Road Density Shapes Soil Fungal Community Composition in Urban Road Green Space
Abstract
1. Introduction
2. Materials and Methods
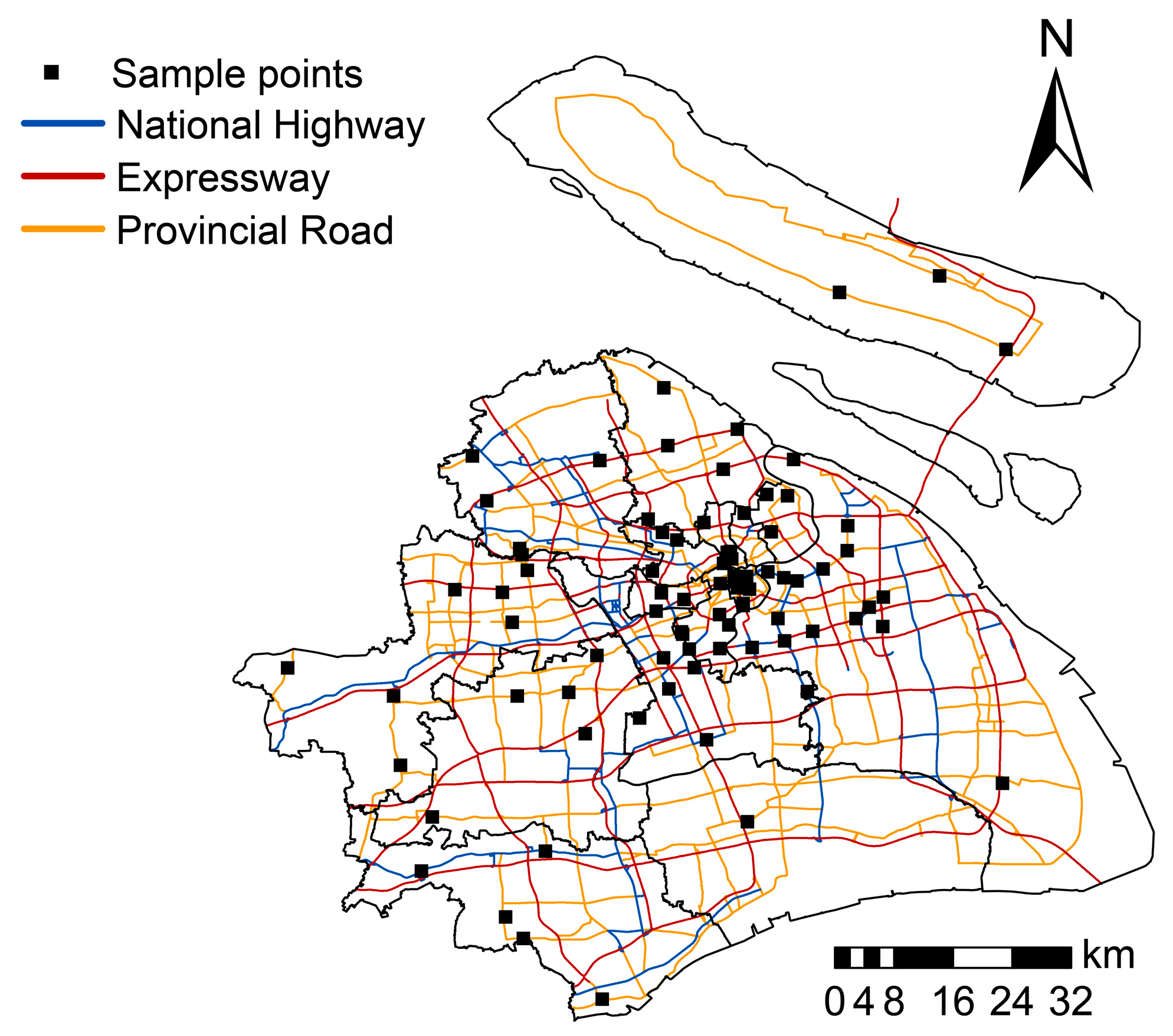
2.1. Study Area and Sample Collection
2.2. Analysis of Edaphic Factor
2.3. DNA Extraction and Illumina Sequencing of Fungal ITS Region
2.4. Statistical Analyses
3. Results
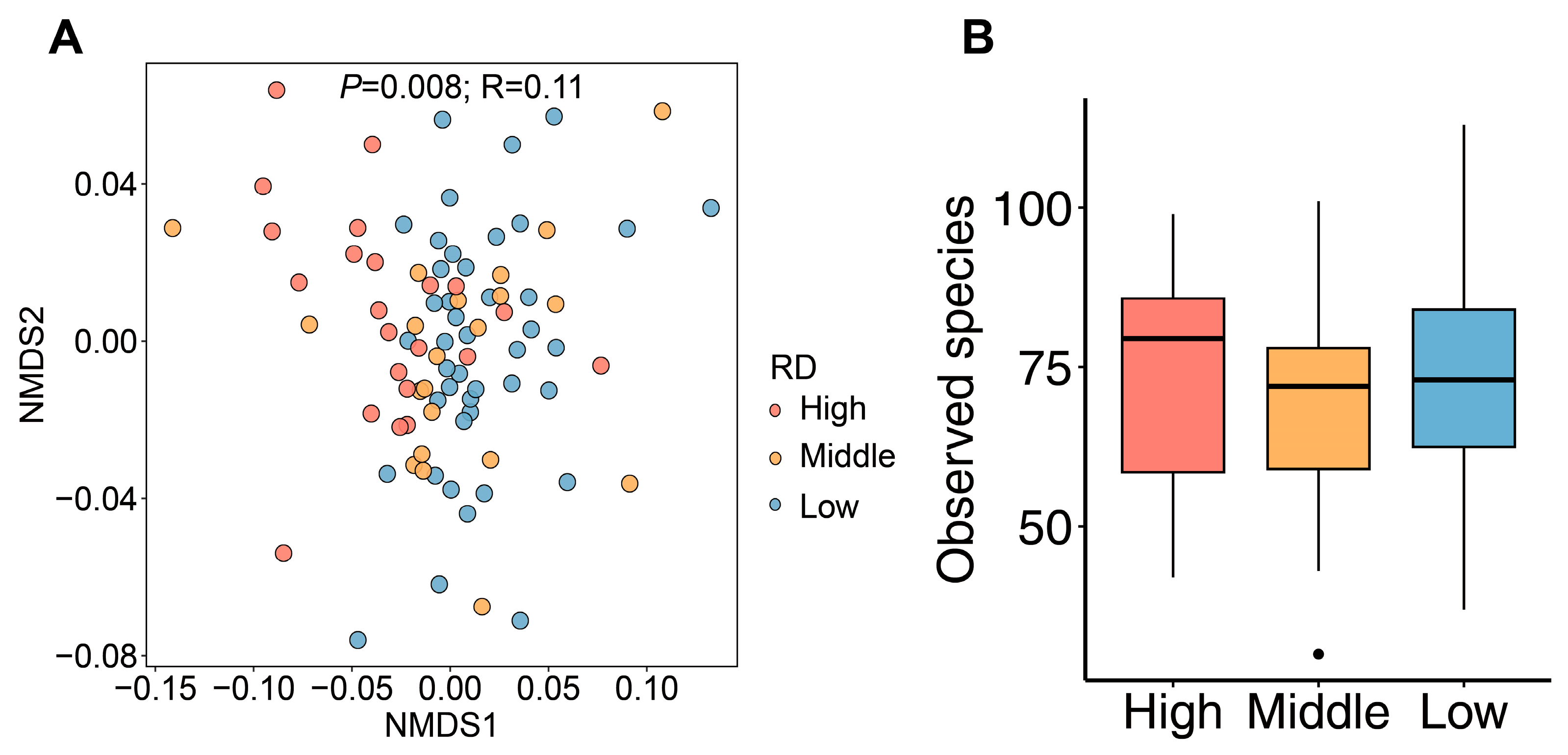
3.1. Diversity Patterns of Soil Fungal Communities in Road Green Spaces
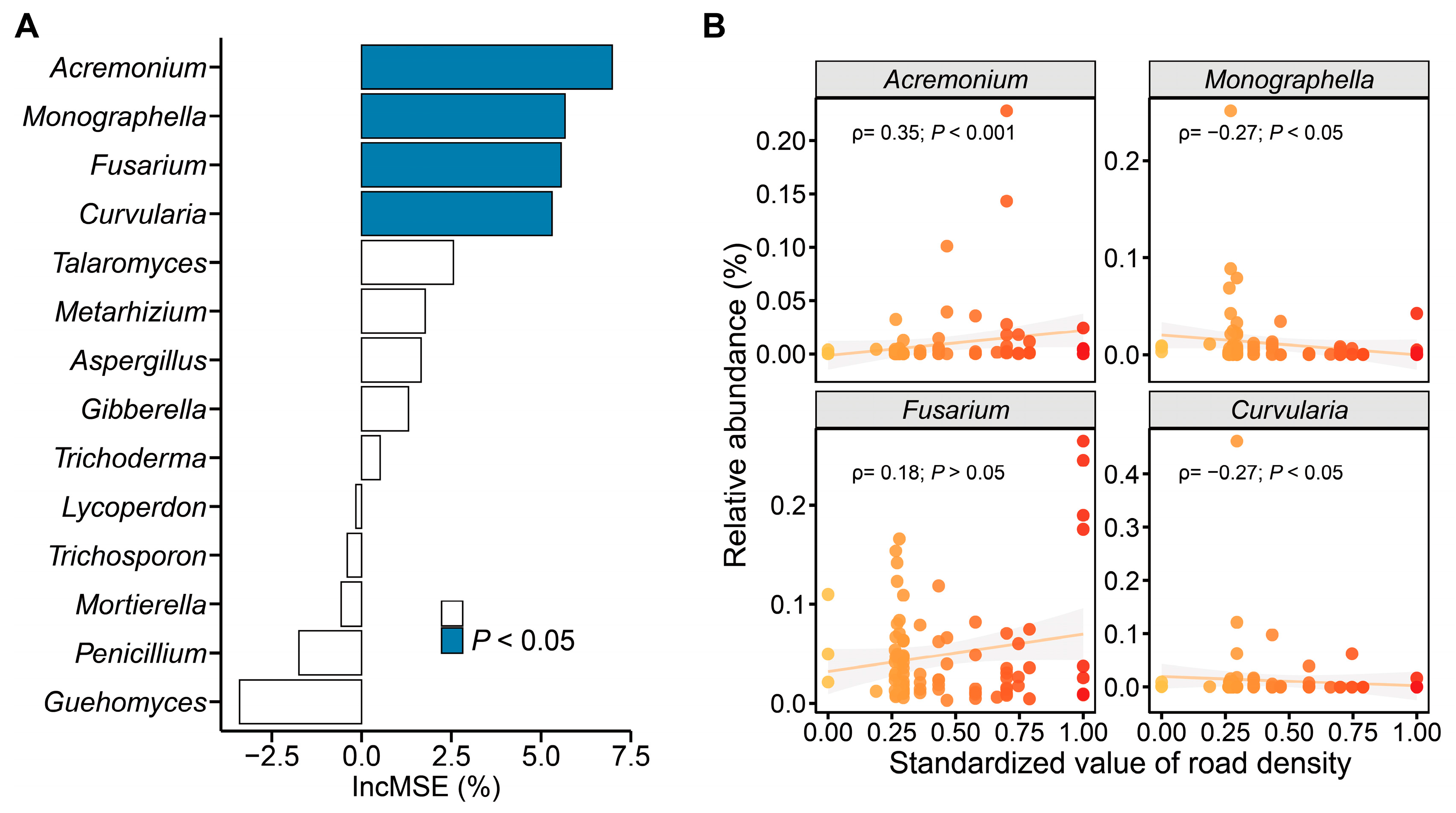
3.2. The Factors That Impact the Fungal Communities in Urban Road Green Spaces
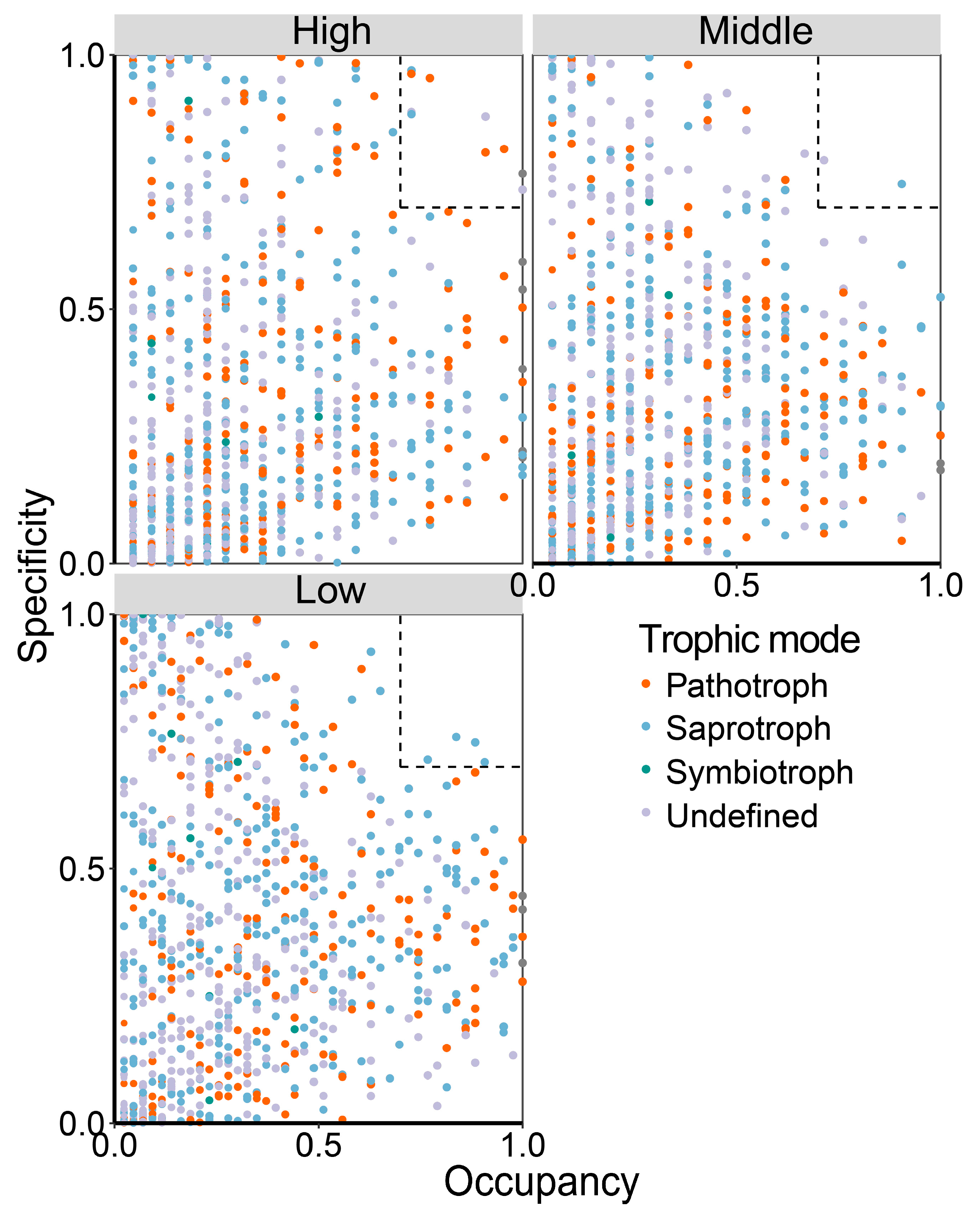
3.3. Specialist Species in Urban Road Green Spaces
4. Discussion
4.1. Direct and Indirect Effect of Road Density on Soil Fungal Community Composition in Road Green Spaces
4.2. Opportunistic Pathogens in Urban Road Green Spaces
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Jia, L.; Daryanto, S.; Chen, L.; Liu, Y. Source–Sink Landscape. In Encyclopedia of Ecology, 2nd ed.; Fath, B., Ed.; Elsevier: Oxford, UK, 2019; pp. 467–473. [Google Scholar]
- Ritchie, H.; Samborska, V.; Roser, M. Urbanization-Our World in Data. 2024. Available online: https://ourworldindata.org (accessed on 10 January 2025).
- Peng, J.; Tian, L.; Liu, Y.X.; Zhao, M.Y.; Hu, Y.N.; Wu, J.S. Ecosystem services response to urbanization in metropolitan areas: Thresholds identification. Sci. Total Environ. 2017, 607, 706–714. [Google Scholar] [CrossRef]
- Hou, X.J.; Feng, L.; Tang, J.; Song, X.P.; Liu, J.G.; Zhang, Y.L.; Wang, J.J.; Xu, Y.; Dai, Y.H.; Zheng, Y.; et al. Anthropogenic transformation of Yangtze Plain freshwater lakes: Patterns, drivers and impacts. Remote Sens. Environ. 2020, 248, 111998. [Google Scholar] [CrossRef]
- Liu, X.P.; Huang, Y.H.; Xu, X.C.; Li, X.C.; Li, X.; Ciais, P.; Lin, P.R.; Gong, K.; Ziegler, A.D.; Chen, A.N.; et al. High-spatiotemporal-resolution mapping of global urban change from 1985 to 2015. Nat. Sustain. 2020, 3, 564–570. [Google Scholar] [CrossRef]
- McDonald, R.I.; Mansur, A.V.; Ascensao, F.; Colbert, M.l.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Guneralp, B.; Haase, D.; Hamann, M.; et al. Research gaps in knowledge of the impact of urban growth on biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef]
- Hazelton, P.; Murphy, B.W. Interpreting Soil Test Results: What Do All the Numbers Mean? 2nd ed.; CSIRO Publishing: Clayton, MO, USA, 2006. [Google Scholar]
- Beazley, K.F.; Snaith, T.V.; MacKinnon, F.; Colville, D. Road density and potential impacts on wildlife species such as American moose in mainland Nova Scotia. Proc. N. S. Inst. Sci. 2004, 42, 339–357. [Google Scholar] [CrossRef][Green Version]
- Ward, A.I.; Dendy, J.; Cowan, D.P. Mitigating impacts of roads on wildlife: An agenda for the conservation of priority European protected species in Great Britain. Eur. J. Wildl. Res. 2015, 61, 199–211. [Google Scholar] [CrossRef]
- Laforge, A.; Barbaro, L.; Bas, Y.; Calatayud, F.; Ladet, S.; Sirami, C.; Archaux, F. Road density and forest fragmentation shape bat communities in temperate mosaic landscapes. Landsc. Urban Plan. 2022, 221, 104353. [Google Scholar] [CrossRef]
- Bennett, V.J. Effects of Road Density and Pattern on the Conservation of Species and Biodiversity. Curr. Landsc. Ecol. Rep. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Laurance, W.F.; Clements, G.R.; Sloan, S.; O’Connell, C.S.; Mueller, N.D.; Goosem, M.; Venter, O.; Edwards, D.P.; Phalan, B.; Balmford, A.; et al. A global strategy for road building. Nature 2014, 513, 229–232. [Google Scholar] [CrossRef]
- Grilo, C.; Koroleva, E.; Andrasik, R.; Bil, M.; Gonzalez-Suarez, M. Roadkill risk and population vulnerability in European birds and mammals. Front. Ecol. Environ. 2020, 18, 323–328. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Delgado, J.D. A forecasting map of avian roadkill-risk in Europe: A tool to identify potential hotspots. Biol. Conserv. 2020, 249, 108729. [Google Scholar] [CrossRef]
- Tian, Y.; Jim, C.Y.; Wang, H. Assessing the landscape and ecological quality of urban green spaces in a compact city. Landsc. Urban Plan. 2014, 121, 97–108. [Google Scholar] [CrossRef]
- Chiesura, A. The role of urban parks for the sustainable city. Landsc. Urban Plan. 2004, 68, 129–138. [Google Scholar] [CrossRef]
- Xu, X.; Duan, X.; Sun, H.; Sun, Q. Green Space Changes and Planning in the Capital Region of China. Environ. Manag. 2011, 47, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Chen, W.B.; He, W. Planning of Green Space Ecological Network in Urban Areas: An Example of Nanchang, China. Int. J. Environ. Res. Public Health 2015, 12, 12889–12904. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, T.; Zhang, S.; Zheng, W.; Di, L.; Guo, P. Spatial distribution of soil heavy metal in green land along a center citysuburb–satellite citygradient. Acta Sci. Circumstantiae 2018, 38, 3294–3301. [Google Scholar]
- Yang, L.; Xin, J.; Tian, R. Research Progress in the Mitigative Effects of Rhizosphere Microorganisms on Heavy Metal Stress in Plants and Their Mechanisms. Biotechnol. Bull. 2022, 38, 213–225. [Google Scholar]
- Sun, S.; Hoy, M.J.; Heitman, J. Fungal pathogens. Curr. Biol. 2020, 30, R1163–R1169. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.M.M.; Procopio, D.P.; Zamuner, C.K.; Nobrega, B.B.; Bettim, M.R.; de Rezende, G.; Lopes, P.M.; Pereira, A.B.D.; Bechara, E.J.H.; Oliveira, A.G.; et al. Fungal bioassays for environmental monitoring. Front. Bioeng. Biotechnol. 2022, 10, 954579. [Google Scholar] [CrossRef]
- Warnasuriya, S.D.; Udayanga, D.; Manamgoda, D.S.; Biles, C. Fungi as environmental bioindicators. Sci. Total Environ. 2023, 892, 164583. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Feijen, F.A.A.; Vos, R.A.; Nuytinck, J.; Merckx, V. Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification. Sci. Rep. 2018, 8, 10698. [Google Scholar] [CrossRef]
- García-Guzmán, G.; Heil, M. Life histories of hosts and pathogens predict patterns in tropical fungal plant diseases. New Phytol. 2014, 201, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Kohl, L.; Oehl, F.; van der Heijden, M.G.A. Agricultural practices indirectly influence plant productivity and ecosystem services through effects on soil biota. Ecol. Appl. 2014, 24, 1842–1853. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Delavaux, C.S.; Weigelt, P.; Dawson, W.; Duchicela, J.; Essl, F.; van Kleunen, M.; König, C.; Pergl, J.; Pysek, P.; Stein, A.; et al. Mycorrhizal fungi influence global plant biogeography. Nat. Ecol. Evol. 2019, 3, 424–429. [Google Scholar] [CrossRef]
- Cai, X.J.; Wu, Z.F.; Cheng, J. Using kernel density estimation to assess the spatial pattern of road density and its impact on landscape fragmentation. Int. J. Geogr. Inf. Sci. 2013, 27, 222–230. [Google Scholar] [CrossRef]
- Kabala, C.; Musztyfaga, E.; Galka, B.; Labunska, D.; Manczynska, P. Conversion of soil pH 1:2.5 KCl and 1:2.5 H2O to 1:5 H2O: Conclusions for Soil Management, Environmental Monitoring, and International Soil Databases. Pol. J. Environ. Stud. 2016, 25, 647–653. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter, in: Methods of Soil Analysis Part 2. Chem. Microb. Prop. 1982, 9, 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis, Part 2: Chemical and Microbial Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Shi, X.-M.; Liu, S.; Song, L.; Wu, C.-S.; Yang, B.; Lu, H.-Z.; Wang, X.; Zakari, S. Contamination and source-specific risk analysis of soil heavy metals in a typical coal industrial city, central China. Sci. Total Environ. 2022, 836, 155694. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal. Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.6-8. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 January 2025).
- Lahouar, A.; Ben Hadj Slama, J. Day-ahead load forecast using random forest and expert input selection. Energy Convers. Manag. 2015, 103, 1040–1051. [Google Scholar] [CrossRef]
- Sáez-Sandino, T.; Maestre, F.T.; Berdugo, M.; Gallardo, A.; Plaza, C.; García-Palacios, P.; Guirado, E.; Zhou, G.; Mueller, C.W.; Tedersoo, L.; et al. Increasing numbers of global change stressors reduce soil carbon worldwide. Nat. Clim. Change 2024, 14, 740–745. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approac. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Gweon, H.S.; Bowes, M.J.; Moorhouse, H.L.; Oliver, A.E.; Bailey, M.J.; Acreman, M.C.; Read, D.S. Contrasting community assembly processes structure lotic bacteria metacommunities along the river continuum. Environ. Microbiol. 2021, 23, 484–498. [Google Scholar] [CrossRef] [PubMed]
- R Foundation for Statistical Computing. R Core R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 January 2025).
- Guo, J.H.; Xin, Y.; Li, X.Y.; Sun, Y.M.; Hu, Y.; Wang, J.F. The Assembly Mechanisms of Arbuscular Mycorrhizal Fungi in Urban Green Spaces and Their Response to Environmental Factors. Diversity 2025, 17, 425. [Google Scholar] [CrossRef]
- Gucinski, H.; Ziemer, M.J.; Ziemer, R.R.; Brookes, M.H. Forest Roads: A Synthesis of Scientific Information; U.S. Forest Service Pacific Northwest Research Station General Technical Report PNW-GTR; DIANE Publishing: Collingdale, PA, USA, 2001; pp. 1–103. [Google Scholar]
- Drinnan, I.N. The search for fragmentation thresholds in a Southern Sydney Suburb. Biol. Conserv. 2005, 124, 339–349. [Google Scholar] [CrossRef]
- Edman, M.; Gustafsson, M.; Stenlid, J.; Ericson, L. Abundance and viability of fungal spores along a forestry gradient—Responses to habitat loss and isolation? Oikos 2004, 104, 35–42. [Google Scholar] [CrossRef]
- Ruhling, A.; Soderstrom, B. Changes in Fruitbody Production of Mycorrhizal and Litter Decomposing Macromycetes in Heavy-Metal Polluted Coniferous Forests in North Sweden. Water Air Soil Pollut. 1990, 49, 375–387. [Google Scholar] [CrossRef]
- Wang, S.S.; Hu, G.R.; Yan, Y.; Wang, S.; Yu, R.L.; Cui, J.Y. Source apportionment of metal elements in PM2.5 in a coastal city in Southeast China: Combined Pb-Sr-Nd isotopes with PMF method. Atmos. Environ. 2019, 198, 302–312. [Google Scholar] [CrossRef]
- Gao, P.P.; Xue, P.Y.; Dong, J.W.; Zhang, X.M.; Sun, H.X.; Geng, L.P.; Luo, S.X.; Zhao, J.J.; Liu, W.J. Contribution of PM2.5-Pb in atmospheric fallout to Pb accumulation in Chinese cabbage leaves via stomata. J. Hazard. Mater. 2021, 407, 124356. [Google Scholar] [CrossRef]
- Zarei, M.; Hempel, S.; Wubet, T.; Schaefer, T.; Savaghebi, G.; Jouzani, G.S.; Nekouei, M.K.; Buscot, F. Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environ. Pollut. 2010, 158, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Lemmel, F.; Maunoury-Danger, F.; Leyval, C.; Cebron, A. Altered fungal communities in contaminated soils from French industrial brownfields. J. Hazard. Mater. 2021, 406, 124296. [Google Scholar] [CrossRef] [PubMed]
- Newbound, M.; McCarthy, M.A.; Lebel, T. Fungi and the urban environment: A review. Landsc. Urban Plan. 2010, 96, 138–145. [Google Scholar] [CrossRef]
- Dighton, J.; Tuininga, A.R.; Gray, D.M.; Huskins, R.E.; Belton, T. Impacts of atmospheric deposition on New Jersey pine barrens forest soils and communities of ectomycorrhizae. For. Ecol. Manag. 2004, 201, 133–144. [Google Scholar] [CrossRef]
- Fakharian, A.; Dorudinia, A.; Alavi Darazam, I.; Mansouri, D.; Masjedi, M.R. Acremonium pneumonia: Case Report and Literature Review. Tanaffos 2015, 14, 156–160. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Lin, Y.; Chen, R.; Han, J.; Liu, Y. Road Density Shapes Soil Fungal Community Composition in Urban Road Green Space. Diversity 2025, 17, 539. https://doi.org/10.3390/d17080539
Luo S, Lin Y, Chen R, Han J, Liu Y. Road Density Shapes Soil Fungal Community Composition in Urban Road Green Space. Diversity. 2025; 17(8):539. https://doi.org/10.3390/d17080539
Chicago/Turabian StyleLuo, Shuhong, Yong Lin, Ruirui Chen, Jigang Han, and Yun Liu. 2025. "Road Density Shapes Soil Fungal Community Composition in Urban Road Green Space" Diversity 17, no. 8: 539. https://doi.org/10.3390/d17080539
APA StyleLuo, S., Lin, Y., Chen, R., Han, J., & Liu, Y. (2025). Road Density Shapes Soil Fungal Community Composition in Urban Road Green Space. Diversity, 17(8), 539. https://doi.org/10.3390/d17080539





