilia vivipara | CZNC | 4819 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Poecilia vivipara | CZNC | 4853 |
| 02 May 2021 | RES 1B | Afluente da Lagoa Juparanã | Córrego | −1,936,511 | −4,011,470 | Poecilia vivipara | CZNC | 4887 |
| 11 April 2021 | SÃO 1A | Rio São José | Córrego | −1,915,960 | −4,021,523 | Poecilia vivipara | CZNC | 4873 |
| 12 April 2021 | SAO 1C | Rio São José | Córrego | −1,911,154 | −4,024,773 | Poecilia vivipara | CZNC | 4836 |
| 06 May 2021 | TAL 1B | Lagoa Terra Alta | Lago | −1,948,494 | −4,029,217 | Poecilia vivipara | CZNC | 4901 |
| 19 January 2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −40,229,444 | Poecilia vivipara | MBML-PEIXES | 14,005 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Prochilodus lineatus | CZNC | 4916 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Prochilodus lineatus | CZNC | 4924 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Prochilodus lineatus | CZNC | 4949 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Pygocentrus nattereri | CZNC | 4954 |
| 30 March 2022 | L. NOVA | Lagoa Nova | Lago | −1,933,768 | −4,017,302 | Pygocentrus nattereri | CZNC | 4919 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Pygocentrus nattereri | CZNC | 4928 |
| 30 March 2022 | L. PALMINHAS | Lagoa Palminhas | Lago | −1,938,574 | −4,021,471 | Pygocentrus nattereri | CZNC | 4944 |
| 01 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Pygocentrus nattereri | CZNC | 4964 |
| 20/12/2020 | PALMAS 4 | Lagoa Palmas | Lago | −1,942,385 | −4,026,169 | Pygocentrus nattereri | CZNC | 4939 |
| 19/01/2020 | Palmas | Lagoa das Palmas, Linhares | Lago | −19,447,222 | −40,229,444 | Pygocentrus nattereri | MBML-PEIXES | 14,001 |
| 10 April 2021 | OLE 1A | Afluente da Lagoa do Batista | Córrego | −1,951,966 | −4,043,761 | Rhamdia quelen | CZNC | 4892 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Synbranchus marmoratus | CZNC | 4951 |
| 01 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Synbranchus marmoratus | CZNC | 4960 |
| 19 April 2020 | L. LIMÃO | Lagoa do Limão | Lago | −1,955,946 | −4,039,027 | Trachelyopterus striatulus | CZNC | 4953 |
| 31 March 2022 | L. PALMAS | Lagoa Palmas | Lago | −1,944,636 | −4,023,067 | Trachelyopterus striatulus | CZNC | 4925 |
| 01 April 2022 | L. PIABANHA | Lagoa Piabanha | Lago | −1,946,710 | −4,024,168 | Trachelyopterus striatulus | CZNC | 4965 |
| 10 April 2022 | PAU 1B | Afluente da Lagoa Pau Grosso | Córrego | −1,949,099 | −4,033,457 | Trachelyopterus striatulus | CZNC | 4855 |
Appendix C
Appendix C.1. Redundancy Analisis Plot
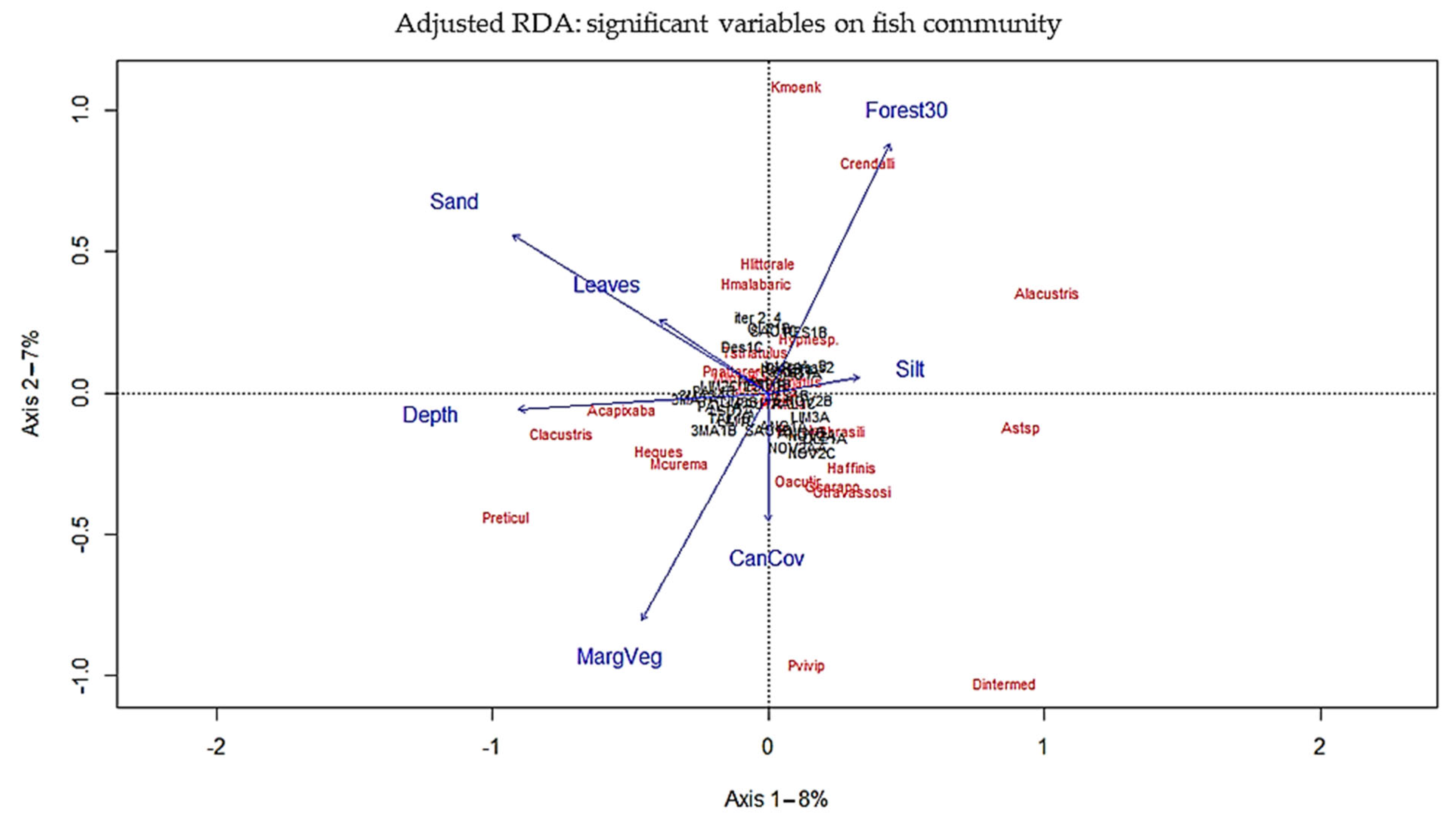
Appendix C.2. Redundancy Analysis (RDA) Abbreviation
| Abbreviation | Variable/Fish Species |
|---|---|
| Forest30 | Proportion of native vegetation within a 30 m riparian buffer upstream of the streams |
| Silt | Proportion of silt in the substrate |
| Depth | Average depth of the reach |
| MargVeg | Riparian vegetation |
| Leaves | Leaf litter cover on the substrate |
| CanCov | Canopy cover |
| Sand | Presence of sandbars |
| Alacustris | Astyanax lacustris |
| Astsp | Astyanax sp. |
| Acapixaba | Australoheros capixaba |
| Ckelberi | Cichla kelberi |
| Crendalli | Coptodon rendalli |
| Clacustris | Crenicichla lacustris |
| Cgilbert | Cyphocharax gilbert |
| Dintermed | Deuterodon intermedius |
| Gbrasili | Geophagus brasiliensis |
| Gcarapo | Gymnotus carapo |
| Hmalabaric | Hoplias malabaricus |
| Hlittorale | Hoplosternum littorale |
| Heques | Hyphessobrycon eques |
| Haffinis | Hypostomus affinis |
| Kmoenk | Knodus moenkhausi |
| Mlippin | Metynnis lippincottianus |
| Mdoceana | Moenkhausia doceana |
| Mcurema | Mugil curema |
| Oacutir | Oligosarcos acutirostris |
| Otravassosi | Otothyris travassosi |
| Pvittata | Pimelodella aff. vittata |
| Pmaculatus | Pimelodus maculatus |
| Preticul | Poecilia reticulata |
| Pvivip | Poecilia vivipra |
| Pnattereri | Pygocentrus nattereri |
| Rquelen | Rhamdia quelen |
| Tstriatus | Trachelyopterus striatulus |
Appendix C.3. Redundancy Analisis Scores
| RDA1 | RDA2 | RDA3 | RDA4 | RDA5 | RDA6 | |
|---|---|---|---|---|---|---|
| Alacustris | 0.2871774 | 0.095098 | −0.2346368 | 0.117138 | −0.0127829 | 6.675 × 10−2 |
| Astsp | 0.2599233 | −0.033125 | 0.2497147 | 0.102720 | −0.0616100 | 2.079 × 10−2 |
| Acapixaba | −0.1502863 | −0.016744 | −0.0946923 | 0.001056 | 0.0206506 | −1.272 × 10−1 |
| Ckelberi | 0.0095291 | 0.018361 | −0.0247578 | 0.095438 | −0.0211341 | 3.295 × 10−2 |
| Crendalli | 0.1020989 | 0.218210 | −0.0589342 | −0.249312 | 0.0239482 | 7.438 × 10−2 |
| Clacustris | −0.2121435 | −0.037414 | −0.1022061 | 0.128475 | 0.0368650 | 5.359 × 10−2 |
| Cgilbert | 0.0067282 | −0.004576 | 0.0032906 | −0.003626 | −0.0136688 | −1.737 × 10−2 |
| Dintermed | 0.2424275 | −0.273372 | −0.0201323 | −0.059099 | −0.0207313 | 2.390 × 10−2 |
| Gbrasili | 0.0742925 | −0.034724 | 0.0691402 | 0.003919 | 0.0405709 | −1.393 × 10−2 |
| Gcarapo | 0.0666279 | −0.087901 | −0.0084166 | −0.012176 | −0.0204309 | −3.183 × 10−2 |
| Hmalabaric | −0.0120460 | 0.103393 | 0.0517551 | 0.036565 | 0.0009784 | 5.490 × 10−2 |
| Hlittorale | 0.0005268 | 0.123535 | −0.0270564 | 0.007960 | 0.0843419 | −4.524 × 10−4 |
| Heques | −0.1112137 | −0.056053 | −0.0315570 | 0.021358 | 0.0397048 | 2.462e × 10−2 |
| Hyphesp. | 0.0423203 | 0.050411 | 0.2355081 | 0.034440 | 0.1861800 | 3.289 × 10−2 |
| Haffinis | 0.0860899 | −0.069194 | −0.0075782 | −0.052764 | −0.0432219 | 4.489 × 10−3 |
| Kmoenk | 0.0286509 | 0.290509 | −0.0002278 | 0.038631 | −0.1936251 | −6.439 × 10−2 |
| Mlippin | −0.0347366 | 0.014004 | −0.0905114 | 0.046688 | 0.0543380 | 6.154 × 10−2 |
| Mdoceana | −0.0038226 | 0.003703 | −0.0099426 | 0.020586 | 0.0030692 | 7.139 × 10−3 |
| Mcurema | −0.0903952 | −0.065431 | −0.0295016 | −0.082196 | 0.0109884 | −2.252 × 10−4 |
| Oacutir | 0.0310391 | −0.082284 | −0.0165534 | −0.033329 | 0.0109820 | 2.075 × 10−2 |
| Otravassosi | 0.0870227 | −0.092579 | −0.0143259 | −0.010101 | −0.0065022 | −5.057 × 10−3 |
| Pvittata | 0.0168954 | −0.008149 | −0.0049234 | −0.007834 | 0.0008022 | 3.438 × 10−3 |
| Pmaculatus | 0.0193803 | 0.012132 | −0.0180206 | −0.010730 | −0.0073229 | 8.691 × 10−5 |
| Preticul | −0.2684137 | −0.116375 | 0.1445372 | −0.033484 | −0.1578284 | 1.019 × 10−1 |
| Pvivip | 0.0392636 | −0.256204 | −0.1306801 | 0.010152 | −0.0237046 | −4.814 × 10−3 |
| Pnattereri | −0.0354041 | 0.021581 | −0.0270983 | 0.032740 | −0.0106518 | 1.061 × 10−2 |
| Rquelen | 0.0350051 | −0.035740 | 0.0008839 | −0.007965 | −0.0183521 | −6.632 × 10−3 |
| Tstriatulus | −0.0124126 | 0.038993 | −0.0160142 | 0.011482 | 0.0256772 | −4.121 × 10−3 |
References
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Zorn, T.G.; Wiley, M.J. Influence of sampling extent on the relative importance of biotic and abiotic factors in explaining variation in stream fish density. In Community Ecology of Stream Fishes: Concepts, Approaches, and Techniques; American Fisheries Society: Bethesda, MA, USA, 2010; Volume 73, pp. 487–502. [Google Scholar]
- Fuller, P.L.; Nico, L.G.; Williams, J.D. Nonindigenous Fishes Introduced into Inland Waters of the United States; American Fisheries Society Special Publication 27; American Fisheries Society: Bethesda, MA, USA, 1999; p. 613. [Google Scholar]
- Simberloff, D. Confronting introduced species: A form of xenophobia? Biol. Invasions 2003, 5, 179–192. [Google Scholar] [CrossRef]
- Dextrase Alan, J.; Mandrak Nicholas, E. Impacts of alien invasive species on freshwater fauna at risk in Canada. Biol. Invasions 2006, 8, 13–24. [Google Scholar] [CrossRef]
- Garcia, D.A.Z.; Pelicice, F.; Brito, M.F.G.; Orsi, M.L. Peixes não-nativos em riachos no Brasil: Estado da arte, lacunas de conhecimento e perspectivas. Oecologia Aust. 2021, 25, 587. [Google Scholar] [CrossRef]
- Jackson, D.A.; Peres-Neto, P.R.; Olden, J.D. What controls who is where in freshwater fish communities—The roles of biotic, abiotic, and spatial factors. Can. J. Fish. Aquat. Sci. 2001, 58, 157–170. [Google Scholar]
- Latini, A.O.; Petrere, M. Reduction of a native fish fauna by alien species: An example from Brazilian freshwater tropical lakes. Fish. Manag. Ecol. 2004, 11, 71–79. [Google Scholar] [CrossRef]
- Giacomini, H.C.; Lima, D.P., Jr.; Latini, A.O.; Espírito-Santo, H.M.V. Spatio-temporal segregation and size distribution of fish assemblages as related to non-native species occurrence in the middle rio Doce Valley, MG, Brazil. Neotrop. Ichthyol. 2011, 9, 135–146. [Google Scholar] [CrossRef]
- Ogutu-Ohwayo, R. The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environ. Biol. Fishes 1990, 27, 81–96. [Google Scholar] [CrossRef]
- Moyle, P.B.; Leidy, R.A. Loss of biodiversity in aquatic ecosystems: Evidence from fish faunas. In Conservation Biology: The Theory and Practice of Nature Conservation Preservation and Management; Springer: Boston, MA, USA, 1992; pp. 127–169. [Google Scholar]
- Power, M.E.; Marks, J.C.; Parker, M.S. Variation in the vulnerability of prey to different predators—Community level consequences. Ecology 1992, 73, 2218–2223. [Google Scholar]
- Ross, S.T. Mechanisms structuring stream fish assemblages: Are there lessons from introduced species? Environ. Biol. Fishes 1991, 30, 359–368. [Google Scholar] [CrossRef]
- Legendre, P. Spatial autocorrelation: Trouble or new paradigm. Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Pianka, E.R. Evolutionary Ecology; Harpercollins College Div: New York, NY, USA, 2011. [Google Scholar]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; Blackwell Publishing: Malden, MA, USA, 1996. [Google Scholar]
- Lubchenco, J.; Olson, A.M.; Brubaker, L.B.; Carpenter, S.R.; Holland, M.M.; Hubbell, S.P.; Levin, S.A.; Macmahon, J.A.; Matson, P.A.; Melillo, J.M.; et al. The sustainable biosphere initiative: An ecological research agenda. Ecology 1991, 72, 371–412. [Google Scholar] [CrossRef]
- Griesemer, S.J.; Fuller, T.K.; Degraaf, R.M. Habitat use by porcupines (Erethizon dorsatun) in central Massachusetts: Effects of topography and forest composition. Am. Midl. Nat. 1998, 140, 271–279. [Google Scholar] [CrossRef]
- Gosse, J.W.; Cox, R.; Avery, S.W. Home-range characteristics and habitat use by American martens in eastern New foundland. J. Mammal. 2005, 86, 1156–1163. [Google Scholar] [CrossRef]
- Jacques, C.N.; Jenks, J.A.; Klaver, R.W. Seasonal movements and home-range use by female pronghorns in sagebrush-steppe communities of western south Dakota. J. Mammal. 2009, 90, 433–441. [Google Scholar] [CrossRef]
- Whitaker, D.M.; Stauffer, D.; Norman, G.W.; Devers, P.K.; Allen, T.J.; Bittner, S.; Buehler, D.; Edwards, J.; Friedhoff, S.; Giuliano, W.M.; et al. Factors affecting habitat use by Appalachian ruffed grouse. J. Wildl. Manag. 2006, 70, 460–471. [Google Scholar] [CrossRef]
- Brandl, S.J.; Casey, J.M.; Meyer, C.P. Dietary and habitat niche partitioning in congeneric cryptobenthic reef fish species. Coral Reefs 2020, 39, 305–317. [Google Scholar] [CrossRef]
- IGAM. Instituto Mineiro de Gestão das Águas; IGAM: Belo Horizonte, Brazil, 2017. Available online: https://igam.mg.gov.br/ (accessed on 10 July 2023).
- Krenak, A. O insustentável abraço do progresso ou era uma vez uma floresta no Rio Doce. In Os Indígenas e as Justiças no Mundo Ibero-Americano (Sécs. XVI-XIX); Centro de História da Universidade de Lisboa: Lisboa, Brazil, 2019; p. 19. [Google Scholar]
- Oliveira, J.T. História do Estado do Espírito Santo, 3rd ed.; Arquivo Público do Estado do Espírito Santo: Vitória, Brazil, 2008; Volume 8. [Google Scholar]
- IPEMA. Conservação da Mata Atlântica no Estado do Espírito Santo: Cobertura Florestal e Unidades de Conservação; IPEMA: Vitória, Brazil, 2005; p. 152. [Google Scholar]
- Heinsdijk, D.; Macêdo, J.G.; Andel, S.; Ascoly, R.B. A Floresta do Norte do Espírito Santo: Dados e Conclusões dum Inventário Florestal Pilôto; Boletim No 7; Setor de Inventários Florestais, Ministério da Agricultura: Rio de Janeiro, Brazil, 1965; p. 69. [Google Scholar]
- Lani, J.L.; de Sérvulo, B.R.; Sartain, J.D.; Lani, J.A. Águas Da Região Do Delta Do Rio Doce Com Ênfase No Vale Do Suruaca, Linhares-ES; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Engenharia Gallioli. Várzea Litorânea Entre os Rios Doce e São Mateus, Região da Lagoa Suruaca (Estado do Espírito Santo), Plano de Saneamento, Relatório; Engenharia Gallioli: Rio de Janeiro, Brazil, 1966. [Google Scholar]
- Engenharia Gallioli. Várzea Litorânea Entre os Rios Doce e São Mateus, Região da Lagoa Suruaca (Estado do Espírito Santo), Plano de Saneamento, Estimativa Sumária de Custo. Engenharia Gallioli: Rio de Janeiro, Brazil, 1970. [Google Scholar]
- Robertson-Schultz. In Estudo Preliminar do Potencial Para Desenvolvimento do Vale do Suruaca, Estado do Espírito Santo; Robertson-Schultz: São Paulo, Brazil, 1973; p. 29.
- Sarmento-Soares, L.M.; Martins-Pinheiro, R.F. A fauna de peixes na bacia do rio Barra Seca e REBIO de Sooretama, Espírito Santo, Brasil. In Boletim do Museu de Biologia Mello Leitão; Museu de Biologia Professor Mello Leitão: Santa Teresa, Brazil, 2014; Volume 35, pp. 49–104. [Google Scholar]
- Gazeta Online. Peixes Resgatados no Rio Doce Começam a Ser Soltos Em Lagoas. Cidades. 14/11/2015 às 19h07; 16/11/2015. Available online: https://g1.globo.com/espirito-santo/noticia/2015/11/peixes-resgatados-no-rio-doce-no-es-comecam-ser-soltos-em-lagoas.html (accessed on 1 April 2019).
- Gazeta Online. Pescadores que Resgataram Peixes do Rio Doce Dizem que não Foram Pagos. Available online: https://g1.globo.com/espirito-santo/desastre-ambiental-no-rio-doce/noticia/2015/12/pecadores-que-resgataram-peixes-do-rio-doce-dizem-que-nao-foram-pagos.html. (accessed on 2 September 2025).
- Samarco. Resgate de Peixes no Rio Doce. Available online: https://www.bbc.com/portuguese/noticias/2015/11/151109_operacao_arca_noe_samarco_rs (accessed on 1 April 2019).
- Mota, T. Samarco Ajuda ao Projeto “Arca de Noé” a Salvar Peixes do rio Doce R7 Minas Gerais. Notícias. Available online: https://oglobo.globo.com/politica/operacao-arca-de-noe-tenta-salvar-peixes-do-rio-doce-no-espirito-santo-18037051 (accessed on 1 April 2019).
- Santana, W. Voluntários se Mobilizam para Resgatar Peixes do rio Doce no ES. Pesca Amadora—O Portal de notíCias do Pescador. Available online: https://www.Pescamadora.com.br/2015/11/voluntarios-se-mobilizam-para-resgatar-peixes-do-rio-doce-no-espirito-santo/ (accessed on 2 September 2025).
- Martin, L.; Suguio, F.; Flexor, J.M.; Archanjo, J.L. Coastal Quaternary Formations of the Southern Part of the State of Espírito Santo (Brazil). An. Acad. Bras. Ciências 1996, 68, 389–404. [Google Scholar]
- Barroso, G.F. Lagoas costeiras do Espírito Santo: Perspectivas para conservação. In Ecossistemas Costeiros do Espírito Santo: Conservação e Restauração Menezes; Tavares de Menezes, L.F., Pires, F.R., Pereira, O.J., Eds.; EDUFES: Vitória, Brazil, 2007; pp. 71–86. [Google Scholar]
- Hatushika, R.S. Investigação Sismoestratigráfica do Lago Juparanã-Baixo Curso do Rio Doce, Linhares (ES). Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Hatushika, R.S.; Silva, C.G.; Mello, C.L. Sismoestratigrafia de alta resolução no lago Juparanã, Linhares (ES—Brasil) como base para estudos sobre a sedimentação e tectônica quartenária. Rev. Bras. Geof. 2007, 25, 433–442. [Google Scholar] [CrossRef]
- Sarmento-Soares, L.M.; Martins-Pinheiro, R.F. Unidades de Conservação e a água: A situação das áreas protegidas de Mata Atlântica do norte do Espírito Santo, Sudeste do Brasil. Biodivers. Bras. 2017, 7, 69–87. [Google Scholar]
- Suguio, K.; Kohler, H.C. Quaternary Barred Lake Systems of the Doce River (Brazil). An. Acad. Bras. Ciênc. 1992, 64, 183–191. [Google Scholar]
- IBGE Base Cartográfica Vetorial Contínua do Estado do Espírito Santo, na Escala de 1:100 000. Dados Geoespaciais Vetoriais na Versão 3. 0 (ET-EDGV 3. 0). 2023. Available online: https://www.ibge.gov.br/geociencias/cartas-e-mapas/bases-cartograficas-continuas/15807-estados.html?edicao=37200 (accessed on 6 November 2023).
- Borges, D.D.A. Análise Batimétrica e Geomorfológica Integrada de um Conjunto de Lagos Barrados na Região Linhares (ES). Bachelor’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Barroso, G.F.; Gonçalves, M.A.; Garcia, F.d.C. The Morphometry of Lake Palmas, a Deep Natural Lake in Brazil. PLoS ONE 2014, 9, e111469. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.P.; Magnusson, W.E.; Zuanon, J. Relationships between habitat characteristics and fish assemblagesin smallstreams of Central Amazonia. Copeia 2005, 4, 750–763. [Google Scholar]
- Sarmento-Soares, L.M.; Martins-Pinheiro, R.F.; Casagranda, M.D. Endemicity analysis of the ictiofauna of the Rio Doce basin, Southeastern Brazil. Anais Academia Brasileira Ciências 2022, 94, 1–18. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 20 August 2020).
- Montgomery, H.A.C.; Thom, N.S.; Cockburn, A. Determination of dissolved oxygen by the Winkler method and the solubility of oxygen in pure water and sea water. J. Appl. Chem. 1964, 14, 280. [Google Scholar] [CrossRef]
- Governo do Estado do Espírito Santo. Secretaria de Estado da Agricultura Abastecimento Aquicultura e Pesca. Sistema Integrado de Bases Geoespaciais do Estado do Espírito Santo—GEOBASES. 2015. Available online: http://biblioteca.incaper.es.gov.br/digital/handle/123456789/3135. (accessed on 5 November 2023).
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1461-0248.2001.00230.x (accessed on 5 November 2023). [CrossRef]
- Melo, A.S. O que ganhamos ‘confundindo’ riqueza de espécies e equabilidade em um índice de diversidade? Biota Neotrop. 2008, 8, 21–27. [Google Scholar] [CrossRef]
- Shannon, C.E. 1948 A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 24, p. 1009. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Dufrene, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2023. Available online: https://www.R-project.org (accessed on 16 May 2025).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; Bortoli, J.; Leprieur, F.; Logez, M.; Santalla, S.M.; Devasa, R.M.; Rodriguez, C.G.; Crujeiras, R.M.; et al. Partitioning Beta Diversity into Turnover and Nestedness Components. Version 1. 6. 2023. Available online: https://cran.r-project.org/web/packages/betapart/betapart.pdf (accessed on 1 January 2024).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Package ‘Vegan’. Community Ecology Package; The Comprehensive R Archive Network. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 January 2024).
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Miranda, L.E. Depth as an organizer of fish assemblages in floodplain lakes. Aquat. Sci. 2011, 73, 211–221. [Google Scholar] [CrossRef]
- Marceniuk, A.P.; Ingenito, L.; Lima, F.C.T.; Gasparini, J. Systematics, biogeography and conservation of Paragenidens grandoculis n. gen. and n. comb. (Siluriformes; Ariidae), a critically endangered species from southeastern Brazil. Zootaxa 2019, 4586, 425–444. [Google Scholar] [CrossRef]
- Nico, L.G.; Taphorn, D.C. Food habits of piranhas in the low llanos of Venezuela. Biotropica 1988, 20, 311–321. [Google Scholar] [CrossRef]
- Canan, B.; Gurgel, H.D.C.B. Estrutura populacional de Metynnis roosevelti Eigenmann, 1915 (Characidae, Myleinae) da lagoa do Jiqui, Parnamirim, Rio Grande do Norte. Rev. Unimar. 1997, 19, 479–491. [Google Scholar]
- Nico, L.G.; Muench, M.A. Nests and Nest Habitats of the Invasive Catfish Hoplosternum littorale in Lake Tohopekaliga, Florida: A Novel Association with Non-native Hydrilla verticillata. Southeast. Nat. 2004, 3, 451–466. [Google Scholar] [CrossRef]
- da Costa, M.R.; Borges, J.L.; Araújo, F.G. Habitat preferences of common native fishes in a tropical river in Southeastern Brazil. Neotrop. Ichthyol. 2013, 11, 871–880. [Google Scholar] [CrossRef]
- Peter, B.B.; Leandro, C.; Vandick, S.B.; Nidia, N.F. Response of Prochilodus nigricans to flood pulse variation in the central Amazon. R. Soc. Open Sci. 2018, 5, 172232. [Google Scholar] [CrossRef]
- Menezes, N.A.; Weitzman, S.H.; Oyakawa, O.T.; Lima, F.C.T.D.; Correa ECastro, R.M.; Weitzman, M.J. Peixes de Água Doce da Mata Atlântica: Lista Preliminar Das Espécies e Comentários Sobre Conservação de Peixes de Água Doce Neotropicais; Museu de Zoologia da Universidade de São Paulo: São Paulo, Brazil, 2007. [Google Scholar]
- Mckinney, M.L.; And, J.L. Lockwood. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Methuen: London, UK, 1958. [Google Scholar]
- Olden, J.D. Biotic homogenization: A new research agenda for conservation biogeography. J. Biogeogr. 2006, 33, 2027–2039. [Google Scholar] [CrossRef]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- Baselga, A. Determinants of species richness, endemism and turnover in European longhorn beetles. Ecography 2008, 31, 263–271. [Google Scholar] [CrossRef]
- Leprieur, F.; Tedesco, P.A.; Hugueny, B.; Beauchard, O.; Durr, H.H.; Brosse, S.; Oberdorff, T. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecol. Lett. 2011, 14, 325–334. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Chen, C.; Xu, A.; Ding, P.; Wang, Y. The small-island effect and nestedness in assemblages of medium- and large-bodied mammals on Chinese reservoir land-bridge islands. Basic Appl. Ecol. 2019, 38, 47–57. [Google Scholar] [CrossRef]
- Pinha, G.D.; Tramonte, R.P.; Bilia, C.G.; Takeda, A.M. Differences in environmental heterogeneity promote the nestedness of Chironomidae metacommunity in Neotropical floodplain lakes. Acta Limnol. Bras. 2017, 29, 118. [Google Scholar] [CrossRef]
- Da Silva, F.R.; Gonçalves-Souza, T.; Paterno, G.B.; Provete, D.B.; Vancine, M.H. Análises Ecológicas no R. Nupeea: Recife, PE, Canal 6; Clube de Autores: São Paulo, Brazil, 2022; p. 640. ISBN 978-85-7917-564-0. [Google Scholar]
- Dardanelli, S.; Bellis, L.M. Nestedness structure of bird assemblages in a fragmented forest in Central Argentina: The role of selective extinction and colonization processes. Anim. Biodivers. Conserv. 2021, 44, 17–29. [Google Scholar] [CrossRef]
- Watling, J.I.; Donnelly, M.A. Fragments as Islands: A Synthesis of Faunal Responses to Habitat Patchiness. Conserv. Biol. 2006, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.J.; Cottee–Jones, H.E.W.; Whittaker, R.J. Quantifying and interpreting nestedness in habitat islands: A synthetic analysis of multiple datasets. Divers. Distrib. 2015, 21, 392–404. [Google Scholar] [CrossRef]
- Godinho, A.L. The ecology of predator fish introductions: The case of Rio Doce valley lakes. In Ecology and Human Impact on Lakes and Reservoirs in Minas Gerais with Special Reference to Future Development and Management Strategies; Pinto-Coelho, R.M., Giani, A., von Sperling, E.C., Eds.; Editora SEGRAC: Belo Horizonte, Brazil, 1994; pp. 77–83. [Google Scholar]
- Latini, A.O.; Lima-Júnior, D.P.; Giacomini, H.C.; Latini, R.O.; Resende, D.C.; Espírito-Santo, H.M.V.; Barros, D.F.; Pereira, T.L. Invasive fishes in lakes of the Doce river basin (Brazil): Range, new occurrences and conservation of native communities. Lundiana 2004, 5, 135–142. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Júlio, H.F. Ameaça ecológica: Peixes de outras águas. Ciência Hoje 1996, 21, 36–44. [Google Scholar]
- Agostinho, A.A. O lado oculto da introdução peixes. Bol. Inf. Abrapoa 1996, 7, 9–11. [Google Scholar]
- Linhares. Planejamento Estratégico de Linhares 2005–2025. Agenda 2007, 21, 97. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2025. Available online: https://www.fishbase.se/search.php (accessed on 1 April 2025).
- Vieira, I. Freqüência, constância, riqueza e similaridade da ictiofauna da bacia do rio Curuá-Una, Amazônia. Rev. Bras. Zoociências 2000, 2, 51–76. [Google Scholar]
- Sazima, I. Similarities in feeding behavior between some marine and freshwater fishes in two tropical communities. J. Fish Biol. 1986, 29, 53–65. [Google Scholar] [CrossRef]
- Resende, E.K.; Pereira, R.; Almeida, V.L. Peixes Herbívoros da Planície Inundável do Rio Miranda, Mato Grosso do Sul, Brasil; CPAP—Boletim de Pesquisa EMBRAPA: Corumbá, Brazil, 1997; Volume 10, pp. 1–21. [Google Scholar]
- Dias, A.C.M.I.; Branco, C.W.C.; Lopes, V.G. Estudo da dieta natural de espécies de peixes no Reservatório de Riberão das Lajes, RJ. Acta Sci. 2005, 27, 355–364. [Google Scholar]
- Silva, E.C.S.; Araújo-Lima, C.A.R.M. Influência do tipo de alimento e da temperatura na evacuação gástrica da Piranha caju (Pygocentrus nattereri) em condições experimentais. Acta Amaz. 2003, 33, 145–156. [Google Scholar] [CrossRef]
- Bittencourt, M.M. Aspectos da Demografia e do Ciclo de Vida de Pygocentrus Nattereri (Kner, 1860) EM um lago de Várzea da Amazônia Central (Lago do Rei, Ilha do Careiro). Ph.D. Thesis, INPA/UFAM, Manaus, Brazil, 1994. [Google Scholar]
- Apone, F.; de Oliveira, A.K.; Garavello, J.C. Composição da ictiofauna do rio Quilombo, tributário do rio Mogi-Guaçu, bacia do alto rio Paraná, sudeste do Brasil. Biota Neotrop. 2008, 8, 93–107. [Google Scholar] [CrossRef]
- He, F. Area-based assessment of extinction risk. Ecology 2012, 93, 974–980. [Google Scholar] [CrossRef] [PubMed]
- John, J. Magnuson Managing with Exotics—A Game of Chance. Trans. Am. Fish. Soc. 1976, 105, 1–9. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Agostinho, C.; Marques, E.E. Selection of netted prey by piranhas, Serrasalmus marginatus (Pisces, Serrasalmidae). Acta Sci. Biol. Sci. 2001, 23, 461–464. [Google Scholar]
- Pompeu, P.S.; Godinho, H.P. Dieta e estrutura das comunidades de peixes de três lagoas marginais do médio São Francisco. In Águas, Peixes e Pescadores do São Francisco das Minas Gerais; Godinho, H.P., Godinho, A.L., Eds.; PUC Minas: Belo Horizonte, Brazil, 2003; pp. 183–194. [Google Scholar]
- Marchetti, M.P.; Moyle, P.B. Effects of flow regime on fish assemblages in a regulated California stream. Ecol. Appl. 2001, 11, 530–539. [Google Scholar] [CrossRef]
- Leal, C.G.; Lennox, G.D.; Ferraz, S.F.B.; Ferreira, J.; Gardner, T.A.; Thomson, J.R.; Berenguer, E.; Lees, A.C.; Hughes, R.M.; Nally, R.M.; et al. Integrated terrestrial-freshwater planning doubles conservation of tropical aquatic species. Science 2020, 370, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Barbour, C.D.; James, B.H. Fish species diversity in lakes. Am. Nat. 1974, 108, 473–489. [Google Scholar] [CrossRef]
- Pascal, I.; Argillier, C.; Oberdorff, T. Native and introduced fish species richness in French lakes: Local and regional influences. Glob. Ecol. Biogeogr. 2004, 13, 335–344. [Google Scholar] [CrossRef]



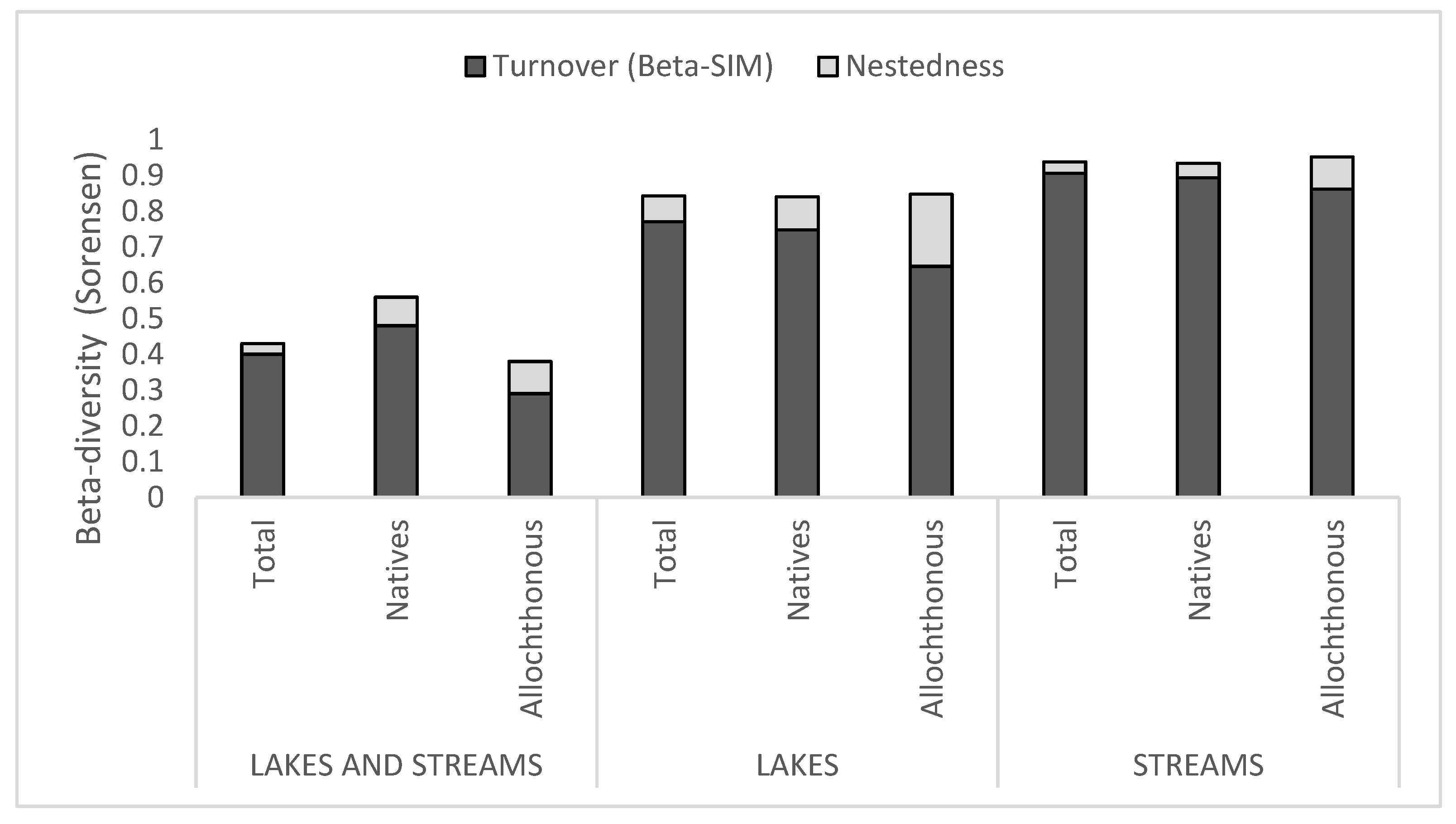




| Type of Environment Considered | Community Considered (According to the Origin of the Species) | Number of Assemblages Considered | Beta Diversity (Sorensen) | Replacement (Simpson) | Nested |
|---|---|---|---|---|---|
| Lakes and streams | Total | 2 | 0.43 | 0.4 | 0.03 |
| Natives | 2 | 0.56 | 0.48 | 0.08 | |
| Non-native | 2 | 0.38 | 0.29 | 0.09 | |
| Lakes | Total | 16 | 0.84 | 0.77 | 0.07 |
| Natives | 16 | 0.84 | 0.75 | 0.09 | |
| Non-native | 16 | 0.85 | 0.65 | 0.20 | |
| Streams | Total | 35 | 0.94 | 0.91 | 0.03 |
| Natives | 35 | 0.93 | 0.89 | 0.04 | |
| Non-native | 35 | 0.95 | 0.86 | 0.09 |
| Environment | ||
|---|---|---|
| Origin | Lakes | Streams |
| Native | 399 (59%) | 790 (88%) |
| Non-native | 278 (41%) | 112 (12%) |
| Position | ||
|---|---|---|
| Origin | Upstream | Downstream |
| Native | 612 (95%) | 174 (68.5%) |
| Non-native | 31 (5%) | 80 (31.5%) |
| Variable | Abbreviation | AIC | F | p |
|---|---|---|---|---|
| Proportion of native vegetation within a 30 m riparian buffer upstream of the streams | Forest30 | −4.23 | 2.16 | 0.010 |
| Proportion of silt in the substrate | Silt | −4.42 | 2.00 | 0.010 |
| Average depth of the reach | Depth | −4.8 | 1.69 | 0.040 |
| Riparian vegetation | MargVeg | −4.68 | 1.79 | 0.045 |
| Leaf litter cover on the substrate | Leaves | −4.98 | 1.54 | 0.075 |
| Canopy cover | CanCov | −4.91 | 1.60 | 0.085 |
| Presence of sandbars | Sand | −4.98 | 1.54 | 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, E.H.d.; Caiola, N.; Betzel, R.L.; Martins-Pinheiro, R.F.; Sarmento-Soares, L.M. Influence of Habitat on the Impact of Non-Native Fishes on Native Ichthyofauna in a Group of Lakes of the Lower Doce River, Espírito Santo, Southeastern Brazil. Diversity 2025, 17, 650. https://doi.org/10.3390/d17090650
Barros EHd, Caiola N, Betzel RL, Martins-Pinheiro RF, Sarmento-Soares LM. Influence of Habitat on the Impact of Non-Native Fishes on Native Ichthyofauna in a Group of Lakes of the Lower Doce River, Espírito Santo, Southeastern Brazil. Diversity. 2025; 17(9):650. https://doi.org/10.3390/d17090650
Chicago/Turabian StyleBarros, Eduardo Hoffmam de, Nuno Caiola, Renan Luxinger Betzel, Ronaldo Fernando Martins-Pinheiro, and Luisa Maria Sarmento-Soares. 2025. "Influence of Habitat on the Impact of Non-Native Fishes on Native Ichthyofauna in a Group of Lakes of the Lower Doce River, Espírito Santo, Southeastern Brazil" Diversity 17, no. 9: 650. https://doi.org/10.3390/d17090650
APA StyleBarros, E. H. d., Caiola, N., Betzel, R. L., Martins-Pinheiro, R. F., & Sarmento-Soares, L. M. (2025). Influence of Habitat on the Impact of Non-Native Fishes on Native Ichthyofauna in a Group of Lakes of the Lower Doce River, Espírito Santo, Southeastern Brazil. Diversity, 17(9), 650. https://doi.org/10.3390/d17090650






