Systematic Review of Crop Pests in the Diets of Four Bat Species Found as Wind Turbine Fatalities
Abstract
1. Introduction
2. Materials and Methods
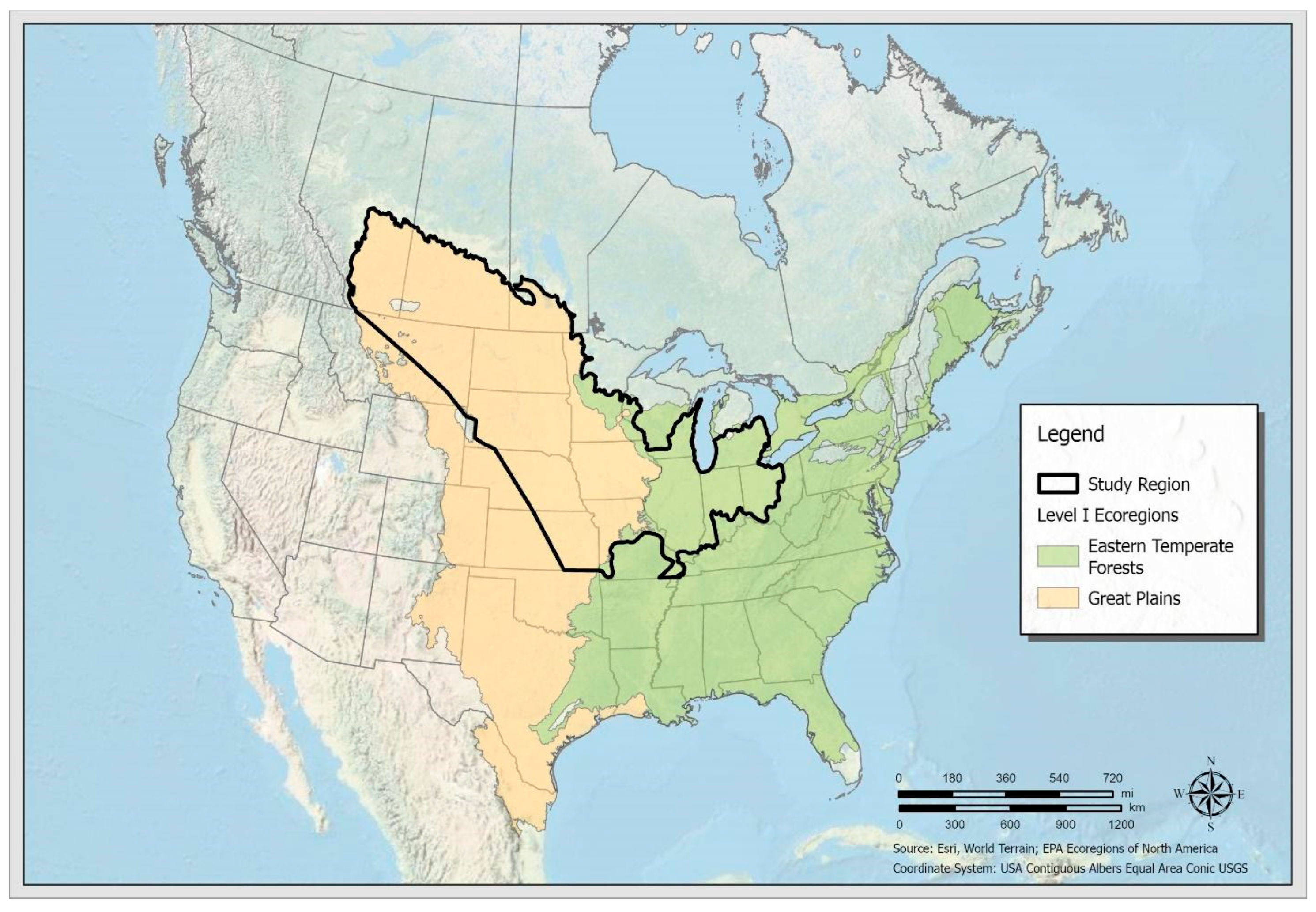
2.1. Study Region and Species
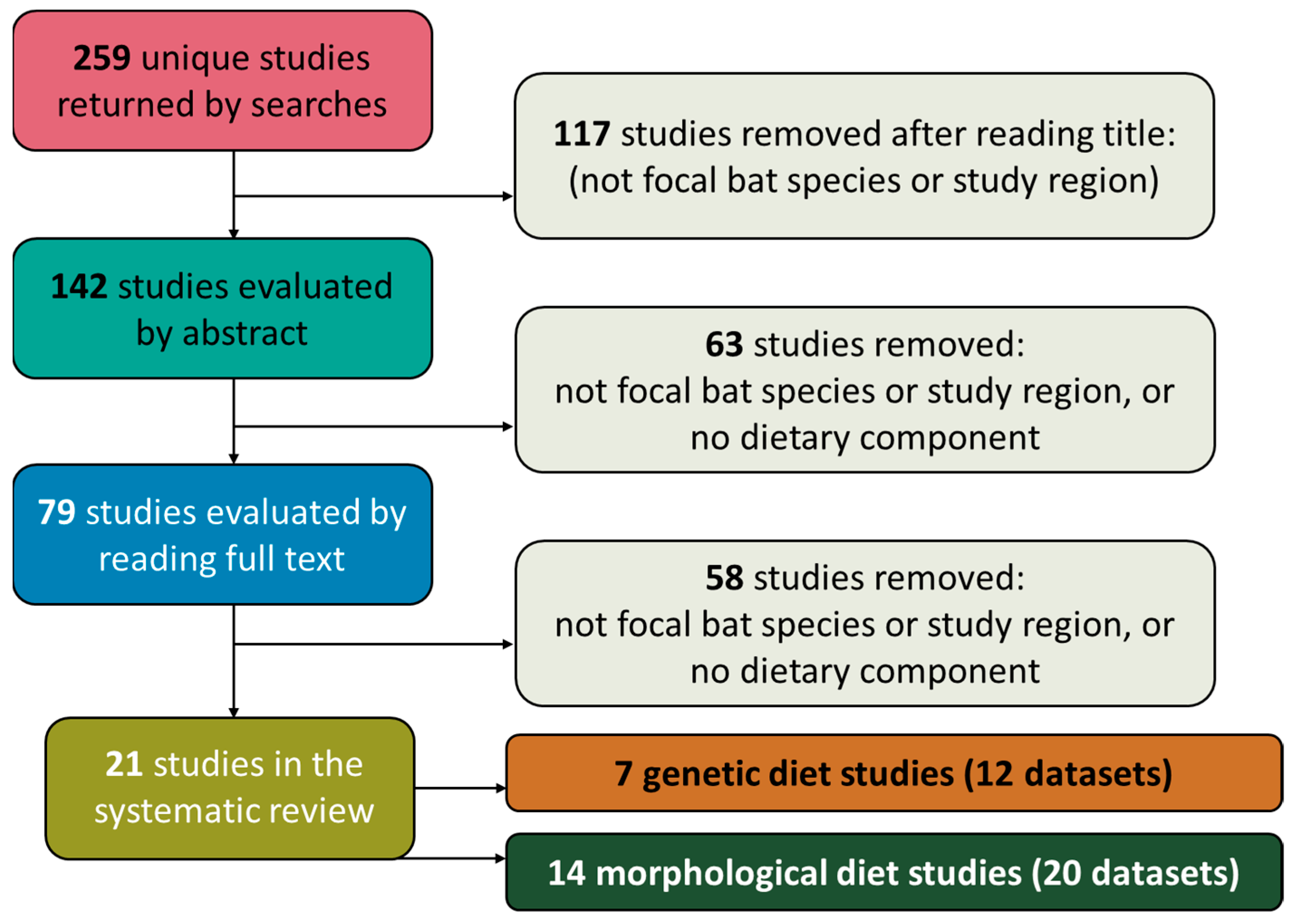
2.2. Data Sources, Search Strategy, and Systematic Review
3. Results
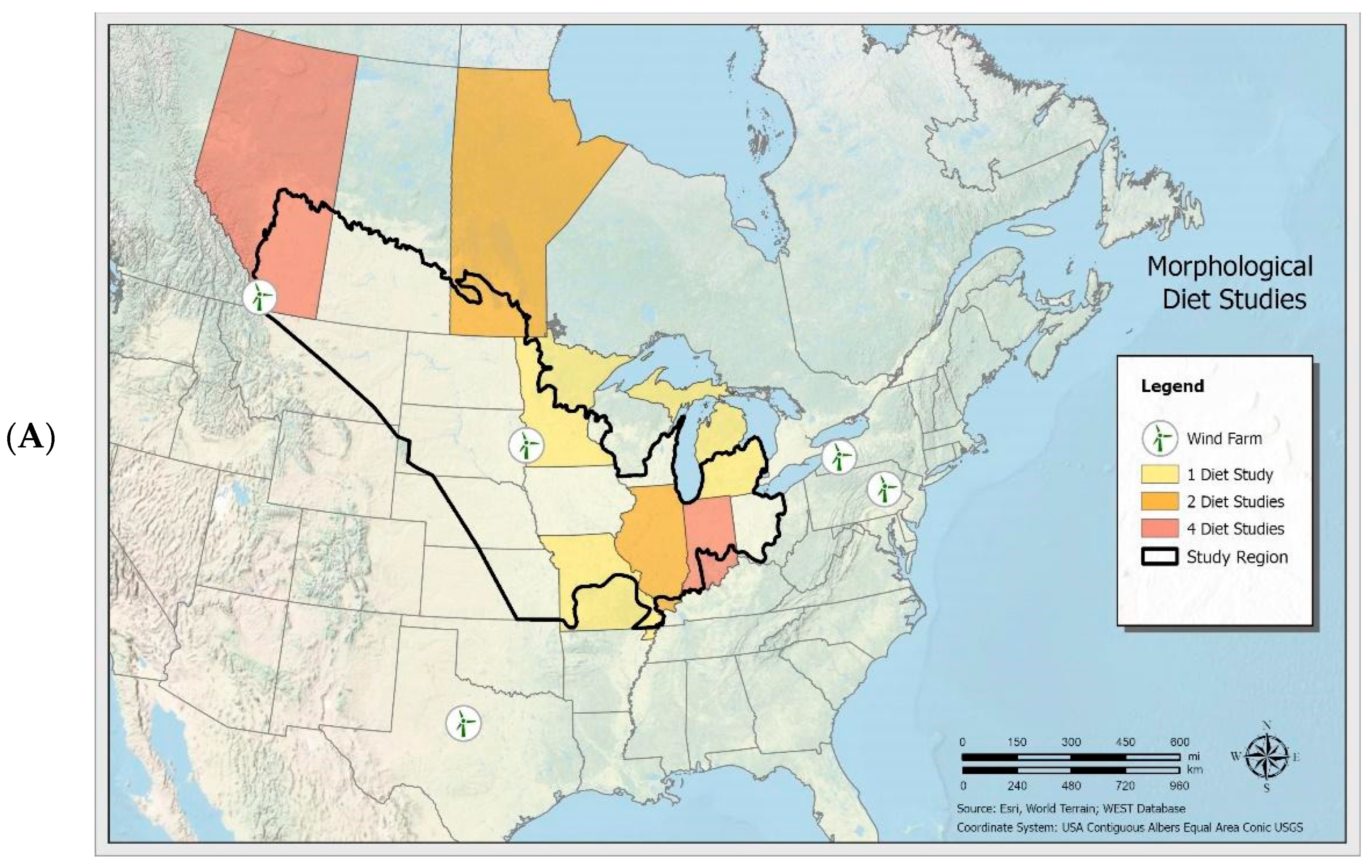
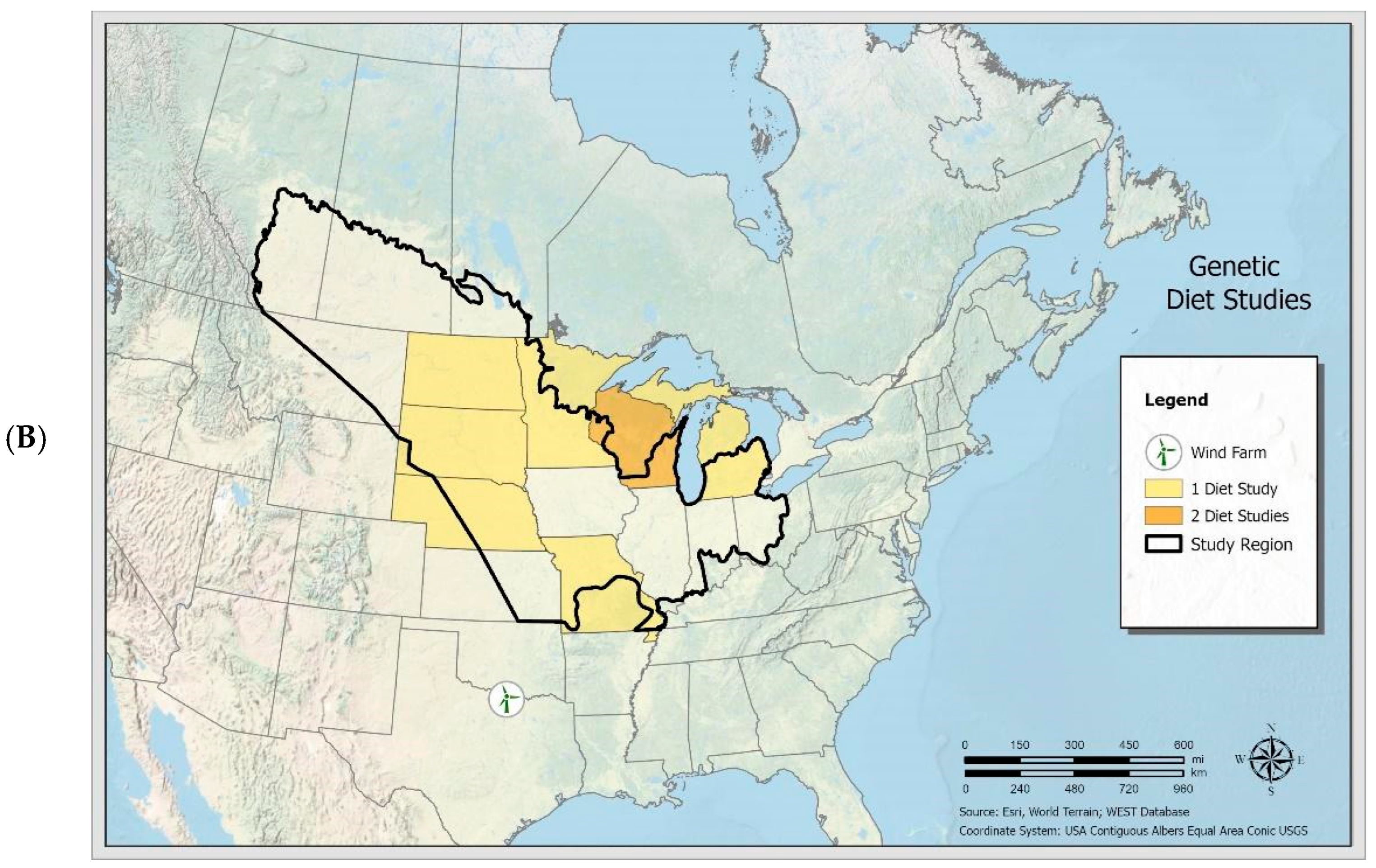
3.1. Crop Pests and Other Prey Consumed by Big Brown Bats
3.2. Crop Pests and Other Prey Consumed by Eastern Red Bats
3.3. Prey Consumed by Hoary Bats and Silver-Haired Bats
4. Discussion
Conservation Implications and Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| USFWS | U.S. Fish and Wildlife Service |
References
- Arnett, E.B.; Baerwald, E.F.; Mathews, F.; Rodrigues, L.; Rodríguez-Durán, A.; Rydell, J.; Villegas-Patraca, R.; Voigt, C.C. Impacts of wind energy development on bats: A global perspective. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 295–323. [Google Scholar]
- Frick, W.F.; Baerwald, E.F.; Pollock, J.F.; Barclay, R.M.R.; Szymanski, J.A.; Weller, T.J.; Russell, A.L.; Loeb, S.C.; Medellin, R.A.; McGuire, L.P. Fatalities at wind turbines may threaten population viability of a migratory bat. Biol. Conserv. 2017, 209, 172–177. [Google Scholar] [CrossRef]
- Thaxter, C.B.; Buchanan, G.M.; Carr, J.; Butchart, S.H.M.; Newbold, T.; Green, R.E.; Tobias, J.A.; Foden, W.B.; O’Brien, S.; Pearce-Higgins, J.W. Bird and bat species’ global vulnerability to collision mortality at wind farms revealed through a trait-based assessment. Proc. Roy Soc. B 2017, 284, 20170829. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Lintott, P.R.; Hosken, D.J.; Economou, T.; Mathews, F. Peaks in bat activity at turbines and the implications for mitigating the impact of wind energy developments on bats. Sci. Rep. 2021, 11, 3636. [Google Scholar] [CrossRef]
- Arnett, E.B.; Brown, W.K.; Erickson, W.P.; Fiedler, J.K.; Hamilton, B.L.; Henry, T.H.; Jain, A.; Johnson, G.D.; Kerns, J.; Koford, R.R.; et al. Patterns of bat fatalities at wind energy facilities in North America. J. Wild Manag. 2008, 72, 61–78. [Google Scholar] [CrossRef]
- Arnett, E.B.; Baerwald, E.F. Impacts of wind energy development on bats: Implications for conservation. In Bat Evolution, Ecology, and Conservation; Adams, R.A., Scott, S.C., Eds.; Springer: New York, NY, USA, 2013; pp. 435–456. [Google Scholar]
- Thompson, M.; Beston, J.A.; Etterson, M.; Diffendorfer, J.E.; Loss, S.R. Factors associated with bat mortality at wind energy facilities in the United States. Biol. Conserv. 2017, 215, 241–245. [Google Scholar] [CrossRef]
- American Wind Wildlife Institute (AWWI). AWWI Technical Report: 2nd Edition: Summary of Bat Fatality Monitoring Data Contained in AWWIC; American Wind Wildlife Institute: Washington, DC, USA, 2020; Available online: https://www.awwi.org (accessed on 26 October 2022).
- Mai, T.; Lopez, A.; Mowers, M.; Lantz, E. Interactions of wind energy project siting, wind resource potential, and the evolution of the U.S. power system. Energy 2021, 223, 119998. [Google Scholar] [CrossRef]
- Goldenberg, S.Z.; Cryan, P.M.; Gorresen, P.M.; Fingersh, L.J. Behavioral patterns of bats at a wind turbine confirm seasonality of fatality risk. Ecol. Evol. 2021, 11, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Cryan, P.M.; Barclay, R.M.R. Causes of bat fatalities at wind turbines: Hypotheses and predictions. J. Mammal. 2009, 90, 1330–1340. [Google Scholar] [CrossRef]
- Guest, E.E.; Stamps, B.F.; Durish, N.D.; Hale, A.M.; Hein, C.D.; Morton, B.P.; Weaver, S.P.; Fritts, S.R. An updated review of hypotheses regarding bat attraction to wind turbines. Animals 2022, 12, 343. [Google Scholar] [CrossRef]
- Horn, J.W.; Arnett, E.B.; Kunz, T.H. Behavioral responses of bats to operating wind turbines. J. Wild Manag. 2008, 72, 123–132. [Google Scholar] [CrossRef]
- Valdez, E.W.; Cryan, P.M. Insect prey eaten by hoary bats (Lasiurus cinereus) prior to fatal collisions with wind turbines. West. N. Am. Nat. 2013, 73, 516–524. [Google Scholar] [CrossRef]
- Rydell, J.; Bogdanowicz, W.; Boonman, A.; Pettersson, S.; Suchecka, E.; Pomorski, J.J. Bats may eat diurnal flies that rest on wind turbines. Mamm. Biol. 2016, 81, 331–339. [Google Scholar] [CrossRef]
- Foo, C.F.; Bennett, V.J.; Hale, A.M.; Korstian, J.M.; Schildt, A.J.; Williams, D.A. Increasing evidence that bats actively forage at wind turbines. PeerJ 2017, 5, e3985. [Google Scholar] [CrossRef]
- Cryan, P.M.; Stricker, C.A.; Wunder, M.B. Evidence of cryptic individual specialization in an opportunistic insectivorous bat. J. Mammal. 2012, 93, 381–389. [Google Scholar] [CrossRef]
- Heim, O.; Lorenz, L.; Kramer-Schadt, S.; Jung, K.; Voigt, C.C.; Eccard, J.A. Landscape and scale-dependent spatial niches of bats foraging above intensively used arable fields. Ecol. Process. 2017, 6, 24. [Google Scholar] [CrossRef]
- Fukui, D.A.I.; Murakami, M.; Nakano, S.; Aoi, T. Effect of emergent aquatic insects on bat foraging in a riparian forest. J. Anim. Ecol. 2006, 75, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Hagen, E.M.; Sabo, J.L. A landscape perspective on bat foraging ecology along rivers: Does channel confinement and insect availability influence the response of bats to aquatic resources in riverine landscapes? Oecologia 2011, 166, 751–760. [Google Scholar] [CrossRef]
- Mayberry, H.W.; McMillan, M.R.; Vikram Chochinov, A.; Hinds, J.C.; Ratcliffe, J.M. Potential foraging niche release in insectivorous bat species relatively unaffected by white-nose syndrome? Can. J. Zool. 2020, 98, 667–680. [Google Scholar] [CrossRef]
- McCracken, G.F.; Westbrook, J.K.; Brown, V.A.; Eldridge, M.; Federico, P.; Kunz, T.H. Bats track and exploit changes in insect pest populations. PLoS ONE 2012, 7, e43839. [Google Scholar] [CrossRef]
- Baroja, U.; Garin, I.; Vallejo, N.; Caro, A.; Ibáñez, C.; Basso, A.; Goiti, U. Molecular assays to reliably detect and quantify predation on a forest pest in bats Faeces. Sci. Rep. 2021, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Blažek, J.; Konečný, A.; Bartonička, T. Bat aggregational response to pest caterpillar emergence. Sci. Rep. 2011, 11, 13634. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.O.; Hamilton, W.J. Mammals of the Eastern United States, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1998; 583p. [Google Scholar]
- Fill, C.; Allen, C.; Twidwell, D.; Benson, J. Spatial distribution of bat activity in agricultural fields: Implications for ecosystem service estimates. Ecol. Soc. 2022, 27, 11. [Google Scholar] [CrossRef]
- Hunninck, L.; Coleman, K.; Boman, M.; O’Keefe, J. Far from home: Bat activity and diversity in row crop agriculture decreases with distance to potential roost habitat. Global Ecol. Conserv. 2022, 39, e02297. [Google Scholar] [CrossRef]
- Omernik, J.M.; Griffith, G.E. Ecoregions of the conterminous United States: Evolution of a hierarchical spatial framework. Environ. Manag. 2014, 54, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Lawrence Berkley National Laboratory (Berkley Lab). Land-based Wind Market Report, 2024 Edition. Available online: https://www.energy.gov/eere/wind/land-based-wind-market-report (accessed on 17 August 2025).
- Canadian Renewable Energy Association (CanREA). By the Numbers. 2025. Available online: https://renewablesassociation.ca/by-the-numbers/ (accessed on 17 August 2025).
- Harvey, M.J.; Altenbach, J.S.; Best, T.L. Bats of the United States and Canada; Johns Hopkins University Press: Baltimore, MD, USA, 2011; 224p. [Google Scholar]
- Whitaker, J.O., Jr. Food of the big brown bat Eptesicus fuscus from maternity colonies in Indiana and Illinois. Am. Midl. Nat. 1995, 134, 346–360. [Google Scholar] [CrossRef]
- Agosta, S.J.; Morton, D. Diet of the big brown bat, Eptesicus fuscus, from Pennsylvania and Western Maryland. Northeast. Nat. 2003, 10, 89–104. [Google Scholar] [CrossRef]
- Zimmerling, J.R.; Francis, C.M. Bat mortality due to wind turbines in Canada. J. Wild Manag. 2016, 80, 1360–1369. [Google Scholar] [CrossRef]
- Kunz, T.H. Lasionycteris noctivagans. Mamm. Species 1952, 172, 1–5. [Google Scholar] [CrossRef]
- Shump, K.A., Jr.; Shump, A.U. Lasiurus borealis. Mamm. Species 1982, 183, 1–6. [Google Scholar] [CrossRef]
- Shump, K.A., Jr.; Shump, A.U. Lasiurus cinereus. Mamm. Species 1982, 185, 1–5. [Google Scholar] [CrossRef]
- Kurta, A.; Baker, R.H. Eptesicus fuscus. Mamm. Species 1990, 356, 1–10. [Google Scholar] [CrossRef]
- Lacki, M.J.; Johnson, J.S.; Dodd, L.E.; Baker, M.D. Prey consumption of insectivorous bats in coniferous forests of north-central Idaho. Northwest Sci. 2007, 81, 199–205. [Google Scholar] [CrossRef]
- Clare, E.L.; Fraser, E.E.; Braid, H.E.; Fenton, M.B.; Hebert, P.D.N. Species on the menu of a generalist predator, the eastern red bat (Lasiurus borealis): Using a molecular approach to detect arthropod prey. Mol. Ecol. 2009, 18, 2532–2542. [Google Scholar] [CrossRef]
- Reimer, J.P.; Baerwald, E.F.; Barclay, R.M.R. Diet of hoary (Lasiurus cinereus) and silver-haired (Lasionycteris noctivagans) bats while migrating through southwestern Alberta in late summer and autumn. Am. Midl. Nat. 2010, 164, 230–237. [Google Scholar] [CrossRef]
- Whitby, M.D.; Kieran, T.J.; Glenn, T.C.; Allen, C. Agricultural pests consumed by common bat species in the United States corn belt: The importance of DNA primer choice. Agr. Ecosyst. Environ. 2020, 303, 107105. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Zeale, M.R.; Butlin, R.K.; Barker, G.L.A.; Lees, D.C.; Jones, G. Taxon-specific PCR for DNA barcoding arthropod prey in bat faeces. Mol. Ecol. Resour. 2011, 11, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lodova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. NY Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]
- Wright, R.J.; DeVries, T.A.; Kalisch, J.A. EC05-1572 Corn Insects I. Historical Materials from University of Nebraska-Lincoln Extension. 2005; p. 1553. Available online: https://digitalcommons.unl.edu/extensionhist/1553 (accessed on 20 October 2022).
- Wright, R.J.; DeVries, T.A.; Kalisch, J.A. EC05-1573 Corn Insects II. Historical Materials from University of Nebraska-Lincoln Extension. 2005; p. 1552. Available online: https://digitalcommons.unl.edu/extensionhist/1552 (accessed on 20 October 2022).
- Wright, R.J.; DeVries, T.A.; Kalisch, J.A. EC08-1574 Soybean Insects I. Historical Materials from University of Nebraska-Lincoln Extension. 2008; p. 4820. Available online: http://digitalcommons.unl.edu/extensionhist/4820 (accessed on 20 October 2022).
- Wright, R.J.; DeVries, T.A.; Kalisch, J.A. EC08-1575 Soybean Insects II. Historical Materials from University of Nebraska-Lincoln Extension. 2008; p. 4819. Available online: http://digitalcommons.unl.edu/extensionhist/4819 (accessed on 20 October 2022).
- Wright, R.J.; DeVries, T.A.; Kalisch, J.A. EC09-1576 Alfalfa Insects I. Historical Materials from University of Nebraska-Lincoln Extension. 2009; p. 4809. Available online: http://digitalcommons.unl.edu/extensionhist/4809 (accessed on 20 October 2022).
- Wright, R.J.; DeVries, T.A.; Kalisch, J.A. EC09-1577 Alfalfa Insects II. Historical Materials from University of Nebraska-Lincoln Extension. 2009; p. 4810. Available online: http://digitalcommons.unl.edu/extensionhist/4810 (accessed on 20 October 2022).
- Cravens, Z.M.; Brown, V.A.; Divoll, T.J.; Boyles, J.G. Illuminating prey selection in an insectivorous bat community exposed to artificial light. J. Appl. Ecol. 2018, 55, 705–713. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr. Food habits of bats from Indiana. Can. J. of Zool. 1972, 50, 877–883. [Google Scholar] [CrossRef]
- Storm, J.J.; Whitaker, J.O., Jr. Prey selection of big brown bats (Eptesicus fuscus) during an emergence of 17-year cicadas (Magicicada spp.). Am. Midl. Nat. 2008, 160, 350–357. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr. Prey selection in a temperate zone insectivorous bat community. J. Mammal. 2004, 85, 460–469. [Google Scholar] [CrossRef]
- Long, B.L.; Kurta, A.; Clemans, D.L. Analysis of DNA from feces to identify prey of big brown bats (Eptesicus fuscus) caught in apple orchards. Am. Midl. Nat. 2013, 170, 287–297. [Google Scholar] [CrossRef]
- Wray, A.K.; Gratton, C.; Jusino, M.A.; Wang, J.J.; Kochanski, J.M.; Palmer, J.M.; Banik, M.T.; Lindner, D.L.; Peery, M.Z. Disease-related population declines in bats demonstrate non-exchangeability in generalist predators. Ecol. Evol. 2022, 12, e8978. [Google Scholar] [CrossRef]
- Wray, A.K.; Peery, M.Z.; Jusino, M.A.; Kochanski, J.M.; Banik, M.T.; Palmer, J.M.; Lindner, D.L.; Gratton, C. Predator preferences shape the diets of arthropodivorous bats more than quantitative local prey abundance. Mol. Ecol. 2021, 30, 855–873. [Google Scholar] [CrossRef]
- Galey, M. Predator to Prey to Poop—Bats as Microbial Hosts and Insectivorous Hunters. Master’s Thesis, University of Minnesota, Twin Cities, MN, USA, 2020. Retrieved from the University Digital Conservancy. Available online: https://hdl.handle.net/11299/217130 (accessed on 20 July 2022).
- Karevold, H.M. Foraging Strategies and Morphometric Characteristics of Bats in North and South Dakota. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2021. [Google Scholar]
- Bast, M.D. Prey Selection within a Southeastern Missouri Bat Community. Master’s Thesis, Truman State University, Kirksville, MO, USA, 2006. [Google Scholar]
- Feldhamer, G.A.; Whitaker, J.O., Jr.; Krejca, J.K.; Taylor, S.J. Food of the evening bat (Nycticeus humeralis) and red bat (Lasiurus borealis) from southern Illinois. Trans. Ill. Acad. Sci. 1995, 88, 139–143. [Google Scholar]
- Perlik, M.K.; McMillan, B.R.; Krenz, J.D. Food habits of the hoary bat in an agricultural landscape. J. Minn. Acad. Sci. 2012, 75, 1–6. Available online: https://digitalcommons.morris.umn.edu/jmas/vol75/iss2/1 (accessed on 15 July 2022).
- Barclay, R.M.R. Long- versus short-range foraging strategies of hoary (Lasiurus cinereus) and silver-haired (Lasionycteris noctivagans) bats and the consequences for prey selection. Can. J. Zool. 1985, 63, 2507–2515. [Google Scholar] [CrossRef]
- Rolseth, S.L.; Koehler, C.E.; Barclay, R.M.R. Differences in the diets of juvenile and adult hoary bats, Lasiurus cinereus. J. Mammal. 1994, 75, 394–398. [Google Scholar] [CrossRef]
- Brigham, R.M.; Saunders, M.B. The diet of big brown bats (Eptesicus fuscus) in relation to insect availability in southern Alberta, Canada. Northwest Sci. 1990, 64, 7–10. [Google Scholar]
- Hamilton, I.M.; Barclay, R.M.R. Diets of juvenile, yearling, and adult big brown bats (Eptesicus fuscus) in southeastern Alberta. J. Mammal. 1998, 79, 764–771. [Google Scholar] [CrossRef][Green Version]
- Mensing-Solick, Y.R.; Barclay, R.M.R. The effect of canine tooth wear on the diet of big brown bats (Eptesicus fuscus). Acta Chiropter 2003, 5, 91–95. [Google Scholar] [CrossRef]
- Long, B.L.; Kurta, A. Activity and diet of bats in conventional versus organic apple orchards in southern Michigan. Can. Field Nat. 2014, 128, 158–164. [Google Scholar] [CrossRef][Green Version]
- Dixon, W.N.; Fasulo, T.R. Tarnished Plant Bug—Lygus lineolaris (Palisot de Beauvois) (Insecta: Hemiptera: Miridae); Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA; University of Florida Department of Entomology and Nematology: Gainesville, FL, USA, 2017; Available online: https://entnemdept.ufl.edu/creatures/trees/tarnished_plant_bug.htm (accessed on 20 October 2022).
- Mann, R.S.; Stelinski, L.L. Spotted-Wing Drosophila—Drospophia suzukii (Matsumura) (Insecta: Diptera: Drosophilidae); University of Florida Department of Entomology and Nematology: Lake Alfred, FL, USA, 2017; Available online: https://entnemdept.ufl.edu/creatures/fruit/flies/drosophila_suzukii.htm (accessed on 20 October 2022).
- Michaud, J.P. Soybean Insects; Kansas State University Department of Entomology: Manhattan, KS, USA, 2013; Available online: https://entomology.k-state.edu/extension/crop-protection/soybeans/webworms.html (accessed on 20 August 2025).
- Jubenville, J.; Szendrei, Z. Ecology of the celery leaftier, Udea Rubigalis, aphids, and natural enemies in Michigan celery fields. In Proceedings of the Entomological Society of America Annual Meeting 2013, Austin, TX, USA, 12 November 2013. [Google Scholar]
- Craig, C.H. The alfalfa plant bug, Adelphocoris lineolatus (Goeze) in Northern Saskatchewan. Can. Entomol. 1963, 95, 6–13. [Google Scholar] [CrossRef]
- Freeman, P.W. Correspondence of food habits and morphology in insectivorous bats. J. Mammal. 1981, 62, 166–173. [Google Scholar] [CrossRef]
- Ober, H.K.; Hayes, J.P. Prey selection by bats in forests of western Oregon. J. Mammal. 2008, 89, 1191–1200. [Google Scholar] [CrossRef]
- Black, H.L. A north temperate bat community: Structure and prey populations. J. Mammal. 1974, 55, 138–157. [Google Scholar] [CrossRef]
- Carter, T.C.; Menzel, M.A.; Owen, S.F.; Edwards, J.W.; Menzel, J.M.; Ford, W.M. Food habits of seven species of bats in the Allegheny Plateau and ridge and valley of West Virginia. Northeast. Nat. 2003, 10, 83–88. [Google Scholar] [CrossRef]
- Whitaker, J.O.; Barnard, S.M. Food of big brown bats (Eptesicus fuscus) from a colony at Morrow, Georgia. Southeast. Nat. 2005, 4, 111–118. [Google Scholar] [CrossRef]
- Clare, E.L.; Symondson, W.O.C.; Fenton, M.B. An inordinate fondness for beetles? Variation in seasonal dietary preferences of night-roosting big brown bats (Eptesicus fuscus). Mol. Ecol. 2014, 23, 3633–3647. [Google Scholar] [CrossRef]
- Winters-Michaud, C.P.; Haro, A.; Callahan, S.; Bigelow, D. Major Uses of Land in the United States, 2017; Report No. EIB-275; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2024. [CrossRef]
- USDA NASS. 2017 Census of Agriculture Report: United States Summary and State Data. AC-17-A-51. Geographic Area Series, Part 51; United States Department of Agriculture National Agricultural Statistics Service: Washington, DC, USA, 2019. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017 (accessed on 24 October 2022).
- Wang, S.; Di Tommaso, S.; Deines, J.M.; Lobell, D.B. Mapping twenty years of corn and soybean across the US Midwest using the Landsat archive. Sci. Data 2020, 7, 307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bullington, L.S.; Seidensticker, M.T.; Schwab, N.; Ramsey, P.W.; Stone, K. Do the evolutionary interactions between moths and bats promote niche partitioning between bats and birds? Ecol. Evol. 2021, 11, 17160–17178. [Google Scholar] [CrossRef] [PubMed]
- Buchler, E.R. Food transit time in Myotis lucifugus Chiroptera: Vespertilionidae). J. Mammal. 1975, 56, 252–255. [Google Scholar] [CrossRef]
- Wilson, L.T.; Barnett, W.W. Degree-days: An aid in crop and pest management. Calif. Agr. 1983, 37, 4–7. [Google Scholar]
- Huff, F.A. Relation between leafhopper influxes and synoptic weather conditions. J. Appl. Meteorol. Clim. 1963, 2, 39–43. [Google Scholar] [CrossRef][Green Version]
- Park, Y.; Tollefson, J.J. Spatial prediction of corn rootworm (Coleoptera: Chrysomelidae) adult emergence in Iowa cornfields. J. Econ. Entomol. 2005, 98, 121–128. [Google Scholar] [CrossRef]
- Tiede, J.; Diepenbruck, M.; Gadau, J.; Wemheuer, B.; Daniel, R.; Scherber, C. Seasonal variation in the diet of the serotine bat (Eptesicus serotinus): A high resolution analysis using DNA metabarcoding. Basic. Appl. Ecol. 2020, 49, 1–12. [Google Scholar] [CrossRef]
- Aizpurua, O.; Budinski, I.; Georgiakakis, P.; Gopalakrishnan, S.; Ibañez, C.; Mata, V.; Rebelo, H.; Russo, D.; Szodoray-Parádi, F.; Zhelyazkova, V.; et al. Agriculture shapes the trophic niche of a bat preying on multiple pest arthropods across Europe: Evidence from DNA metabarcoding. Mol. Ecol. 2018, 27, 815–825. [Google Scholar] [CrossRef]
- Aguiar, L.M.S.; Bueno-Rocha, I.D.; Oliveira, G.; Pires, E.S.; Vasconcelos, S.; Nunes, G.L.; Frizzas, M.R.; Togni, P.H.B. Going out for Dinner—The Consumption of Agriculture Pests by Bats in Urban Areas. PLoS ONE 2021, 16, e0258066. [Google Scholar] [CrossRef]
- Kolkert, H.; Andrew, R.; Smith, R.; Rader, R.; Reid, N. Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol. Evol. 2020, 10, 371–388. [Google Scholar] [CrossRef]
- Maslo, B.; Valentin, R.; Leu, K.; Kerwin, K.; Hamilton, G.C.; Bevan, A.; Fefferman, N.H.; Fonseca, D.M. Chirosurveillance: The use of native bats to detect invasive agricultural pests. PLoS ONE 2017, 12, e0173321. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.; Voigt, C.C. Diet analysis of bats killed at wind turbines suggests large-scale losses of trophic interactions. Conserv. Sci. Practice 2022, 4, e12744. [Google Scholar] [CrossRef]
- Satterfield, D.A.; Sillett, T.S.; Chapman, J.W.; Altizer, S.; Marra, P.P. Seasonal insect migrations: Massive, influential, and overlooked. Front. Ecol. Environ. 2020, 18, 335–344. [Google Scholar] [CrossRef]
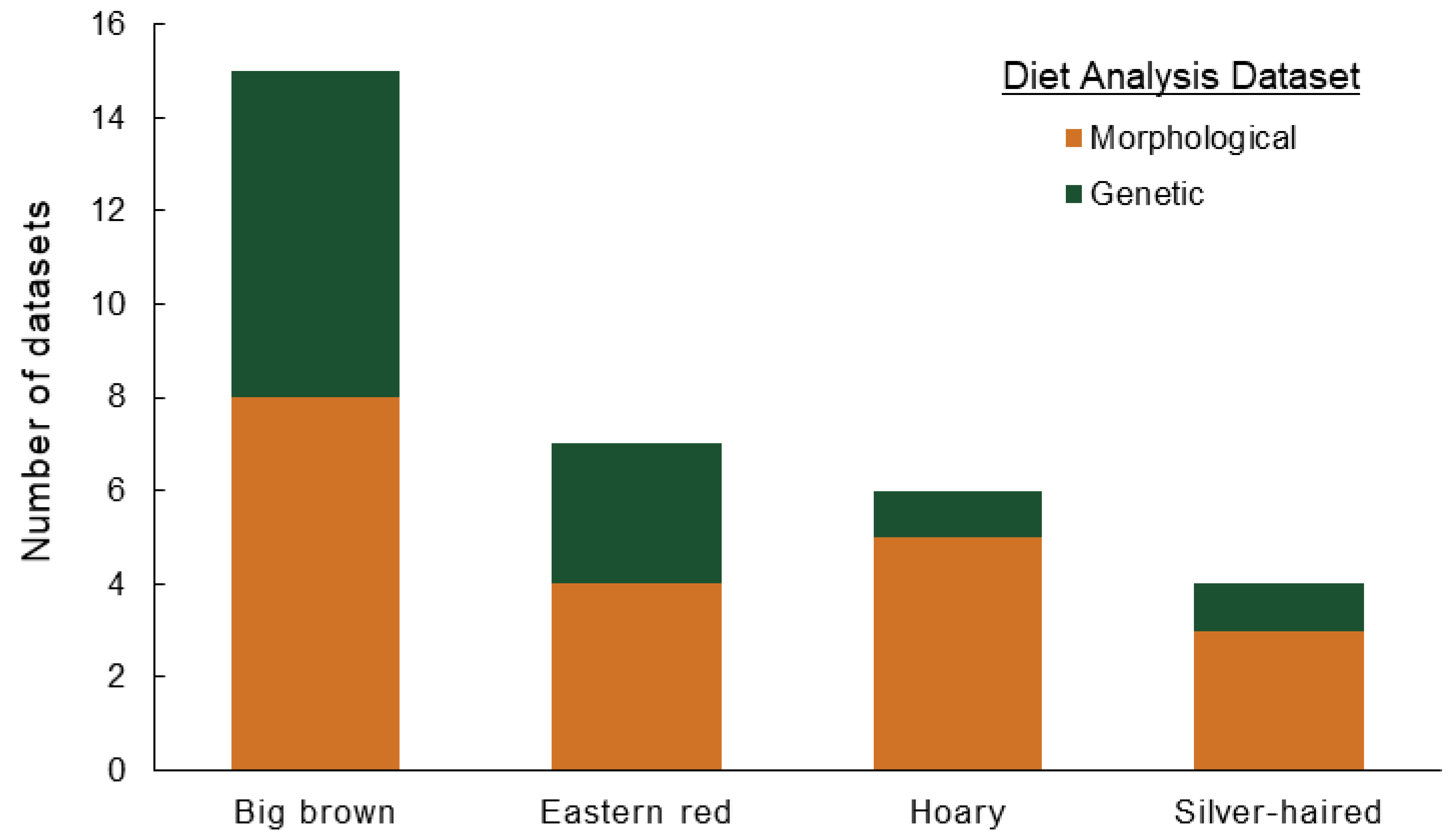
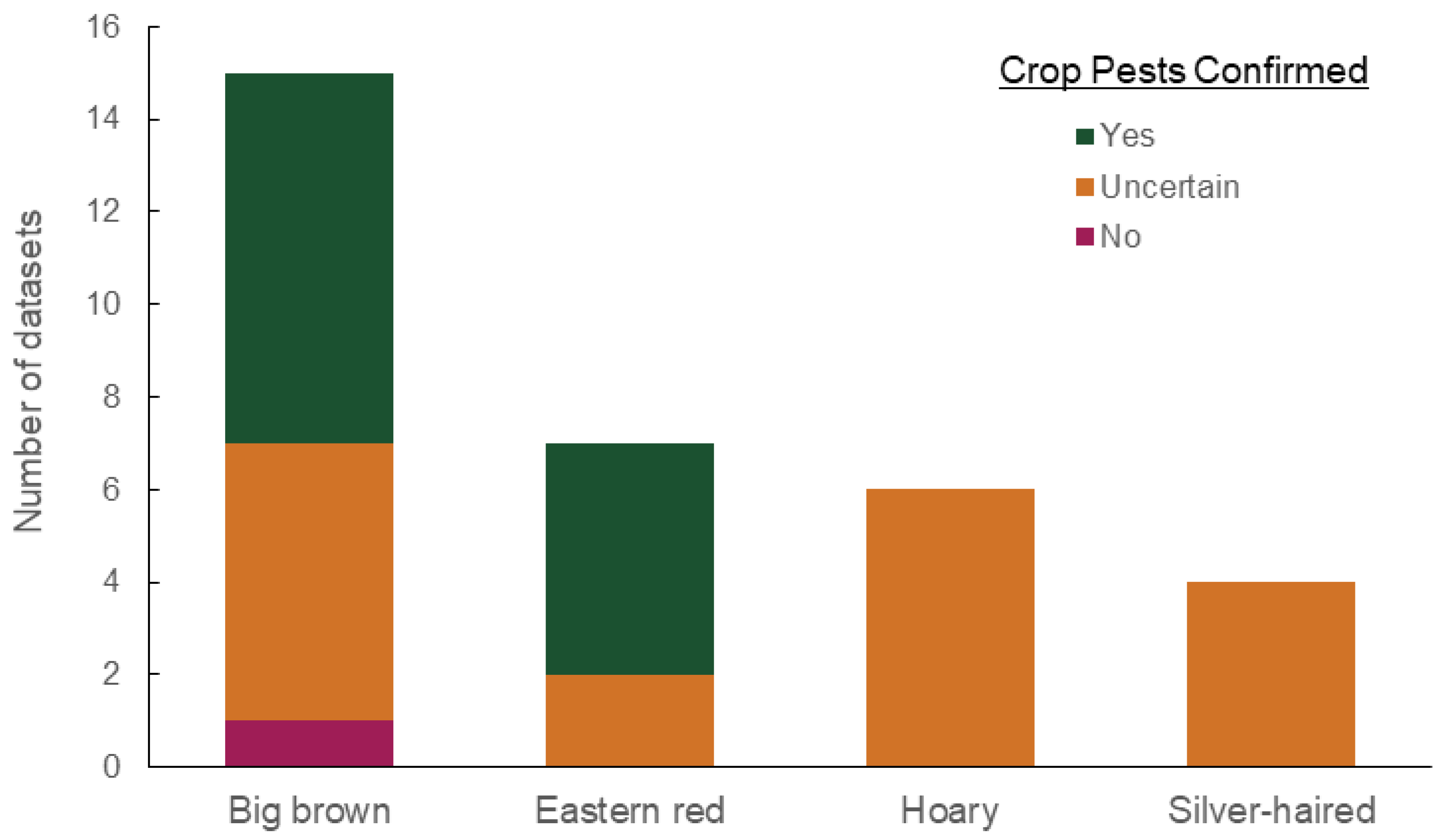
| Analysis Method | Order | Family | Genus | Species | Common Name | Count of Datasets | Representative Host Plant |
|---|---|---|---|---|---|---|---|
| Morphological | |||||||
| Coleoptera | Chrysomelidae | Diabrotica | undecimpunctata | spotted cucumber beetle | 3 a,b,c | soybeans 1 | |
| Scarabaeidae | Phyllophaga | May beetles/June beetles | 1 c | corn 2 | |||
| Homoptera | Cicadellidae | leafhoppers | 3 a,b,d | soybeans/alfalfa 1 | |||
| Curculionidae | weevils | 1 a | soybeans/alfalfa 1 | ||||
| Hemiptera | Pentatomindae | Acrosternum | hilare | green stink bug | 2 b,c | soybeans 3 | |
| Genetic | |||||||
| Coleoptera | Scarabaeidae | Phyllophaga | Implicita | June bug | 1 f | corn 2 | |
| Scarabaeidae | Phyllophaga | anxia | cranberry white grub | 1 g | corn 2 | ||
| Chrysomelidae | Diabrotica | undecimpunctata | spotted cucumber beetle | 2 f,h | soybeans 1 | ||
| Elateridae | Melanotus | similis | wireworms | 1 h,i | corn 2 | ||
| Elateridae | Hemicrepidius | memnonius | click beetles | 1 e i | corn 2 | ||
| Homoptera | Cicadellidae | leafhoppers | 3 g,h | soybeans, alfalfa 1 | |||
| Hemiptera | Pentatomindae | Acrosternum | hilare | green stink bug | 1 h | soybeans, crops 3 | |
| Miridae | Lygus | lineolaris | tarnished plant bug | 2 g,h | soybeans 4 | ||
| Lepidoptera | Tortricidae | multiple | 1 g,h,i | alfalfa, corn 1 | |||
| Noctuidae | multiple | 3 h,i | alfalfa, corn 1 | ||||
| Noctuidae | Agrotis | ipsilon | black cutworm | 1 g | corn 5 | ||
| Order | Family | Genus | Species | Common Name |
|---|---|---|---|---|
| Coleoptera | Carabidae | Bradycellus | verbasci | ground beetles 1 |
| Carabidae | Bradycellus | ground beetles 1 | ||
| Carabidae | Harpalus | compar | ground beetles 1,2 | |
| Carabidae | Harpalus | pensylvanicus | ground beetles 1,2,3 | |
| Carabidae | Harpalus | ground beetles 1,3 | ||
| Carabidae | Notiobia | terminata | ground beetles 1,2,3 | |
| Carabidae | Ophonus | ground beetles 1 | ||
| Carabidae | Pterostichus | melanarus | ground beetles 1 | |
| Cerambycidae | Saperda | tridenta | elm borer 1 | |
| Cerambycidae | Saperda | longhorn beetle 1 | ||
| Nitidulidae | Epuraea | corticina | sap-feeding beetle 1 | |
| Diptera | Culicidae | Aedes | excrucians | Mosquito 1 |
| Culicidae | Aedes | trivittatus | floodwater mosquito 1 | |
| Culicidae | Aedes | vexans | inland floodwater mosquito 1 | |
| Tipulidae | Nephratoma | Cranefly 1 | ||
| Drosophilidae | Drosophila | suzukii | spotted wing drosophila 4 | |
| Ephemeroptera | Isonychiidae | Isonychia | arida | Mayfly 1 |
| Analysis Method | Order | Family | Genus | Species | Common Name | Count of Datasets | Representative Host Plant |
|---|---|---|---|---|---|---|---|
| Morphological | |||||||
| Homoptera | Cicadellidae | leafhoppers | 2 a,b,c | soybeans, alfalfa 1 | |||
| Curculionidae | weevils | 1 a,c | soybeans/alfalfa 1 | ||||
| Delphacidae | plant hoppers | 1 a | rice 1 | ||||
| Coleoptera | Chrysomelidae | Diabrotica | undecimpunctata | spotted cucumber beetle | 2 a,d | soybeans 1 | |
| Hemiptera | Pentatomidae | stink bugs | 1 a | soybeans 1 | |||
| Genetic | |||||||
| Lepidoptera | Tortricidae | multiple | 1 e | alfalfa, corn 1 | |||
| Noctuidae | multiple | 1 e | alfalfa, corn 1 | ||||
| Noctuidae | Spodoptera | ornithogalli | yellow-striped armyworm | 1 e | soybean 1 | ||
| Noctuidae | Helicoverpa | zea | corn earworm | 1 e | soybean, corn 1,2 | ||
| Erebidae | Hypena | scabra | green cloverworm black snout moth | 1 e | soybean, alfalfa 3,4 | ||
| Crambidae | Achyra | rantalis | garden webworm | 1 e | soybean, alfalfa 5 | ||
| Crambidae | Udea | rubigalis | celery leaftier | 1 e | celery 6 | ||
| Coleoptera | Elateridae | Melanotus | OPC-2015 | click beetle | 1 e | corn 1 | |
| Hemiptera | Miridae | Lygus | lineolarus | tarnished plant bug | 1 e | soybeans 7 | |
| Miridae | Adelphocoris | lineolatus | alfalfa plant bug | 1 e | alfalfa 8 | ||
| Order | Family | Genus | Species | Common Name |
|---|---|---|---|---|
| Hemiptera | Rhopalidae | Harmostes | reflexulus | |
| Lepidoptera | Saturniidae | Syssphinx | bicolor | honey locust moth |
| Diptera | Culicinae | Aedes | vexans | inland floodwater mosquito |
| Culicinae | Aedes | trivittatus | floodwater mosquito |
| Analysis Method | Order | Family | Common Name | Count of Datasets |
|---|---|---|---|---|
| Morphological | ||||
| Neuroptera | net-winged insects | 4 a,b,c,d | ||
| Coleoptera | beetles | 4 a,b,c,d | ||
| Diptera | flies | 2 a,b | ||
| Muscoidea (superfamily) | Muscoid flies | 1 c | ||
| Culicidae | mosquitos | 1 c | ||
| Tipulidae | crane flies | 1 c | ||
| Chironomidae | non-biting midges | 2 c,d | ||
| Lepidoptera | moths | 4 a,b,c,d | ||
| Hemiptera | Corixidae | water boatmen | 2 a,c | |
| Homoptera | sucking insects | 3 a,b,c | ||
| Odonata | dragonflies | 2 c,d | ||
| Trichoptera | caddisflies | 1 a,e | ||
| Genetic | ||||
| Coleoptera | beetles | 1 e | ||
| Lepidoptera | moth | 1 e | ||
| Analysis Method | Order | Family | Common Name | Count of Datasets |
|---|---|---|---|---|
| Morphological | ||||
| Neuroptera | net-winged insects | 2 a,b | ||
| Coleoptera | beetles | 2 a,b | ||
| Diptera | flies | 1 b | ||
| Chironomidae | non-biting midges | 1 a | ||
| Muscoidea (superfamily) | Muscoid flies | 1 a | ||
| Tipulidae | crane flies | 1 a | ||
| Culicidae | mosquitos | 1 a | ||
| Hemiptera | true bugs | 2 a,b | ||
| Lepidoptera | moths | 2 a,b | ||
| Homoptera | leafhoppers | 2 a,b | ||
| Tricoptera | caddisflies | 2 a,b | ||
| Genetic | ||||
| Coleoptera | beetles | 1 c | ||
| Diptera | flies | 1 c | ||
| Ephemeroptera | mayflies | 1 c | ||
| Hemiptera | true bugs | 1 c | ||
| Hymenoptera | ants/wasps | 1 c | ||
| Isopoda | isopods | 1 c | ||
| Lepidoptera | moths | 1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hale, A.M.; Foo, C.; Lloyd, J.; Stucker, J. Systematic Review of Crop Pests in the Diets of Four Bat Species Found as Wind Turbine Fatalities. Diversity 2025, 17, 590. https://doi.org/10.3390/d17080590
Hale AM, Foo C, Lloyd J, Stucker J. Systematic Review of Crop Pests in the Diets of Four Bat Species Found as Wind Turbine Fatalities. Diversity. 2025; 17(8):590. https://doi.org/10.3390/d17080590
Chicago/Turabian StyleHale, Amanda M., Cecily Foo, John Lloyd, and Jennifer Stucker. 2025. "Systematic Review of Crop Pests in the Diets of Four Bat Species Found as Wind Turbine Fatalities" Diversity 17, no. 8: 590. https://doi.org/10.3390/d17080590
APA StyleHale, A. M., Foo, C., Lloyd, J., & Stucker, J. (2025). Systematic Review of Crop Pests in the Diets of Four Bat Species Found as Wind Turbine Fatalities. Diversity, 17(8), 590. https://doi.org/10.3390/d17080590







