Habitat and Conservation Assessment of Annual Killifishes of the Genus Xenurolebias (Rivulidae: Cynolebiinae) from Coastal Floodplains, Including the First Record South of the Rio Doce, Southeastern Brazil †
Abstract
1. Introduction
2. Materials and Methods
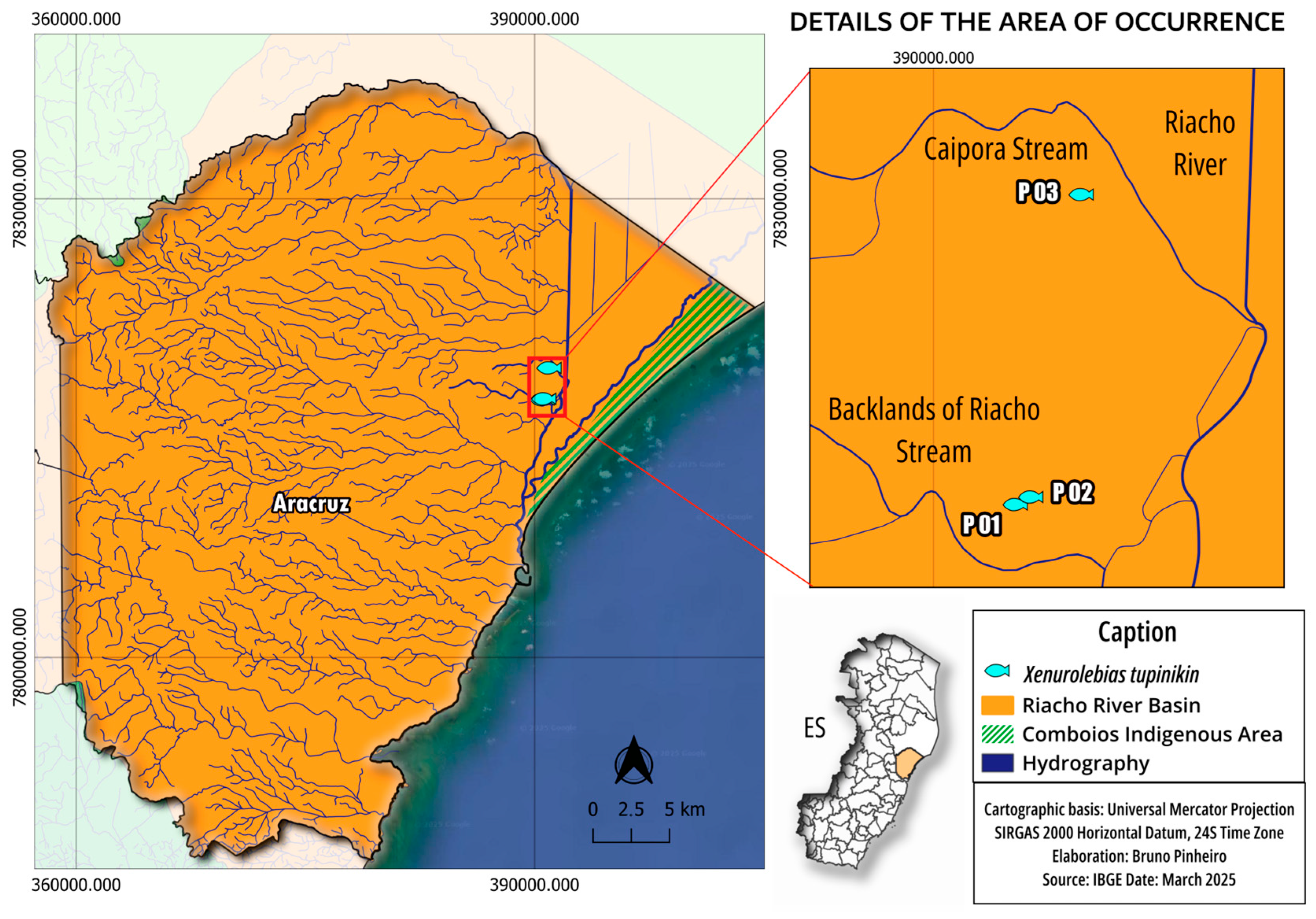
2.1. Study Area
2.2. Sample Design and Data Collection
3. Results and Discussion
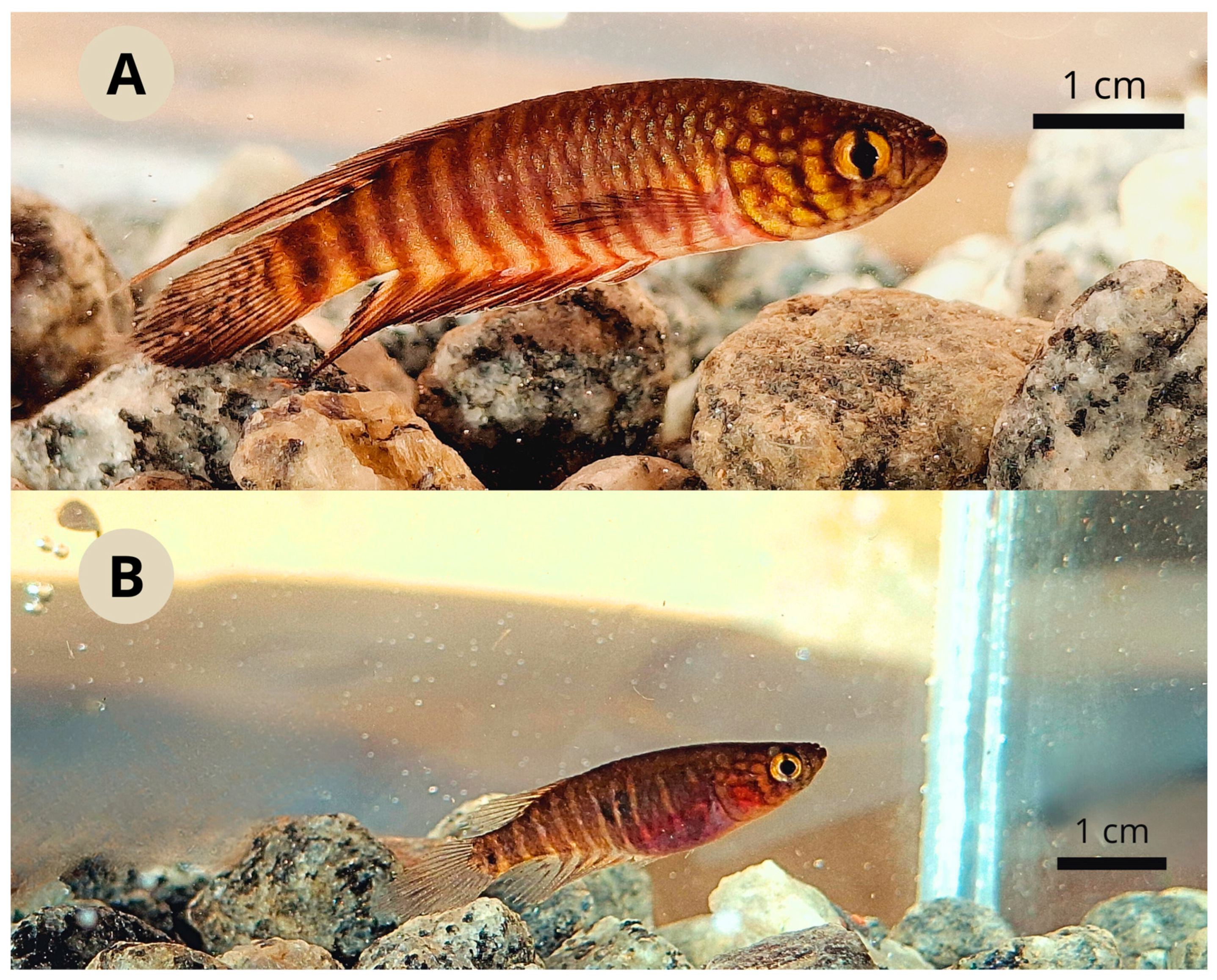
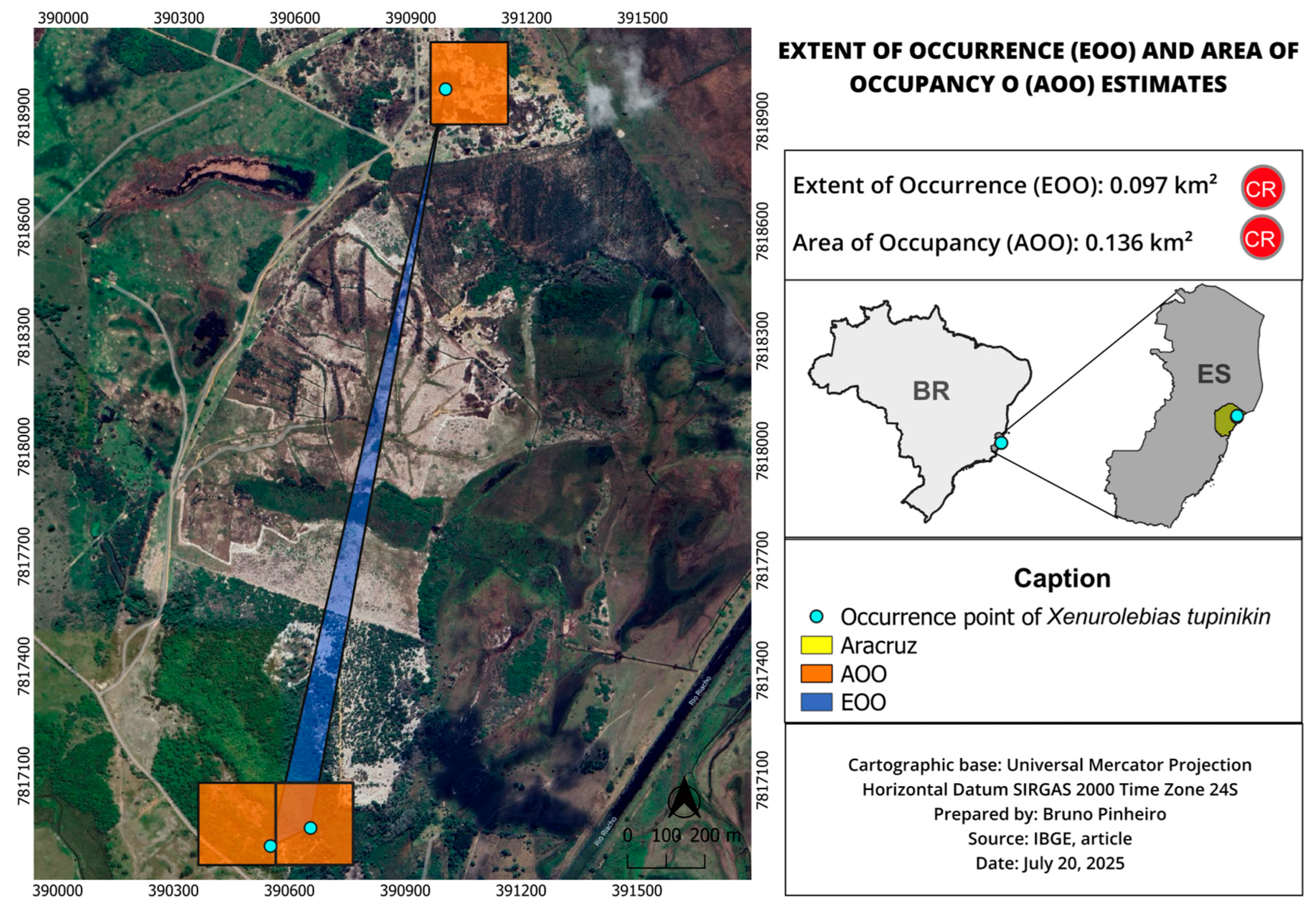
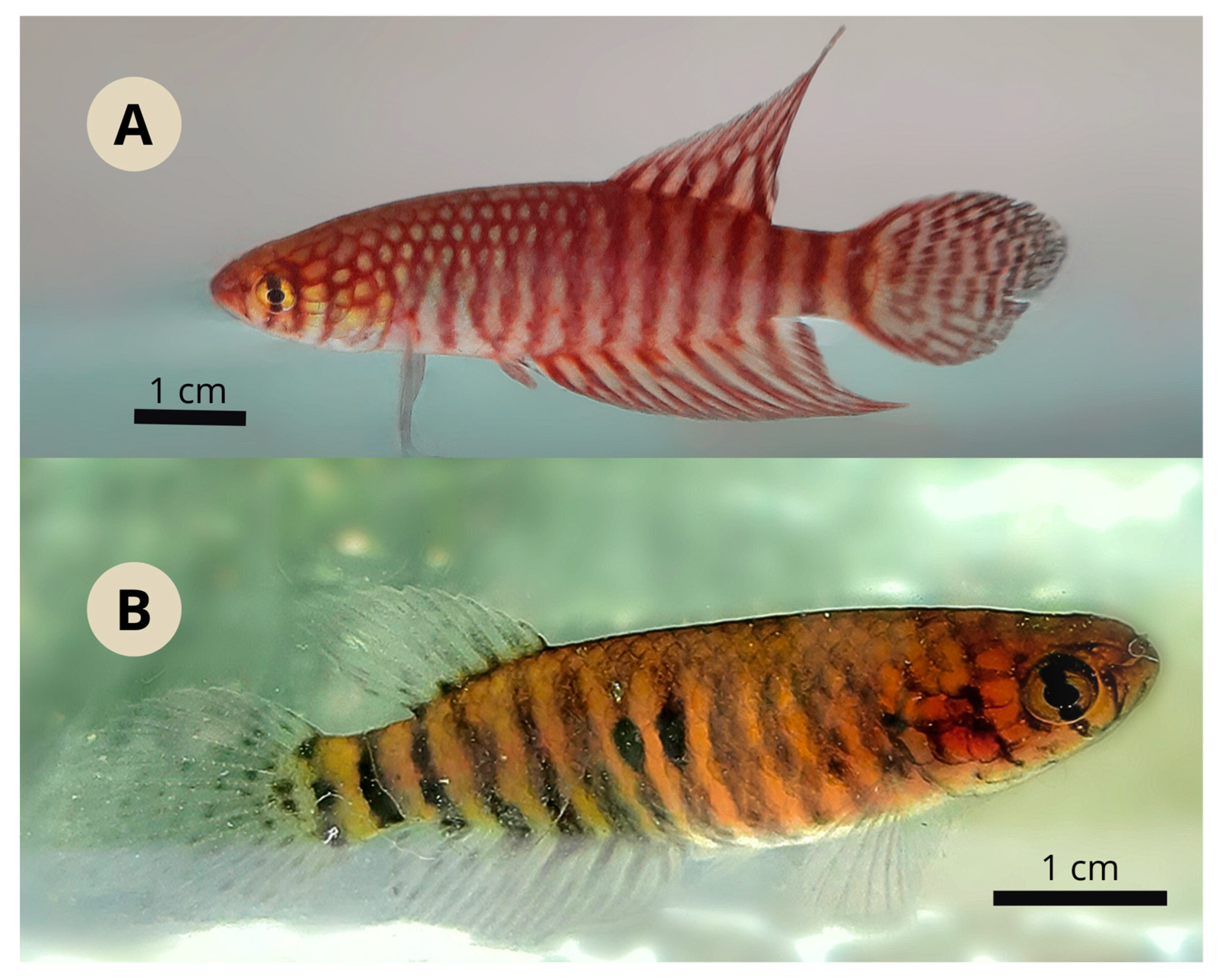
3.1. Xenurolebias tupinikin sp. nov. (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7, Table 1 and Table 2)
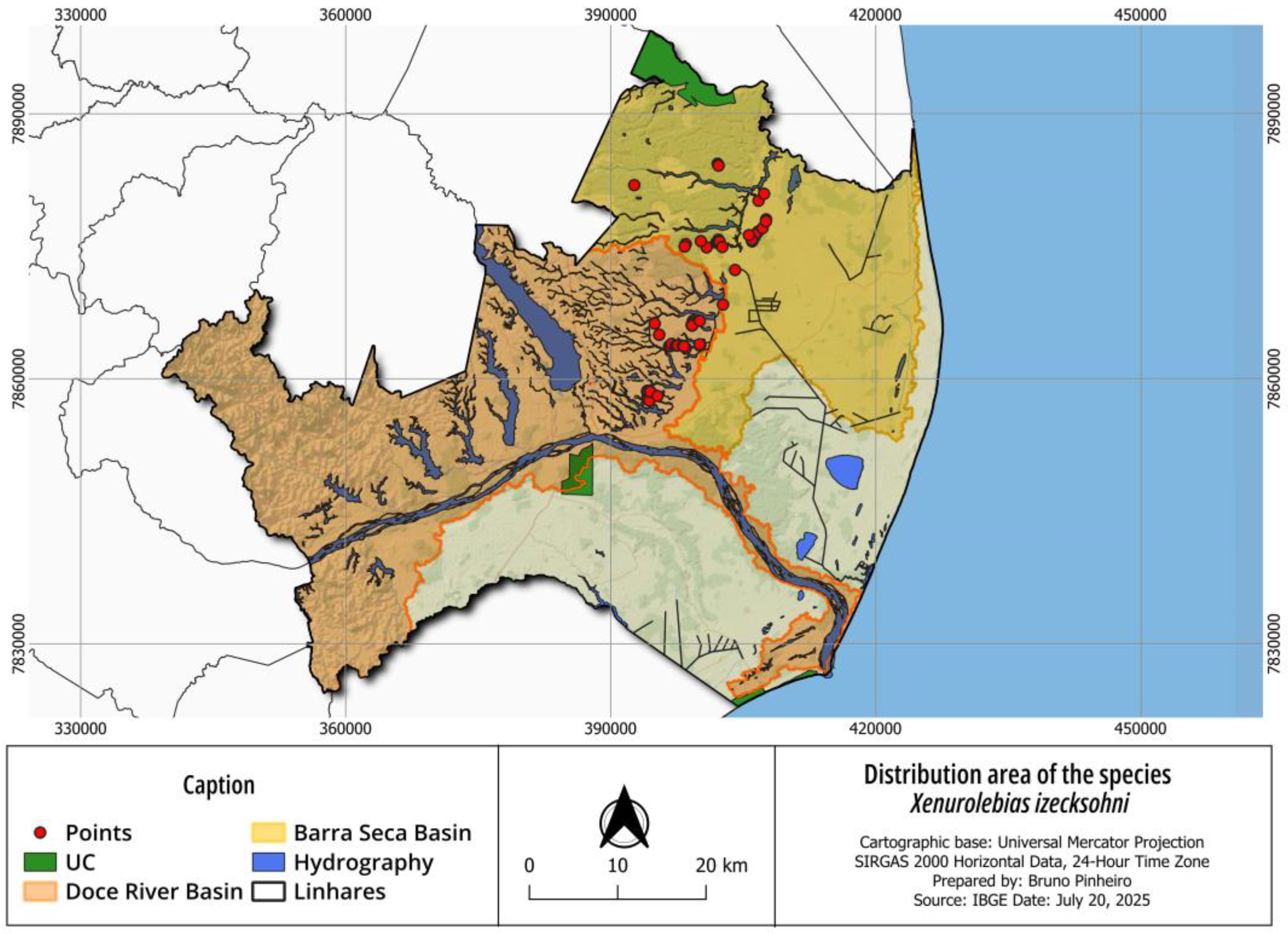
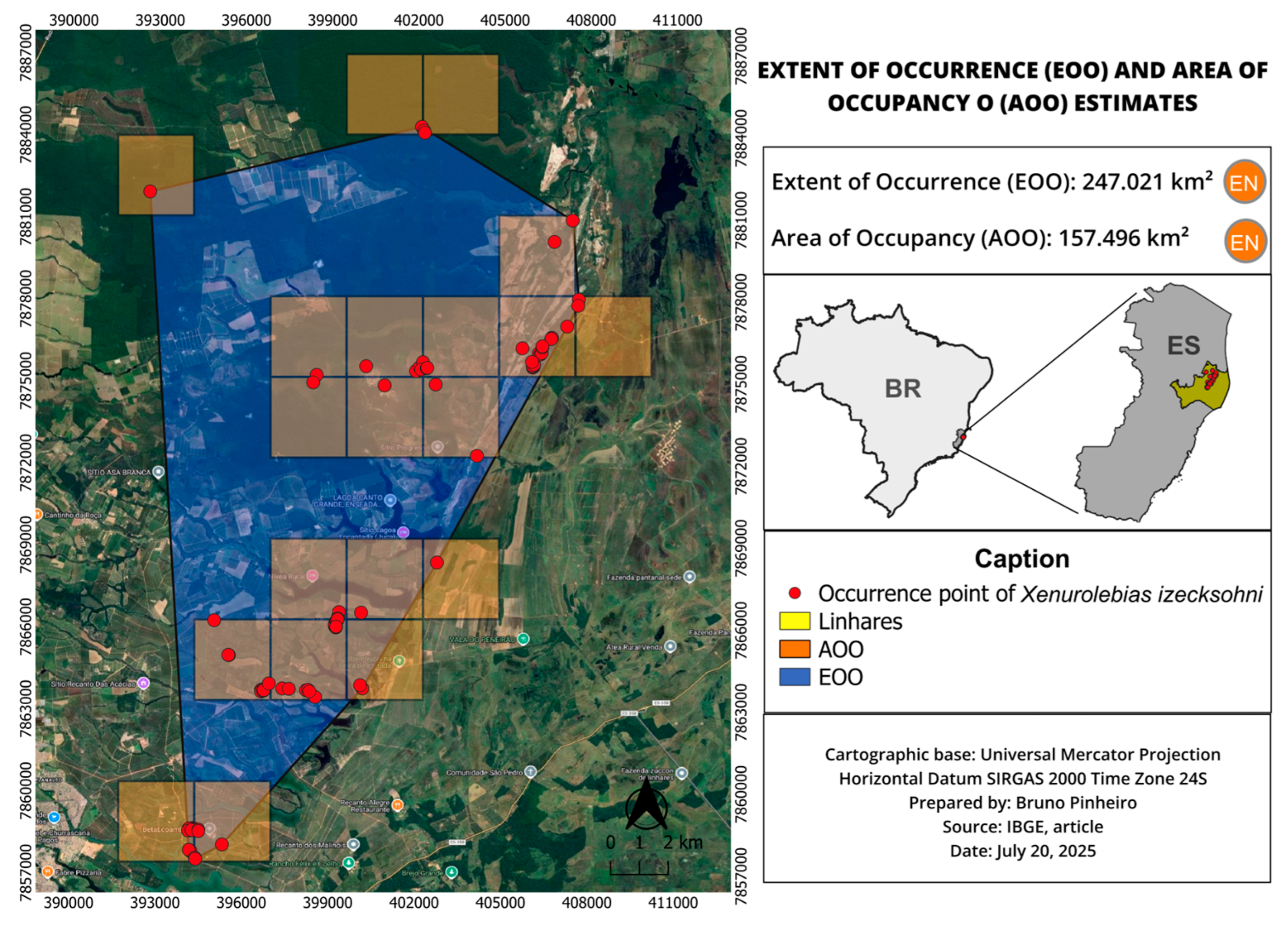
3.2. Xenurolebias izeckshohni (Figure 8, Figure 9, Figure 10 and Figure 11)
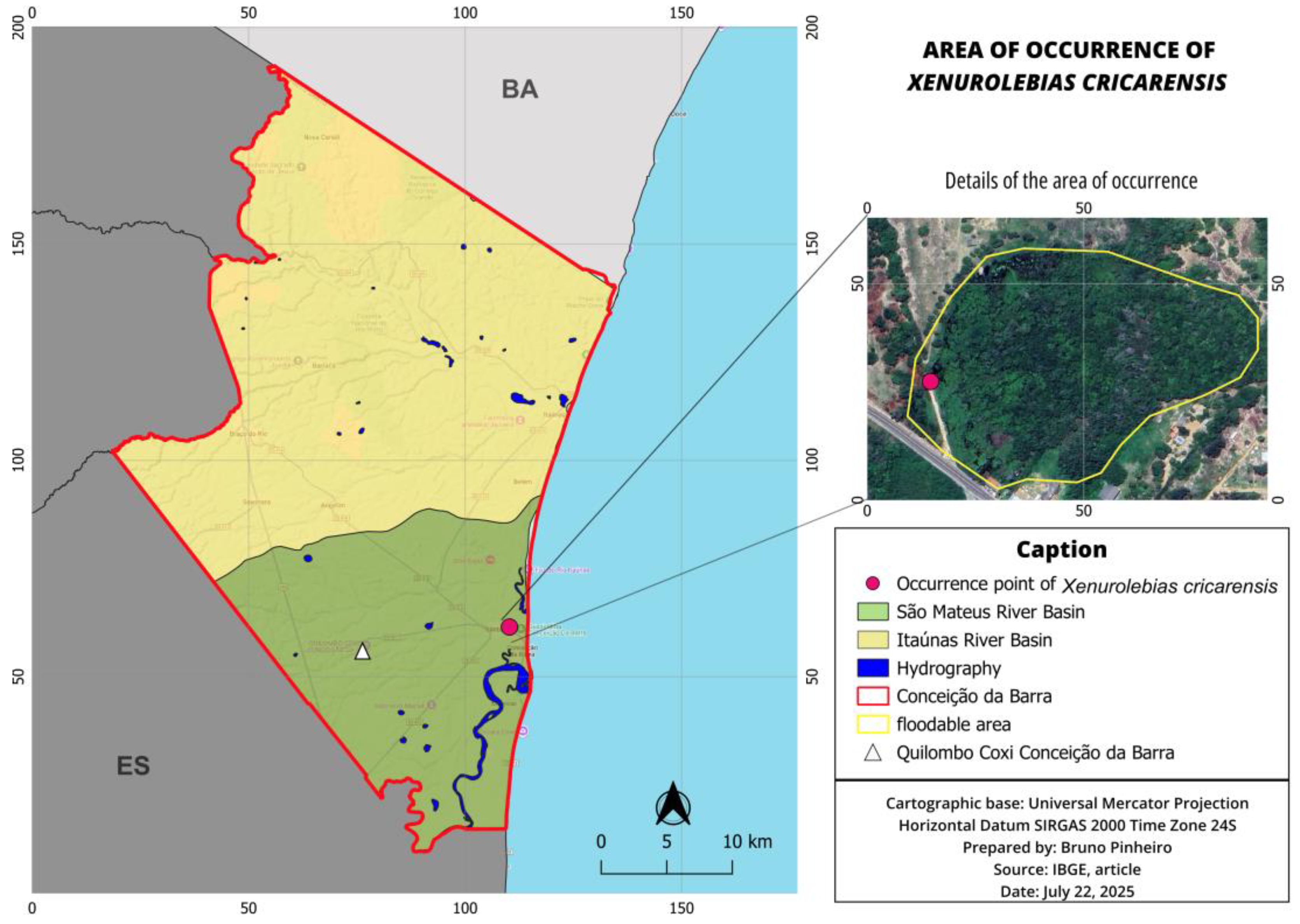
3.3. Xenurolebias cricarensis [8] (Figure 12, Figure 13 and Figure 14)
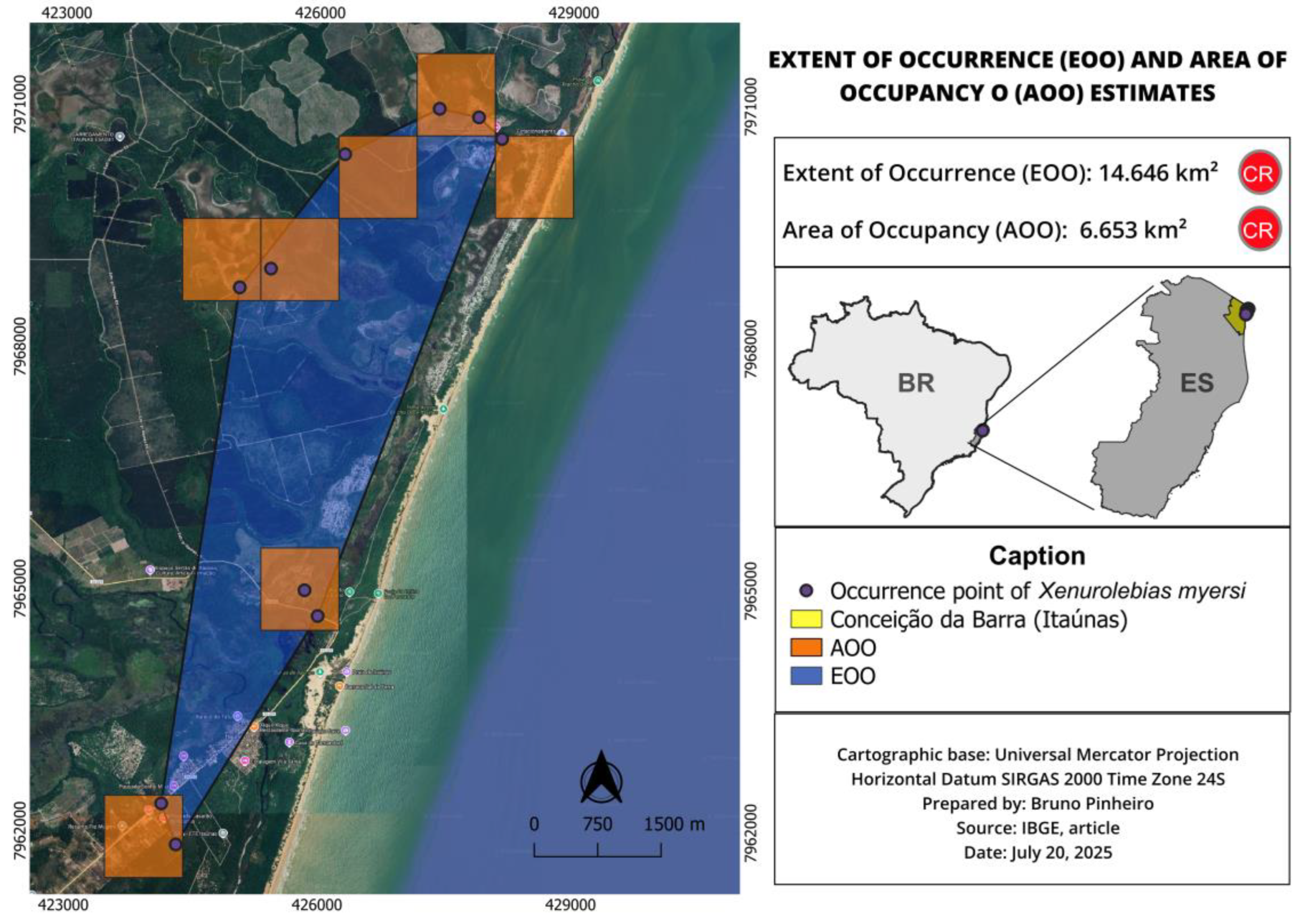
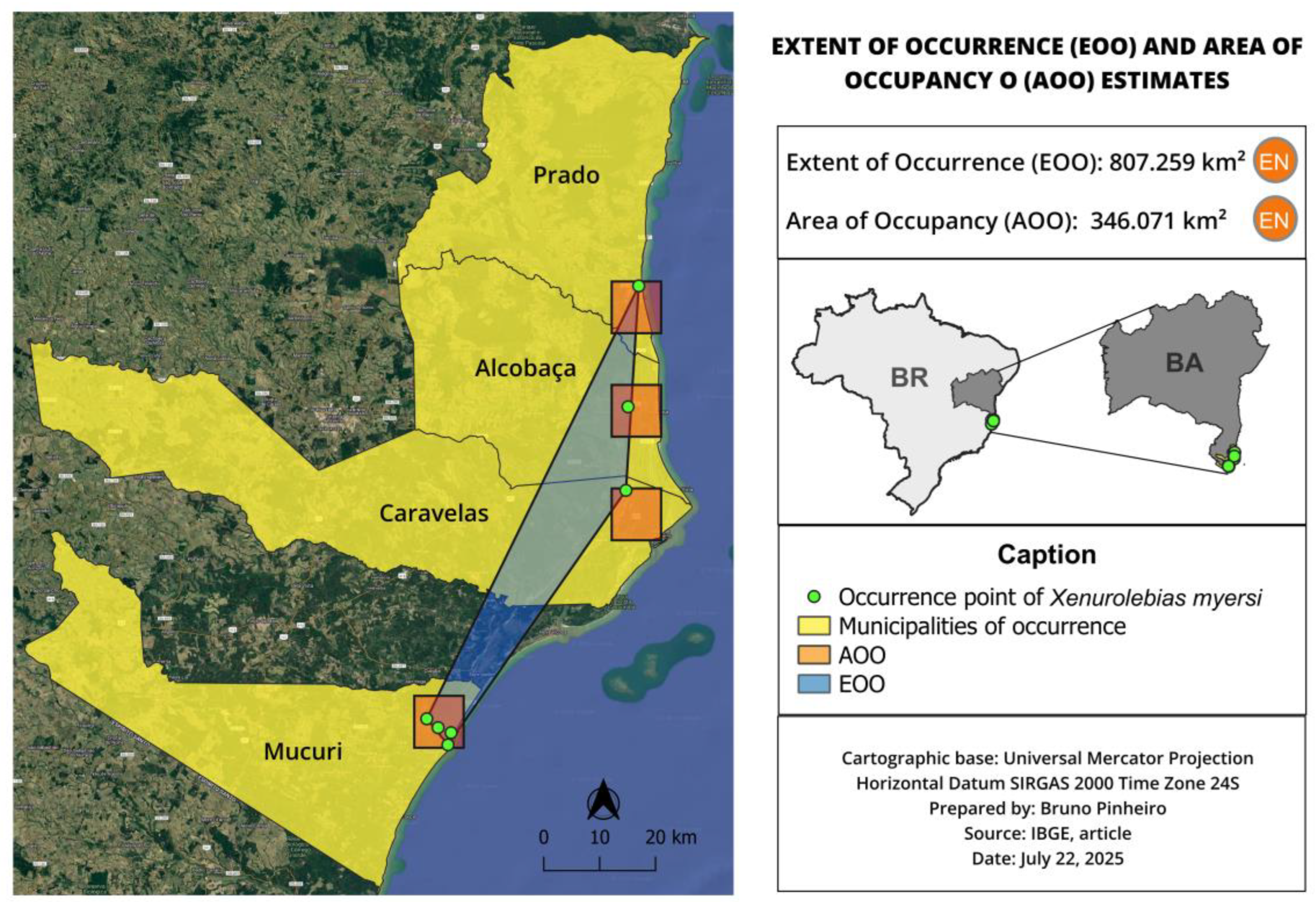
3.4. Xenurolebias myersi (Figure 15, Figure 16 and Figure 17)
3.5. Xenurolebias pataxo (Figure 18, Figure 19 and Figure 20, Table 2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furness, A.I. The evolution of an annual life cycle in killifish: Adaptation to ephemeral aquatic environments through embryonic diapause. Biol. Rev. 2016, 91, 796–812. [Google Scholar] [CrossRef]
- Costa, W.J.E.M. Peixes Anuais Brasileiros: Diversidade e Conservação; Editora Universidade Federal do Paraná: Curitiba, Brazil, 2002; 180p. [Google Scholar]
- Volcan, M.V.; Gonçalves, A.C.; Lanés, L.E.K.; Guadagnin, D.L. Annual Fishes (Rivulidae) from Southern Brazil: A Broad-Scale Assessment of Their Diversity and Conservation. In Annual Fishes: Life History Strategy, Diversity, and Evolution; Berois, N., García, G., de Sá, R.O., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 185–203. [Google Scholar]
- Bizerril, C.R.S.F. Análise taxonômica e biogeográfica da ictiofauna de água doce do leste brasileiro. Acta Biol. Leopoldensia 1994, 16, 51–80. [Google Scholar]
- Menezes, N.A.; Weitzman, S.H.; Oyakawa, O.T.; Lima, F.C.; Castro, R.M.C.; Weitzman, M.J. Peixes de Água Doce da Mata Atlântica; Neotrópica: São Paulo, Brazil, 2007; 407p. [Google Scholar]
- Fernandes, M.; Fernandes, M.; Almeida, A.; Gonzaga, M.I.S.; Gonçalves, F. Ecologia da Paisagem de uma Bacia Hidrográfica dos Tabuleiros Costeiros do Brasil. Floresta Ambiente 2017, 24, e00025015. [Google Scholar] [CrossRef][Green Version]
- Vieira-Guimarães, F.; Sarmento-Soares, L.M.; Martins-Pinheiro, R.F. Freshwater fishes of the Northeastern Mata Atlântica ecoregion, Brazil: An updated checklist with distributional patterns of a highly endemic ichthyofauna. Zootaxa 2024, 5475, 1–72. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Amorim, P.F. Integrative taxonomy and conservation of seasonal killifishes, Xenurolebias (Teleostei: Rivulidae), and the Brazilian Atlantic Forest. Syst. Biodivers. 2014, 12, 350–365. [Google Scholar] [CrossRef]
- Carvalho, A.L. Um novo peixe anual do estado do Espírito Santo (Pisces, Cyprinodontidae, Rivulinae). Revta Bras. Biol. 1971, 31, 401–404. [Google Scholar]
- Cruz, C.A.G. Uma nova espécie de Cynolebias do estado do Espirito Santo, Brazil (Pisces, Cyprinodontidae). Pap. Avul. Zool. 1983, 35, 73–77. [Google Scholar] [CrossRef]
- Costa, W.J.E.M. Taxonomic revision of the seasonal South American killifish genus Simpsonichthys (Teleostei: Cyprinodontiformes: Aplocheiloidei: Rivulidae). Zootaxa 2007, 1669, 1–134. [Google Scholar] [CrossRef]
- Ferreira, J.O. GPS TrackMakerPRO, Version 4.9.610; GeoStudio Technology; Geo Studio Tecnologia Ltd.: Belo Horizonte, Brazil, 2022. [Google Scholar]
- Rabelo, S.T.; Fernandes, M.F.; Moro, M.F. Biogeography of restinga vegetation in Northern and Northeastern Brazil and their floristic relationships with adjacent ecosystems. An. Acad. Bras. Cienc. 2024, 96, e20230925. [Google Scholar] [CrossRef]
- Sarmento-Soares, L.M.; Martins-Pinheiro, R.F. A fauna de peixes na REBIO Córrego Grande e seu entorno direto, Espírito Santo, Brasil. Bol. Mus. Biol. Mello Leitão 2013, 31, 25–57. [Google Scholar]
- Vieira-Guimarães, F.; Sarmento-Soares, L.M.; Martins-Pinheiro, R.F.; Duboc, L.F. Assessment of Stream Environmental Condition Using Fishbased Metrics in a Protected Area And Its Disturbed Buffer Zone, Northeastern Atlantic Rainforest. Oecol. Aust. 2022, 26, 461–475. [Google Scholar] [CrossRef]
- Monteiro, M.M.; Giaretta, A.; Pereira, O.J.; Menezes, L.F.T. Composição e estrutura de uma restinga arbustiva aberta no norte do Espírito Santo e relações florísticas com formações similares no Sudeste do Brasil. Rodriguesia 2014, 65, 61–72. [Google Scholar] [CrossRef]
- Azevedo, N.H.; Martini, A.M.Z.; Oliveira, A.A.; Scarpa, D.L. Ecologia na Restinga: Uma Sequência Didática Argumentativ8a, 1st ed.; Petrobrás and Instituto de Biociências, Universidade de São Paulo, USP: São Paulo, Brazil, 2014; 140p, ISBN 978-85-916948-0-8. Available online: https://www.livrosabertos.abcd.usp.br/portaldelivrosUSP/catalog/view/60/53/252 (accessed on 26 August 2025).
- Rolim, S.G.; de Menezes, L.F.T.; Srbek-Araujo, A.C. Floresta Atlântica de Tabuleiro: Diversidade e Endemismos na Reserva Natural Vale; Rupestre: Belo Horizonte, Brazil, 2016; 496p. [Google Scholar]
- García, G.; Gutiérrez, V.; Ríos, N. Conservation Strategies in the South American Annual Killifish of the Austrolebias sensu lato Linked to the Riparian Wetlands Zones. Aquat. Conserv. Mar. Freshw. Ecosyst. 2025, 35, e70141. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Carlos Rogério de Mello, C.R.; Terra, M.C.N.S.; Beskow, S. Water balance of an Atlantic forest remnant under a prolonged drought period. Cien. Agrotecnol. 2021, 45, e008421. [Google Scholar] [CrossRef]
- Costa, W.J.E.M. Pearl Killifishes, the Cynolebiatinae: Systematics and Biogeography of the Neotropical Annual Fish Subfamily (Cyprinodontiformes: Rivulidae); TFH: Neptune City, NJ, USA, 1995. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. 2025. Available online: https://www.iucnredlist.org (accessed on 21 June 2025).
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria; Version 15.1; IUCN: Gland, Switzerland, 2022; 122p, Available online: https://www.iucnredlist.org/resources/redlistguidelines (accessed on 19 July 2025).
- MMA-Ministério do Meio Ambiente. Portaria MMA Nº 148, de 7 de Junho de 2022. Alteração dos Anexos da Portaria Nº 445, referentes à atualização da Lista Nacional de Espécies Ameaçadas de Extinção. Anexo III. MMA, Brasília. [Unknown Pagination]. Available online: https://www.gov.br/icmbio/pt-br/assuntos/centros-de-pesquisa/aves-silvestres/arquivos/portaria-148-2022.pdf (accessed on 30 July 2025).
- MMA—Ministério Do Meio Ambiente. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume VI—Peixes; MMA: Brasília, Brazil; ICMBio: Brasília, Brazil, 2018. [Google Scholar]
- Costa, W.J.E.M. Descriptive morphology and phylogenetic relationships among species of the Neotropical annual killifish genera Nematolebias and Simpsonichthys (Cyprinodontiformes: Aplocheiloidei: Rivulidae). Neotrop. Ichthyol. 2006, 4, 1–26. [Google Scholar] [CrossRef]
- Guedes, G.H.S. The recapture of Leptopanchax opalescens (Aplocheiloidei: Rivulidae), a critically endangered seasonal killifish: Habitat and aspects of population structure. Zool. Curitiba 2020, 37, e54982. [Google Scholar] [CrossRef]
- Severo-Neto, F.; Volcan, M.V. Population dynamics of Melanorivulus rossoi, a restricted geographic distribution killifish species. Environ. Biol. Fishes 2018, 101, 245–255. [Google Scholar] [CrossRef]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 16. Prepared by the Standards and Petitions Committee. 2024. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 26 August 2025).
- Costa, W.J.E.M. Historical biogeography of Cynolebiasine annual killifishes inferred from dispersal-vicariance analysis. J. Biogeogr. 2010, 37, 1995–2004. [Google Scholar] [CrossRef]
- Sarmento-Soares, L.M.; Castro, R.D.R.; Martins-Pinheiro, R.F.; Garcia, I.B. Ways to Protect the Environments of the Itaúnas Cloud Fish—Xenurolebias myersi (Carvalho, 1971)—Inhabitant of the Restinga Swamps, Conceição Da Barra, Northern Espírito Santo, Southeastern Brazil. Qeios 2024. [Google Scholar] [CrossRef]
- Hostim-Silva, M.; Duboc, L.F.; Pimentel, C.R.; Vilar, C.C.; Machado, D.F.; Dario, F.D.; Guimarães, F.V.; Pinheiro, I.E.G.; Adelir-Alves, J.; Musiello-Fernandes, J.; et al. Peixes ameaçados de extinção no estado do Espírito Santo. In Fauna e Flora Ameaçadas de Extinção no Estado do Espírito Santo; Fraga, C.N., Formigoni, M.H., Chaves, F.G., Eds.; Instituto Nacional da Mata Atlântica: Rio de Janeiro, Brazil, 2019; pp. 230–255. [Google Scholar]
- Silva, A.T.; Chagas, R.J.; Santos, A.C.A.; Zanata, A.M.; Rodrigues, B.K.; Polaz, C.N.M.; Alves, C.B.M.; Vieira, C.S.; Souza, F.B.; Vieira, F.; et al. Freshwater fishes of the Bahia State, Northeastern Brazil. Biota Neotrop. 2020, 20, e20200969. [Google Scholar] [CrossRef]
- Volcan, M.V.; Lanés, L.E.K. Brazilian killifishes risk extinction. Science 2018, 361, 340–341. [Google Scholar] [CrossRef]
- ICMBio—Instituto Chico Mendes de Conservação da Biodiversidade. Sumário Executivo do Plano de Ação Nacional de Conservação de Peixes Rivulídeos Ameaçados de Extinção. 2013. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/pan/pan-rivulideos/1-ciclo/pan-rivulideos-sumario.pdf (accessed on 21 June 2025).
- IEMA—Instituto Estadual De Meio Ambiente E Recursos Hídricos. Plano de Ação Territorial para Conservação de Espécies Ameaçadas de Extinção do Território Capixaba-Gerais: Sumário Executivo; IEMA: Cariacica, Brazil, 2021. [Google Scholar]











| Xenurolebias tupinikin | |||
|---|---|---|---|
| Holotype Male | Male (n = 5) | Female (n = 10) | |
| Standard length (mm) | 23.3 | 20.3–23.3 | 16.5–23.9 |
| Percentages of standard length | |||
| Body depth | 26.9 | 25.9–27.9 | 19.0–32.9 |
| Caudal peduncle depth | 14.3 | 11.4–14.4 | 6.6–14.7 |
| Predorsal length | 59.0 | 56.6–64.1 | 48.8–73.9 |
| Prepelvic length | 44.0 | 42.7–49.4 | 44.1–56.2 |
| Length of dorsal-fin base | 24.2 | 24.2–29.2 | 09.1–16.8 |
| Length of anal-fin base | 51.0 | 47.6–56.1 | 17.8–26.1 |
| Caudal-fin length | 40.7 | 37.3–40.7 | 29.2–39.5 |
| Pectoral-fin length | 32.9 | 27.8–33.2 | 15.8–29.7 |
| Head length | 29.9 | 29.2–31.5 | 28.5–33.8 |
| Percentages of head length | |||
| Head depth | 76.8 | 64.4–76.8 | 61.27–85.5 |
| Lower jaw length | 25.5 | 18.4–25.5 | 15.1–21.6 |
| Eye diameter | 32.2 | 31.5–46.1 | 10.8–34.8 |
| Environmental Features | Values Per Species | ||||
|---|---|---|---|---|---|
| X. myersi | X. izecksohni | X. pataxo | X. cricarensis | X. tupinikin | |
| Depth (cm) | 40–110 | 20–70 | 30–50 | 50–60 | 30–60 |
| Temperature (°C) | 24–28 | 22–37 | 25 | 24.6 | 26.5–29.2 |
| pH | 3.5–5.6 | 3.5–5.6 | 4.2–5.2 | 5.6 | 4.3–6.2 |
| Humidity (%) | 60–80 | 60–88 | 43–60 | 68 | 54–60 |
| Species | EOO (km2) | AOO (km2) | Current Status | Suggested IUCN Status | Justification (IUCN Criteria) |
|---|---|---|---|---|---|
| Xenurolebias tupinikin | 0.097 | 0.136 | Not assessed | CR B1ab(iii) | Very restricted range, only three known localities, degraded habitat |
| X. cricarensis | single puddle | single puddle | Data Deficient (DD) | CR B1ab(iii) | Known for a seasonal pond, anthropogenic pressure, exotic fish |
| X. pataxo | ~10.0 | 346.071 | Vulnerable (VU) | EN B1ab(iii,v) | Drastic decline, limited occurrence, habitat loss |
| X. izecksohni | 247.021 | 157.496 | Near Threatened (NT) | VU B1ab(iii) | Habitat fragmentation, anthropogenic pressures |
| X. myersi | 14.646 | 6.653 | Endangered (EN) | CR B1ab(iii) | Habitat loss, anthropogenic pressures, small isolated populations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.P.; Sarmento-Soares, L.M.; Martins-Pinheiro, R.F.; Leite, G.R. Habitat and Conservation Assessment of Annual Killifishes of the Genus Xenurolebias (Rivulidae: Cynolebiinae) from Coastal Floodplains, Including the First Record South of the Rio Doce, Southeastern Brazil. Diversity 2025, 17, 644. https://doi.org/10.3390/d17090644
Gomes BP, Sarmento-Soares LM, Martins-Pinheiro RF, Leite GR. Habitat and Conservation Assessment of Annual Killifishes of the Genus Xenurolebias (Rivulidae: Cynolebiinae) from Coastal Floodplains, Including the First Record South of the Rio Doce, Southeastern Brazil. Diversity. 2025; 17(9):644. https://doi.org/10.3390/d17090644
Chicago/Turabian StyleGomes, Bruno Pinheiro, Luisa Maria Sarmento-Soares, Ronaldo Fernando Martins-Pinheiro, and Gustavo Rocha Leite. 2025. "Habitat and Conservation Assessment of Annual Killifishes of the Genus Xenurolebias (Rivulidae: Cynolebiinae) from Coastal Floodplains, Including the First Record South of the Rio Doce, Southeastern Brazil" Diversity 17, no. 9: 644. https://doi.org/10.3390/d17090644
APA StyleGomes, B. P., Sarmento-Soares, L. M., Martins-Pinheiro, R. F., & Leite, G. R. (2025). Habitat and Conservation Assessment of Annual Killifishes of the Genus Xenurolebias (Rivulidae: Cynolebiinae) from Coastal Floodplains, Including the First Record South of the Rio Doce, Southeastern Brazil. Diversity, 17(9), 644. https://doi.org/10.3390/d17090644






