Abstract
The species diversity of the recently described genus Drouetiella (Oculatellaceae, Cyanobacteriota), including thin filamentous cyanobacteria, has been rapidly increasing due to the subsequent discovery of new-to-science species in the last several years. This study focuses on one more strain that was isolated from a small lake on the Chukotka Peninsula in the Russian Arctic and tested by an integrative approach. In the result of molecular phylogenetic analysis of the 16S rRNA gene and the 16S–23S ITS rRNA region, this strain was found in a separate clade in the genus Drouetiella. The strain is characterized by unique secondary structures of conserved regions D1–D1′, Box–B, V2, and V3 helices of the 16–23S ITS rRNA. Morphologically, the tested strain was distinct from other Drouetiella species by long cells in mature trichomes, absence of false branching, and hormogonia. Drouetiella elegans occurred in the plankton of a small lake and shared ecological similarities with some aquatic strains of Drouetiella lurida. We provide a taxonomic description of a new species, Drouetiella elegans sp. nov.
1. Introduction
Thin filamentous cyanobacteria are among the most easily cultivated groups, which is why research into their diversity and phylogeny is progressing rapidly. However, the limited set of differentiating features and their high plasticity make it impossible to morphologically identify not only species but also entire genera. The integrative approach is the most reliable method for studying their diversity and refining the systematics of this group. Only five years have passed since the family Oculatellaceae Mai & J.R. Johansen was separated in 2018 [1] on the basis of the previously described five genera and six new ones, and it was separated into an independent order Oculatellales Strunecký & Mareš [2] with 17 genera. At present, the family Oculatellaceae includes 24 genera with 66 species [3,4]. The most species-rich genus is Oculatella Zammit, Billi & Albertano, which, since its description in 2012 [5], counts 14 species. The younger genera Timaviella Sciuto & Moro [6], Pegethrix Mai, J.R. Johansen & Bohunická [1], and Albertania Zammit [7] each include six species. While the 14 genera are presently monotypic, the rapid pace of biodiversity discovery and the frequent description of new species in recent genera make it certain that the genera of the Oculatellaceae family will be expanded with new species.
The genus Drouetiella can serve as a successful example of increasing species diversity by describing new taxa from diverse biomes and ecotopes. This genus is represented by thin, solitary filamentous, often with false-branching trichomes covered by colorless sheaths that are untapered and slightly constricted at the cross-walls. Phormidium luridum Gomont (Leptothrix lurida Kützing) with the type strain Lukesova 1986/6 from the tailings of Cainozoic clay in the Czech Republic was chosen as the type of species of the genus Drouetiella [1]. Simultaneously, Drouetiella fasciculata Mai, J.R. Johansen & Bohunická from a large seep wall and waterfall in Navajo, Utah, U.S.A., and D. hepatica Mai, J.R. Johansen & Pietrasiak from subaerial limestone in the National Park Slovak Paradise were described [1]. Five years later, the description of two new species was published: Drouetiella ramosa D. Davydov, Vilnet, Novakovskaya & Patova in biological soil crusts from the Urals, Russia [8], and D. epilithica D.-H. Kim, N.-J. Lee, H.-R. Wang, A.S. Lim & O.-M. Lee on a stone monument from the Republic of Korea [9].
The study presents the results of an integrative analysis of a strain isolated from a small lake on the Chukotka Peninsula in the Russian Arctic. The strain’s morphological and ecological traits were examined, and molecular analyses were performed using 16S rRNA and 16–23S internal transcribed spacer (ITS) sequences. Based on the results of a comprehensive approach, one more new species of the genus Drouetiella has been proposed.
2. Materials and Methods
2.1. Origin of the Strain and Culture Conditions
A strain of Drouetiella was isolated from a small, unnamed lake (66°10.591′ N, –170°22.359′ E) located in the lower course of the Uuchynveem River (Figure 1). The lake is situated 20 km west of the Uelen rural locality, Chukotsky District, Chukotka Autonomous Area, Russia.
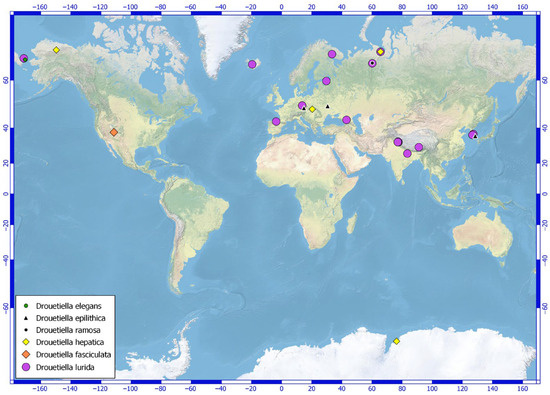
Figure 1.
Distribution of molecularly confirmed Drouetiella specimens across various geographic regions.
Field samples were collected by Dr. E.V. Chemeris on 8 August 2019. The natural sample consisted of hollow translucent mucous balls found in the plankton. The sample was dried in a sterile paper bag and later transferred to liquid Z8 medium in a flask in the laboratory, following the method described by Kotai [10] and Waterbury [11]. The accumulated culture was initially seeded onto solid BG-11 medium in Petri dishes. Unialgal cultures were subsequently obtained by isolating material from the edges of discrete colonies that had been growing for three weeks on the solid agar medium.
The strain was maintained at 22 °C under artificial illumination with a 16:8 h light/dark photocycle and a photon flux density of 35 μmol m−2s−1. The isolated strain has been deposited in the Collection of Cyanoprokaryotes of the Polar-Alpine Botanical Garden-Institute (KPABG) and assigned the accession number LID–150280. A portion of the type material was dried and deposited in the KPABG herbarium. The label information was included in the ‘L.’ database [12] and assigned the accession number KPABG 4519! for future reference.
2.2. DNA Extraction and Sequencing
DNA was extracted from the unialgal culture using the DNeasy Plant Mini Kit (Qiagen, Venlo, Limburg, The Netherlands) according to the manufacturer’s protocol. The partial 16S rRNA gene and the 16S–23S ITS rRNA region were amplified using a pair of primers: one modified by [13] after [14] (1: 5′–CTC TGT GTG CCT AGG TAT CC–3′) and 27F (5′–AGA GTT TGA TCC TGG CTC AG–3′) suggested by [15]. PCR amplification was performed in 20 µL reaction volumes with MasDDTaqMIX (Dialat Ltd., Moscow, Russia), 10 pmol of each oligonucleotide primer, and 1 ng of DNA. The amplification protocol included an initial denaturation step at 94 °C for 3 min, followed by 40 cycles of 30 s at 94 °C, 40 s at 56 °C, and 60 s at 72 °C, with a final extension at 72 °C for 2 min. The amplified fragments were purified using the Cleanup Mini Kit (Evrogen, Moscow, Russia) and subsequently sequenced using the ABI PRISM® BigDye™ Terminator v3.1 Sequencing Ready Reaction Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the standard protocol provided for the Applied Biosystems 3730 DNA Analyser (Applied Biosystems, Waltham, MA, USA). Additionally, the internal primer 2 (5′–GGG GGA TTT TCC GCA ATG GG–3′) [16] was used for sequencing. A single amplicon was obtained without ambiguities for the entire 16S–23S rRNA region. To avoid sequencing errors, the amplicon for the 16S–23S ITS rRNA region of the tested strain was amplified and sequenced again under the same conditions; the nucleotide sequence was identical to that obtained the first time.
2.3. Molecular Analyses
The nucleotide sequence of 16S–23S rRNA for strain LID–150280 was assembled in the program BioEdit 7.0.1 [17]. The BLAST 2.17.0 searches the most similar accessions of 16S rRNA to clarify the identification of the tested strain. The type strain of the recently described Drouetiella epilithica (NR186875) provided the highest nucleotide sequence similarity. The taxonomic position of strain LID–150280 and its genetic divergence from close relatives were estimated through phylogenetic reconstructions. The dataset included 32 accessions from the genus Drouetiella with reference strains of five known species, as well as accessions of 31 species from 22 genera of the family Oculatellaceae and an accession of Pantanalinema rosaneae V. Vaz, Genuário, Andreote, C.F. Malone, C.L. Sant’Anna, L. Barbiero & M.F. Fiore from the phylogenetically allied family Leptolyngbyaceae J. Komárek, Kaštovský, Mareš & J.R. Johansen chosen as an outgroup according to current taxonomical views [2,4]. The 16S rRNA gene alignment consists of 65 specimens and has a length of 1179 sites. The alignment of the 16S–23S ITS rRNA region of the Drouetiella species includes 26 specimens with a total length of 554 sites.
The maximum likelihood (ML) method using IQ-TREE [18] and the Bayesian approach with MrBayes v3.2.1 [19] were employed to reconstruct the molecular phylogeny based on the 16S rRNA gene and the 16S–23S ITS rRNA region. The ML analysis was performed with the best-fit evolutionary model of nucleotide substitutions and ultrafast bootstrapping [20] using 1000 replicates. ModelFinder [21] identified K2P+R4 as the best model for the 16S rRNA gene and TPM2u+F+I+G4 for the 16S–23S ITS rRNA region. For the Bayesian analysis, the GTR+I+G model was applied to both datasets, as recommended by the software’s developers. Two independent runs of the Metropolis-coupled MCMC were conducted to sample parameter values according to their posterior probability. Each run included three heated chains and one unheated chain, with two starting trees chosen randomly. The chains were run for two million generations for the 16S rRNA gene analysis and one million generations for the 16S–23S ITS rRNA region analysis, with trees sampled every 100th generation. In the 16S rRNA gene analysis, the first 5000 trees from each run were discarded as burn-in, resulting in 30,000 sampled trees. For the 16S–23S ITS rRNA region analysis, the first 2500 trees from each run were discarded as burn-in, with 15,000 trees sampled from both runs. The average standard deviation of split frequencies between the two runs was 0.008053 for the 16S rRNA gene and 0.005849 for the 16S–23S ITS rRNA region. Bayesian posterior probabilities (BA PP) were calculated from trees sampled after the burn-in period. Majority rule (MJ) consensus trees were obtained after combining the runs, excluding the 25% burn-in.
The nucleotide sequence similarity of the 16S rRNA gene was estimated using the formula: , where p represents the average pairwise p–distances. These distances were calculated using MEGA 11 [22] with the pairwise deletion option to account for gaps. The dissimilarity of the 16S–23S ITS region was also determined as the average pairwise p–distances.
To search for conserved helices D1–D1′, Box–B, V2, and V3 in the 16S–23S ITS regions, the model proposed by [23] was followed. The hypothetical secondary structures of all conserved helices were reconstructed using Mfold [24] specifically for reference strains of the genus Drouetiella and strain LID–150280.
3. Results
3.1. Taxonomic Description
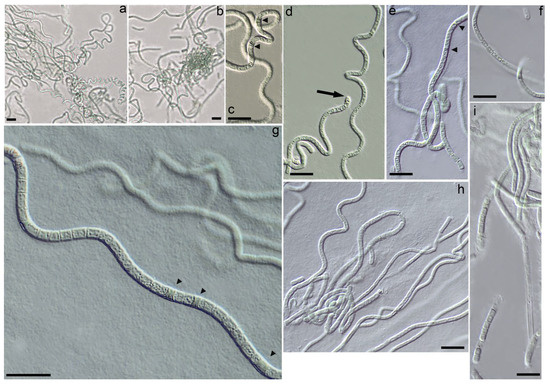
Figure 2.
Light micrographs of the Drouetiella elegans LID–150280. Scale bar = 10 μm: (a,b) Variation in the degree of trichomes coiling from bent, flexuous to spiral coils; (c,e) Necridic cells in trichomes; (d) The terminal cells are untapered and rounded; (f) Elongated cells in mature trichomes; (g) Necridic cells in flexuous trichomes; (h) Irregular length of cells in mature trichomes; (i) Sheath tightly embraces trichome.
Diagnosis: Drouetiella elegans is phenotypically distinct from other Drouetiella species due to its long cells in mature trichomes and the absence of false branching, hormogonia, and meristematic zones. It is characterized by distinct phylogenetic positions based on 16S rRNA and 16S–23S ITS rRNA gene analyses and the unique hypothetical secondary structures of the D1–D1′, Box–B, V2, and V3 helices. The 16S rRNA similarity with other Drouetiella species varies from 96.31% to 98.97%, and the dissimilarity in the 16S–23S ITS rRNA exceeds 12%.
Description: Colonies on solid media formed bright blue-green growths on the agar surface, which gradually transitioned to an olive-green color with continued cultivation. The trichomes were curved, wavy, sometimes coiled, and less frequently straight, exhibiting a blue-green hue and a thin structure (Figure 2a–d). In young colonies, the trichomes displayed irregular curving, forming tangled knots reminiscent of the growth pattern observed in Pegethrix species (Figure 2a). There was no false branching in the trichomes, and hormogonia were absent. The trichomes maintained a consistent width throughout their length, appearing cylindrical and either not constricted or only slightly constricted at the cross-walls (Figure 2e–g). The sheath was firm, colorless, usually attached to the trichome, and occasionally distinct (Figure 2i), but absent in immature filaments or hormogonia. The cells were elongated and varied in length (Figure 2d,h), measuring (1.6) 2.5–4.5 (5.35) µm in length and (1.25) 1.76–2.3 (2.7) µm in width (Figure 3). Cell length varied between young and mature trichomes. In young filaments, the cells were shortened or square, with elongation observed only at the ends of the trichomes. As the trichomes matured, the cells elongated, resulting in trichomes with predominantly elongated cells. The terminal cells were untapered and rounded (Figure 2d). Necridic cells were present and frequent (Figure 2c,e,g).
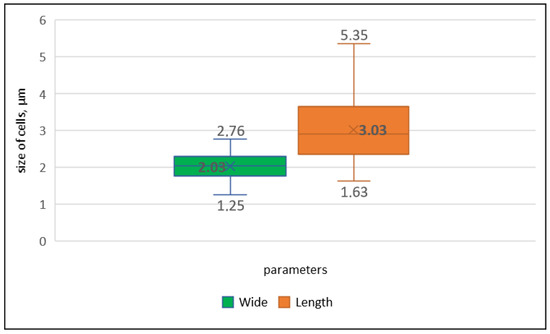
Figure 3.
Distribution of the average (in bold), maximum (above boxes), and minimum (under boxes) values of length and width of cells in the Drouetiella elegans strain LID–150280.
Holotype: KPABG–4519 (dried cultured material, preserved in a metabolically inactive state), collected on 8 August 2019 by E.V. Chemeris, deposited in the herbarium of Polar-Alpine Botanical Garden-Institute (KPABG), Apatity, Russia.
Type locality: 66°10.591′ N, –170°22.359′ E; the lower course of the Uuchynveem River, a small lake, planktic; Chukotsky District, Chukotka Autonomous Area, Russia.
Etymology: From Latin adjective elegans, elegant, fine.
Nucleotide sequence: GenBank accession number is PQ589721 for the 16S and 16S–23S ITS rRNA genes.
3.2. Molecular Analyses
The assembled nucleotide sequence of the entire 16S–23S rRNA region for strain Drouetiella elegans LID–150280 had been deposited in the NCBI database. The Maximum Likelihood (ML) analysis of the 16S rRNA gene dataset yielded a tree with a log-likelihood of −7878.117. In the Bayesian analysis (BA) of the 16S rRNA gene, the arithmetic mean log-likelihoods for each sampling run were −7964.64 and −7962.25. The trees obtained by both methods displayed congruent topologies (Figure 4). The ML analysis of the 16S–23S ITS rRNA region produced a tree with a log-likelihood of −2747.981, and the log-likelihoods after Bayesian estimation were −2766.24 and −2767.24. The tree topologies for the 16S–23S ITS rRNA region obtained from both methods were similar (Figure 5).
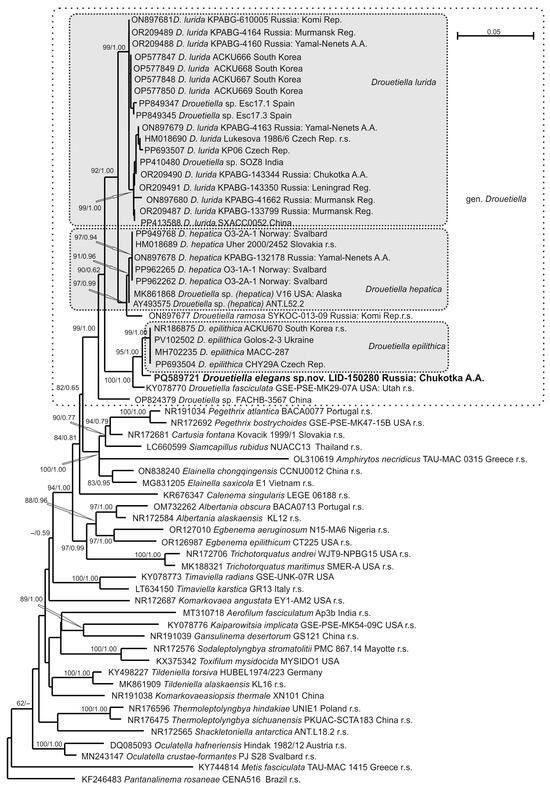
Figure 4.
Phylogram obtained under the maximum likelihood approach for 64 accessions of Oculatellaceae based on the 16S rRNA gene. Bootstrap support values from the maximum likelihood and Bayesian posterior probabilities of more than 50% (0.50) are indicated. The reference strains are marked as r.s.
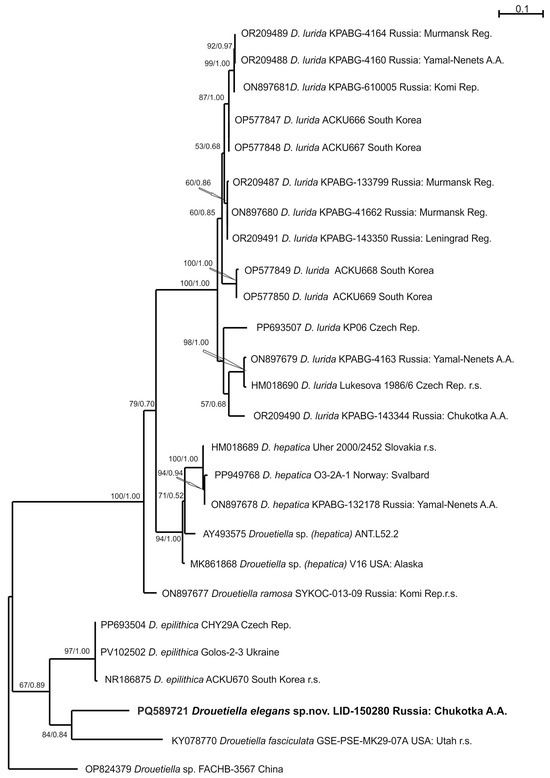
Figure 5.
Phylogram obtained under the maximum likelihood approach for 26 accessions of the genus Drouetiella based on the 16S-23S ITS rRNA region. The reference strains are marked as r.s.
The strain LID–150280 was found within the clade of the genus Drouetiella, which received the highest support (bootstrap support (BS) 99% ML/posterior probability (PP) 1.00 BA).
The genus Drouetiella clade was positioned in relation (82/0.65) to a clade composed of the genera Pegethrix, Cartusia, Elainella, and the recently described Siamcapillus and Amphirytos (100/1.00) [9,25,26]. Within the Drouetiella clade, strain D. elegans LID–150280 was placed in a sister relationship to four strains of the species D. epilithica (95/1.00). The subsequent relation was composed of strain of Drouetiella fasciculata (100/1.00). Recently described Drouetiella ramosa kept its relation to the clade composed of seven strains of D. hepatica (91/0.96). Eighteen sequencing to date strains of Drouetiella lurida were located in one highly supported clade (99/1.00). The topology of the 16S–23S ITS rRNA region indicated a relation of strain Drouetiella elegans LID-150280 to D. fasciculata, but with poor support (84/0.84). This pair was subsequently related to Drouetiella epilithica with support (67/0.89). Drouetiella ramosa was changed to a sister relation (100/1.00) of the clade composed of D. lurida and D. hepatica compared with the previously shown affinity to D. hepatica-clade [8].
The similarity of the 16S rRNA gene of strain Drouetiella elegans LID–150280 to reference strains of Drouetiella species varied from 96.31% to 98.97%, while the dissimilarity of the 16S–23S ITS rRNA region ranged from 12.27% to 20.43% (Table 1). The highest similarity was observed between strain Drouetiella elegans LID–150280 and D. epilithica ACKU670 (98.97%/12.27%), followed by D. fasciculata GSE-PSE-MK29-07A (98.28%/15.80%). The infraspecific nucleotide sequence similarity for 16S of Drouetiella lurida is 99.43%, D. hepatica—99.84%, D. epilithica—100%. The nucleotide sequence dissimilarity the 16S–23S ITS rRNA region of Drouetiella lurida is 4.04%, D. hepatica—3.43%, D. epilithica—0.29%.

Table 1.
Values of nucleotide sequence similarity/dissimilarity (%) for the genus Drouetiella, based on 16S rRNA gene (1179 bp) and 16S–23S ITS rRNA region (555 bp).
The sequence length of the 16S–23S ITS rRNA region was robustly estimated for reference strains of Drouetiella hepatica Uher 2000/2452, D. ramosa SYKOA C-013-09, D. epilithica ACKU670, and D. fasciculata GSE-PSE-MK29-07A. However, incomplete data were available for Drouetiella lurida Lukesova 1986/6 in GenBank (Table 2). Strain Drouetiella elegans LID–150280 exhibited a D1–D1′ region (63 bp) that was 1–2 bp shorter than that of other species. The V2 region (10 bp) was reduced and similar in length to that of Drouetiella epilithica and D. fasciculata. The Box-B helix length was similar only to that of Drouetiella epilithica (35 bp), and the V3 region was characterized by a stable length (52 bp) for the genus.

Table 2.
Sequence length variability (base pairs, bp) within 16S–23S ITS rRNA among reference strains of Drouetiella.
The secondary structure of the D1–D1′ helix of strain Drouetiella elegans LID–150280 was common with other Drouetiella species and contained three loops (Figure 6). The basal stem structure was consistent with that of the genus Drouetiella, and the nucleotide sequence of the basal loop was similar to that of D. lurida, D. hepatica, and D. ramosa, differing in both length and sequence from the most closely related species, D. epilithica (C–A substitution) and D. fasciculata (U–A substitution). The middle stem was the longest, with 16 steps; the middle loop was the shortest (5 bp); and the terminal loop had a unique motif due to seven substitutions compared with other species.
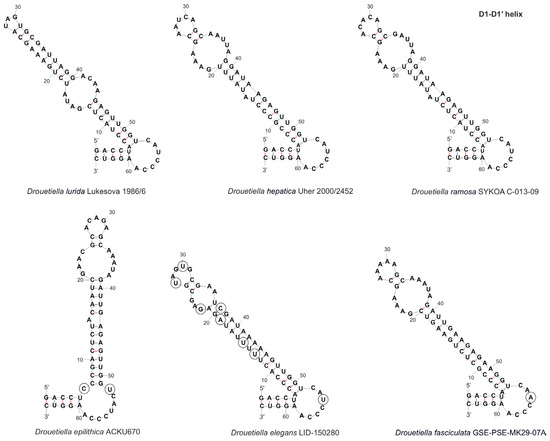
Figure 6.
The secondary structure of D1–D1′ helices of the 16S–23S ITS rRNA region of Drouetiella strains. Circles indicate differences.
The Box–B helix of strain Drouetiella elegans LID–150280 resembled those of D. lurida and D. epilithica, with two loops and a bulb composed of a single unpaired nucleotide (A) in the middle part of the stem (Figure 7). The basal loop of strain Drouetiella elegans LID–150280 differed in the C–A substitution compared to D. lurida and D. epilithica, and the terminal loop had a unique sequence among other species due to four substitutions (UUUG).
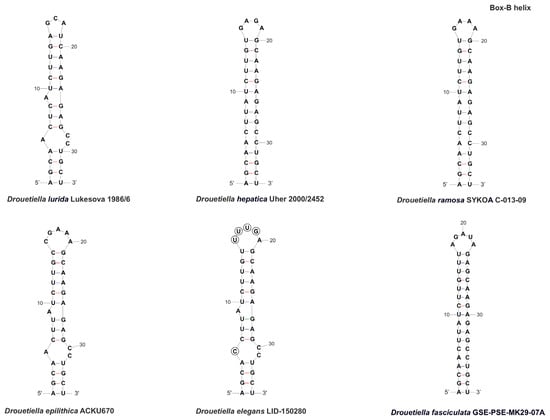
Figure 7.
The secondary structure of Box–B helices of the 16S–23S ITS rRNA region of Drouetiella strains. Circles indicate differences.
The V2 region was reduced in strain Drouetiella elegans LID–150280, similar to D. epilithica and D. fasciculata (Figure 8). It had a stem only two steps long and a terminal loop with five nucleotides, with only two nucleotides clearly distinguishing strain Drouetiella elegans LID–150280 from D. epilithica and D. fasciculata. The V2 region in Drouetiella lurida, D. hepatica, and D. ramosa had a stem length of 6–7 steps and a variable loop region, both in length and nucleotide sequence.
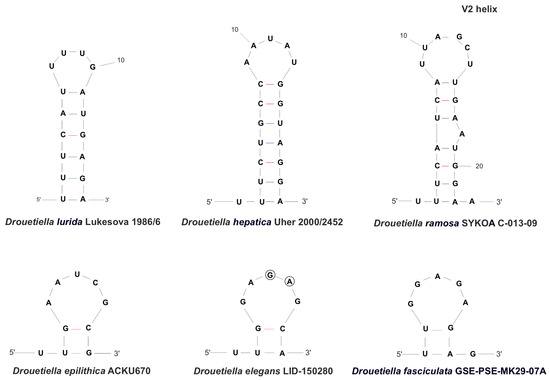
Figure 8.
The secondary structure of V2 helices of the 16S-23S ITS rRNA region of Drouetiella strains. Circles indicate differences.
The V3 region of strain Drouetiella elegans LID–150280 was similar to that of D. epilithica, D. hepatica, and D. ramosa in the nucleotide sequence of the basal stem and loop but differed in the sequence of the middle loop (Figure 9).
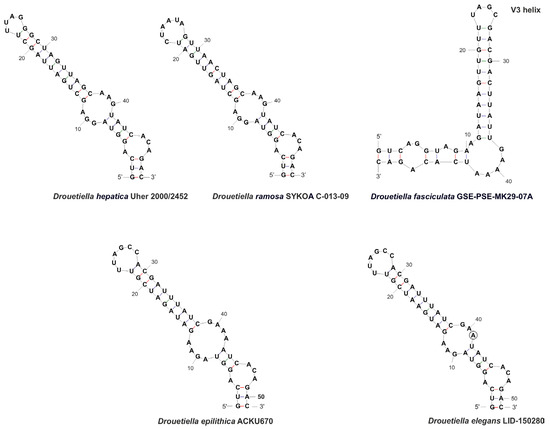
Figure 9.
The secondary structure of V3 helices of the 16S-23S ITS rRNA region of Drouetiella strains. Circles indicate differences.
The length of the stem between the middle and terminal loops and the sequence of the terminal loop of strain Drouetiella elegans LID–150280 were similar to those of D. epilithica. However, a single substitution at position 43 led to differences in the middle loop motif and the length of the stem between the middle and basal loops.
Thus, the separate position on constructed trees, the similarity of the 16S rRNA gene, the dissimilarity in the 16S–23S ITS rRNA region, and the features of secondary structures of conserved helices in the 16S–23S ITS region, together with morphological, ecological, and geographical traits, allowed us to consider the strain LID–150280 as a new species for science: Drouetiella elegans.
4. Discussion
Thin filamentous non-heterocytous cyanobacteria exhibit significant taxonomic diversity within the family Oculatellaceae. Delimitation of taxa within this family is reliably achievable only through molecular genetic data. The genus Drouetiella is a notable example of cryptic diversity. The morphological characteristics typical of Drouetiella species are not sufficient for unambiguous species identification. Therefore, species within this genus are primarily identified through the analysis of 16S rRNA and 16S–23S ITS rRNA sequences.
In a previous study, we attempted to compare the morphology of strains from different Drouetiella species [8]. Considering the newly described species Drouetiella epilithica and D. elegans, it can be stated that the main characteristics of this genus include thin cylindrical trichomes with a width ranging from 1.25 to 3.1 (4.3) µm, without constrictions at the cross-walls, the presence of necridic cells that facilitate trichome disintegration, and a rounded terminal cell. Another important anatomical feature for cyanobacterial taxonomy is the arrangement of thylakoids. As with all members of the family Oculatellaceae, species of the genus Drouetiella are characterized by a parietal thylakoid arrangement [1,9].
The publishing of new data from strains gathered from remote localities allows for the correction of initial species and genus descriptions to clarify variability and diversification. The Drouetiella lurida strains from Yamal-Nenets Autonomous Area, Komi Republic, and Murmansk Region of Russia [8] and from the Republic of Korea [9] are characterized by the presence of false branching, necridic cells, and hormogonia, which were not found in the type strain Lukesova 1986/6. Newly marked morphological features provide misleading separation of Drouetiella lurida from other species. Opposite strain Drouetiella hepatica from Yamal-Nenets Autonomous Area does not have false branching, which is also rare in type strain Uher 2000/2452 from Slovakia. Other species are still presented by type strain only, and their morphological variability is not sufficiently studied. Key features for species differentiation are summarized in Table 3. False branching is very often observed in Drouetiella ramosa and D. epilithica, rarely in D. hepatica and D. lurida, and is absent in D. fasciculata and D. elegans. Meristematic zones in the trichomes, characterized by highly shortened cells, are typical of Drouetiella hepatica and D. epilithica. Hormogonia are absent in Drouetiella hepatica and D. elegans, or are very rarely observed in D. lurida, D. ramosa, and D. fasciculata, being common only in D. epilithica. Drouetiella elegans differs from phylogenetically allied D. epilithica by the absence of false branching, meristematic zones, and hormogonia, and more narrow and elongate cells. Less pronounced differences were found between Drouetiella elegans and D. fasciculata, consisting of the absence of constrictions on the transverse walls and rarely forming hormogonia in the latter species. Newly described species Drouetiella elegans is similar to strain D. hepatica V16 from Alaska by coiled trichomes [27].

Table 3.
Morphological comparison among named Drouetiella strains.
As mentioned earlier, Drouetiella elegans cannot definitely be differentiated from other species by subtle variations in morphological traits. Therefore, due to the minor overlap in morphological characteristics among Drouetiella species, their primary distinction relies on molecular data.
As a result of phylogenetic estimations, Drouetiella elegans was found in a separate clade clustered in sister relation with the clade containing the type strain of D. epilithica ACKU670 in the 16S rRNA calculation or in poorly supported relation with D. fasciculata GSE-PSE-MK29-07A in the 16S–23S ITS rRNA region. The distinct position among known taxa supported the description of the obtained genetic unit as a new species.
The 16S rDNA sequence similarity of Drouetiella elegans varies from 96.31% with D. lurida Lukesova 1986/6 to 98.97% with D. epilithica ACKU670, which fits well into the 98.7–99.00% threshold for Cyanobacteriota species delimitation [28,29]. The highest value of 16S–23S ITS rRNA sequence dissimilarity of Drouetiella elegans was registered with D. epilithica ACKU670 (12.27%), and the lowest was 20.43% with D. hepatica Uher 2000/2452. The threshold of sequence dissimilarity in the 16S–23S ITS rRNA region of more than 7% suggested strains as belonging to distinct species [30], which additionally supports species status for Drouetiella elegans. The species Drouetiella lurida, D. hepatica and D. epilithica demonstrates the high level of infraspecific 16S gene similarity (99.43–100%) and low level of 16–23S ITS dissimilarity (0.2–4%) among specimens from remote localities, which characterizes all species as genetically stable units and further confirms the molecular genetic differentiation of D. elegans at the species level.
The secondary structures of the conserved helices in the 16S–23S ITS rRNA region are species-specific. The main feature of phylogenetically allied species Drouetiella elegans, D. epilithica, and D. fasciculata is a highly reduced V2 helices compared to other species in the genus.
The distribution map (Figure 1) highlights the locations where various Drouetiella strains have been identified, offering insight into the global dispersal and diversity of this genus. The species Drouetiella lurida has been known since 1849 [31]; numerous records of its occurrence have been documented in the literature. Based on these data, the species is characterized by a multizonal distribution. However, it is important to note that the number of findings confirmed by molecular data is relatively small, and most of the records should be considered provisional. Nevertheless, even the confirmed records indicate that the species exhibits broad ecological plasticity, thriving in both aerophytic and subaerophytic conditions as well as underwater as an epilithic organism.
The type specimen was found in soil in a temperate forest in the Czech Republic [1]. In the Leningrad Region, Drouetiella lurida was discovered in flowing spring water [32]. The species was also identified as an epilithic organism on a submerged boulder in a river in the Polar Ural Mountains [8]. In the Murmansk Region, it was collected from an anthropogenic habitat at the ash dump of the Apatity Power Station, and in the Subpolar Ural Mountains, it was found on the soils of a grass-moss community [8]. The species has also been reported on gravels from water in the Republic of Korea [9] and China (GenBank accession number is PP413588). Strains of Drouetiella lurida were also collected from high-altitude lakes in the cold desert region of Himachal Pradesh, India [33], or soil (PP410480) and in the water of a floodplain lake in Chukotka [32]. Drouetiella lurida was also found in biofilms from Spanish caves (PP849347 and PP849345).
Besides the locus classicus in a temperate forest in Slovakia, Drouetiella hepatica has been found in Antarctica [34], Alaska [27], Svalbard [35], and the Polar Urals [8]. Drouetiella epilithica was gathered from the base of the stone monument in Daegu (the Republic of Korea) [9], and recently was suggested from caves of the Czech Republic [36] and pine bark from Ukraine [37]. As for Drouetiella fasciculata and D. ramosa, these species have not yet been identified in locations other than those where the type specimens were collected. Drouetiella fasciculata is known from a large seep wall and waterfall in Navajo Sandstone (Utah, USA), where the species composes mats with other filamentous algae [1]. Drouetiella ramosa was found on a quartz mine on damp quartz sand in the Subpolar Urals Mountains (Komi Republic, Russia) [8]. The majority of species of the genus Drouetiella prefer aerophyte or subaerophyte habitats. Drouetiella elegans lives as a plankton in the water of a small lake (Chukotka, Russia) and in its ecology partly resembles some aquatic strains of D. lurida from Russia or the Republic of Korea.
Thin filamentous non-heterocytic cyanobacteria of the order Oculatellales are widespread in various ecotopes and have the ability to be successfully cultivated in laboratory conditions, which, apparently, is a reliable basis for studying their diversity and clarifying their taxonomy. In addition to the Drouetiella elegans described here, another new species can be proposed from the Drouetiella sp. strain FACHB-3567 based on the results of the provided molecular evaluation of all currently published nucleotide data.
Strains of this genus show considerable promise for bioremediation applications, particularly in harsh environments in Antarctica, Alaska, Svalbard, and the Russian Arctic. As pioneer species, they possess a remarkable ability to colonize various anthropogenic substrates, including mining waste from ore processing, overburden materials, and other industrially disturbed landscapes. Their ecological resilience is well-documented by their widespread distribution in extreme Arctic ecosystems and successful establishment on ash deposits at the Apatity Thermal Power Plant [8,38].
The capacity to form viable populations under such extreme conditions indicates the presence of robust metabolic adaptations to stressful environments. This combination of traits—including stress tolerance, rapid colonization ability, and survival in nutrient-poor conditions—positions these strains as particularly valuable for remediation efforts in northern regions where conventional approaches often prove ineffective.
Author Contributions
Conceptualization, D.D.; methodology, D.D. and A.V.; software, D.D. and A.V.; validation, D.D. and A.V.; formal analysis, D.D. and A.V.; investigation, D.D. and A.V.; resources, D.D.; data curation, D.D. and A.V.; writing—original draft preparation, D.D. and A.V.; writing—review and editing, D.D. and A.V.; visualization, D.D. and A.V.; supervision, D.D.; project administration, D.D.; funding acquisition, D.D. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 25-14-20011, (https://rscf.ru/project/25-14-00011/ accessed on 14 July 2025).
Data Availability Statement
The data supporting the reported results can be found in the publicly archived dataset of the “L” Information system https://isling.org/ (accessed on 14 July 2025).
Acknowledgments
We are grateful to Elena Chemeris for gathering a sample of cyanobacterium.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunická, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through Recognition of Four Families Including Oculatellaceae Fam. Nov. and Trichocoleaceae Fam. Nov. and Six New Genera Containing 14 Species. Phytotaxa 2018, 365, 1. [Google Scholar] [CrossRef]
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An Updated Classification of Cyanobacterial Orders and Families Based on Phylogenomic and Polyphasic Analysis. J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef]
- Geng, R.; Cheng, Y.; Chen, S.; Zhang, H.; Xiao, P.; Chen, S.; Ma, Z.; Han, B.; Li, R. Maricoleus vaginatus Gen. et sp. nov. (Oculatellaceae, Synechococcales), a Novel Cyanobacterium Isolated from a Marine Ecosystem in China. Fottea 2024, 24, 27–41. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. WorldWide Electronic Publication 2023. Available online: http://www.algaebase.org (accessed on 12 December 2024).
- Zammit, G.; Billi, D.; Albertano, P. The Subaerophytic Cyanobacterium oculatella Subterranea (Oscillatoriales, Cyanophyceae) Gen. et sp. nov.: A Cytomorphological and Molecular Description. Eur. J. Phycol. 2012, 47, 341–354. [Google Scholar] [CrossRef]
- Sciuto, K.; Moschin, E.; Moro, I. Cryptic Cyanobacterial Diversity in the Giant Cave (Trieste, Italy): The New Genus Timaviella (Leptolyngbyaceae). Cryptogam. Algol. 2017, 38, 285–323. [Google Scholar] [CrossRef]
- Zammit, G. Systematics and Biogeography of Sciophilous Cyanobacteria; an Ecological and Molecular Description of Albertania skiophila (Leptolyngbyaceae) gen. & pp. nov. Phycologia 2018, 57, 481–491. [Google Scholar] [CrossRef]
- Davydov, D.; Vilnet, A.; Novakovskaya, I.; Patova, E. Terrestrial Species of Drouetiella (Cyanobacteria, Oculatellaceae) from the Russian Arctic and Subarctic Regions and Description of Drouetiella ramosa sp. nov. Diversity 2023, 15, 132. [Google Scholar] [CrossRef]
- Kim, D.; Lee, N.; Wang, H.; Lim, A.S.; Lee, O. Drouetiella epilithica sp. nov. and Drouetiella lurida (Oculatellaceae, Synechococcales) Isolated in the Republic of Korea Based on the Polyphasic Approach. Phycol. Res. 2023, 71, 140–153. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae. Nor. Inst. Water Res. 1972, 11, 1–5. [Google Scholar]
- Waterbury, J.B. The Cyanobacteria—Isolation, Purification and Identification. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 1053–1073. ISBN 978-0-387-25494-4. [Google Scholar]
- Melekhin, A.V.; Davydov, D.A.; Borovichev, E.A.; Shalygin, S.S.; Konstantinova, N.A. CRIS—Service for Input, Storage and Analysis of the Biodiversity Data of the Cryptogams. Folia Cryptogam. Est. 2019, 56, 99–108. [Google Scholar] [CrossRef]
- Boyer, S.L.; Flechtner, V.R.; Johansen, J.R. Is the 16S–23S rRNA Internal Transcribed Spacer Region a Good Tool for Use in Molecular Systematics and Population Genetics? A Case Study in Cyanobacteria. Mol. Biol. Evol. 2001, 18, 1057–1069. [Google Scholar] [CrossRef]
- Wilmotte, A.; Van der Auwera, G.; De Wachter, R. Structure of the 16 S Ribosomal RNA of the Thermophilic Cyanobacterium Chlorogloeopsis HTF (‘Mastigocladus Laminosus HTF’) Strain PCC7518, and Phylogenetic Analysis. FEBS Lett. 1993, 317, 96–100. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; Therese, D.D.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. rRNA Sequences and Evolutionary Relationships among Toxic and Nontoxic Cyanobacteria of the Genus Microcystis. Int. J. Syst. Bacteriol. 1997, 47, 693–697. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR Primers to Amplify 16S rRNA Genes from Cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Iteman, I.; Rippka, R.; Tandeau De Marsac, N.; Herdman, M. Comparison of Conserved Structural and Regulatory Domains within Divergent 16S rRNA–23S rRNA Spacer Sequences of Cyanobacteria the GenBank Accession Numbers for the Sequences Reported in This Paper Are AF180968 and AF180969 for ITS-L and ITS-S, Respectively. Microbiology 2000, 146, 1275–1286. [Google Scholar] [CrossRef]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Jasser, I.; Panou, M.; Khomutovska, N.; Sandzewicz, M.; Panteris, E.; Niyatbekov, T.; Łach, Ł.; Kwiatowski, J.; Kokociński, M.; Gkelis, S. Cyanobacteria in Hot Pursuit: Characterization of Cyanobacteria Strains, Including Novel Taxa, Isolated from Geothermal Habitats from Different Ecoregions of the World. Mol. Phylogenet. Evol. 2022, 170, 107454. [Google Scholar] [CrossRef]
- Tawong, W.; Pongcharoen, P.; Nishimura, T.; Saijuntha, W. Siamcapillus rubidus Gen. et sp. nov. (Oculatellaceae), a Novel Filamentous Cyanobacterium from Thailand Based on Molecular and Morphological Analyses. Phytotaxa 2022, 558, 33–52. [Google Scholar] [CrossRef]
- Strunecky, O.; Raabova, L.; Bernardova, A.; Ivanova, A.P.; Semanova, A.; Crossley, J.; Kaftan, D. Diversity of Cyanobacteria at the Alaska North Slope with Description of Two New Genera: Gibliniella and Shackletoniella. FEMS Microbiol. Ecol. 2020, 96, fiz189. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Ebersole, J.J. Taxonomic Parameters Revisited: Tarnished Gold Standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the Classification of Cultured and Uncultured Bacteria and Archaea Using 16S rRNA Gene Sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- González-Resendiz, L.; Johansen, J.R.; León-Tejera, H.; Sánchez, L.; Segal-Kischinevzky, C.; Escobar-Sánchez, V.; Morales, M. A Bridge Too Far in Naming Species: A Total Evidence Approach Does Not Support Recognition of Four Species in Desertifilum (Cyanobacteria). J. Phycol. 2019, 55, 898–911. [Google Scholar] [CrossRef]
- Kützing, F.T. Species Algarum; F.A. Brockhaus: Leipzig, Germany, 1849. [Google Scholar]
- Kotkova, V.M.; Afonina, O.M.; Androsova, V.I.; Arslanov, S.N.; Belyakov, E.A.; Chernova, A.M.; Czernyadjeva, I.V.; Davydov, E.A.; Doroshina, G.Y.; Erokhina, O.V.; et al. New cryptogamic records. 10. Nov. Sist. Nizshikh Rastenii 2022, 56, 477–517. [Google Scholar] [CrossRef]
- Singh, Y.; Khattar, J.I.S.; Singh, D.P.; Rahi, P.; Gulati, A. Limnology and Cyanobacterial Diversity of High Altitude Lakes of Lahaul-Spiti in Himachal Pradesh, India. J. Biosci. 2014, 39, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Grubisic, S.; Ertz, D.; Hodgson, D.A.; Piccardi, R.; Biondi, N.; Tredici, M.R.; Mainini, M.; Losi, D.; Marinelli, F.; et al. Polyphasic Study of Antarctic Cyanobacterial Strains. J. Phycol. 2006, 42, 1257–1270. [Google Scholar] [CrossRef]
- Mikhailyuk, T.; Glaser, K.; Demchenko, E.; Hotter, V.; Pushkareva, E.; Karsten, U. Diversity of Algae and Cyanobacteria from Biological Soil Crusts in the High Arctic (Svalbard) along Two Different Moisture Gradients. Eur. J. Phycol. 2025, 60, 221–244. [Google Scholar] [CrossRef]
- Wipplingerová, M.; Pokorný, J.; Kaštovský, J. Cyanobacteria and Algae from Chýnov and Koněprusy Caves (Czech Republic). Int. J. Speleol. 2025, 54, ijs2514. [Google Scholar] [CrossRef]
- Mikhailyuk, T.I.; Vinogradova, O.M.; Demchenko, E.M.; Petlovana, V.R.; Glaser, K.; Karsten, U. Terrestrial Algae and Cyanobacteria of the Holosiiv National Nature Park (Kyiv, Ukraine), with the Description of Leptochlorella arboricola sp. nov. (Trebouxiophyceae, Chlorophyta). Ukr. Bot. J. 2025, 82, 3–30. [Google Scholar] [CrossRef]
- Davydov, D.; Vilnet, A. Cyanobacterial Assemblages Inhabiting the Apatity Thermal Power Plant Fly Ash Dumps in the Russian Arctic. Microorganisms 2025, 13, 1762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).