Distinct Patterns of Co-Evolution Among Protist Symbionts of Neoisoptera Termites
Abstract
1. Introduction
2. Materials and Methods
2.1. Termite Collections
2.2. Protist Observation and Sanger Seqeuencing
2.3. Hindgut Community 18S Amplicon Sequencing
2.4. Phylogenetic Analyses
3. Results
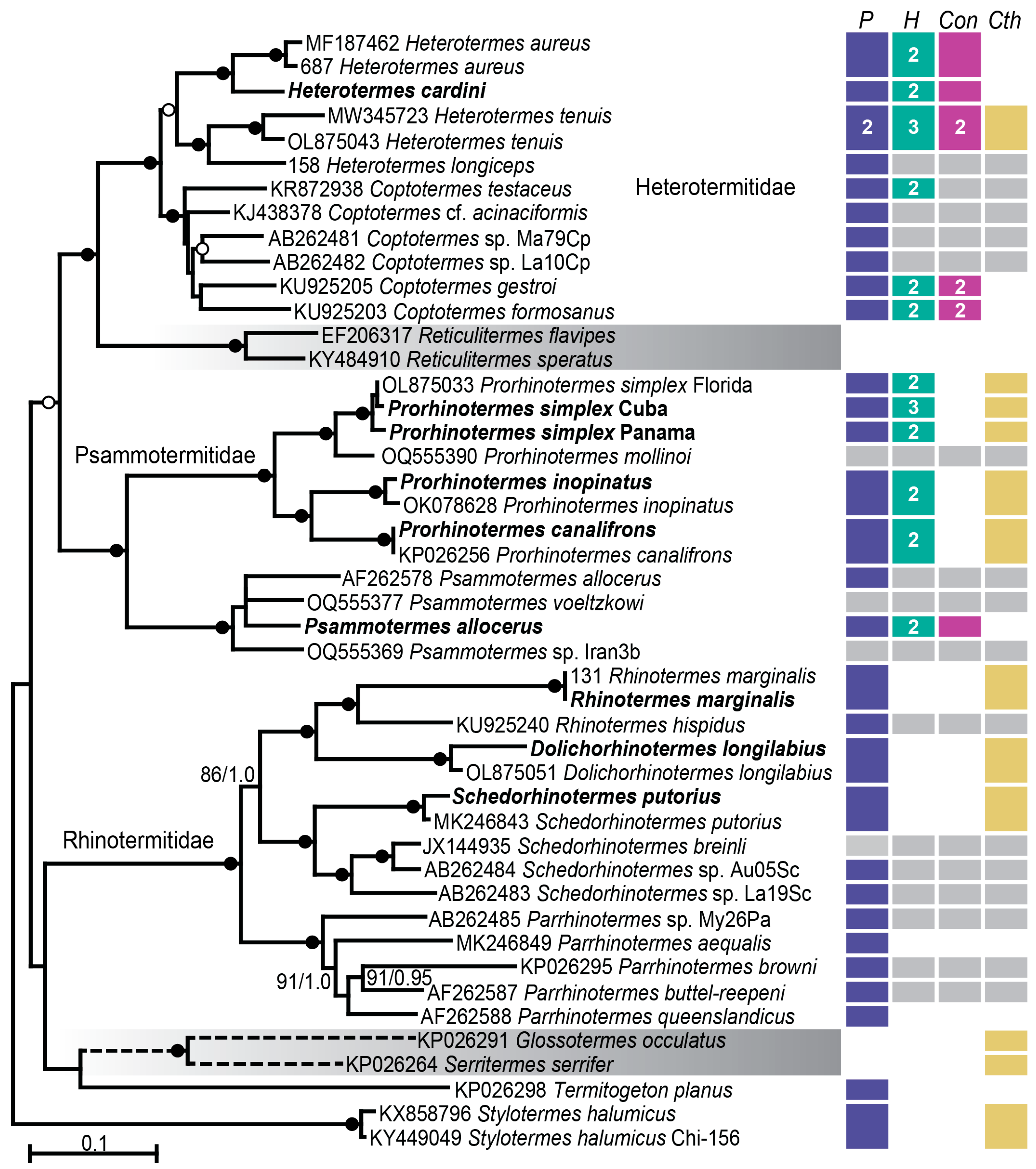
3.1. Termite Identification and Phylogeny
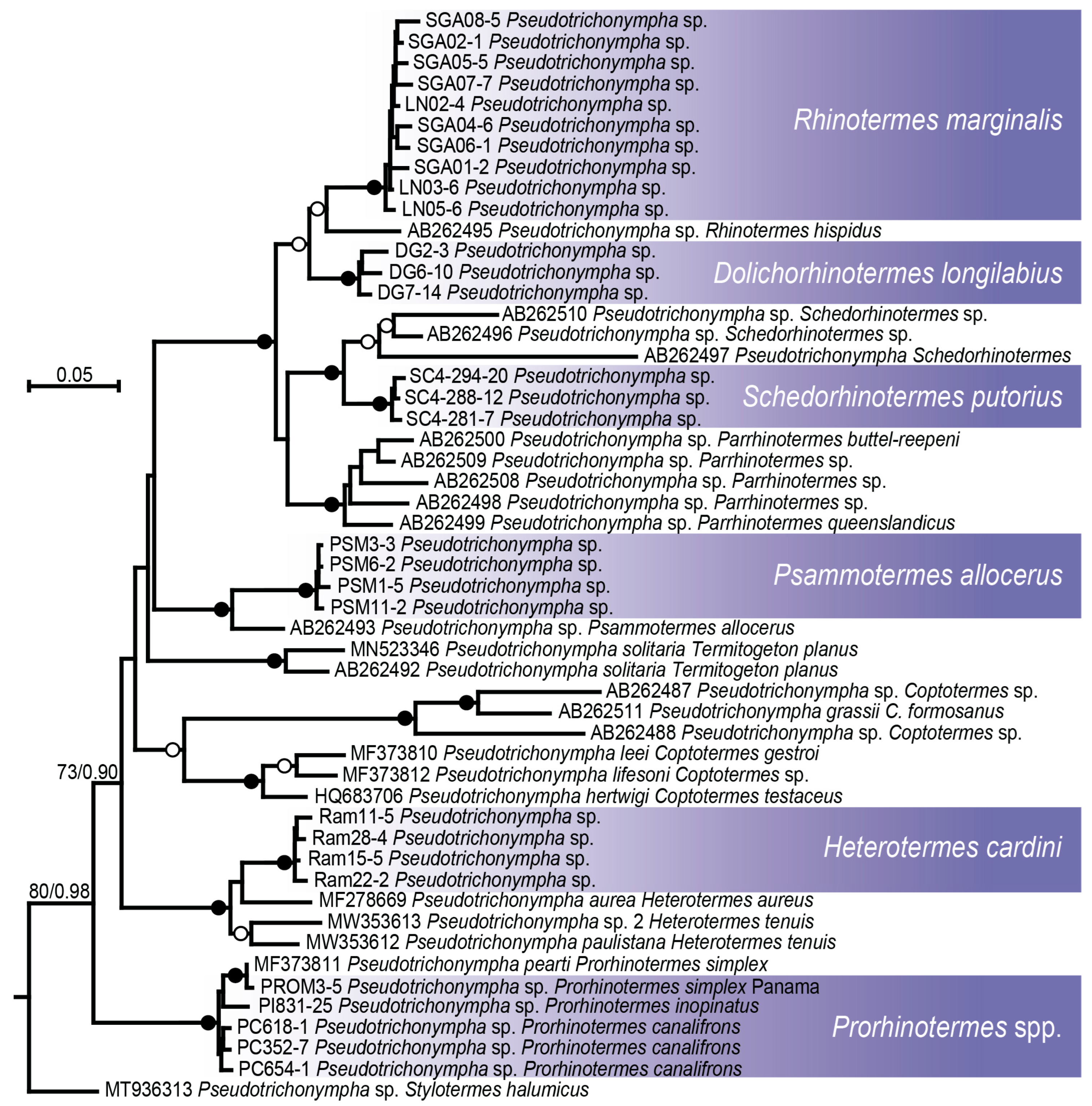
3.2. Protist Identification and Phylogeny
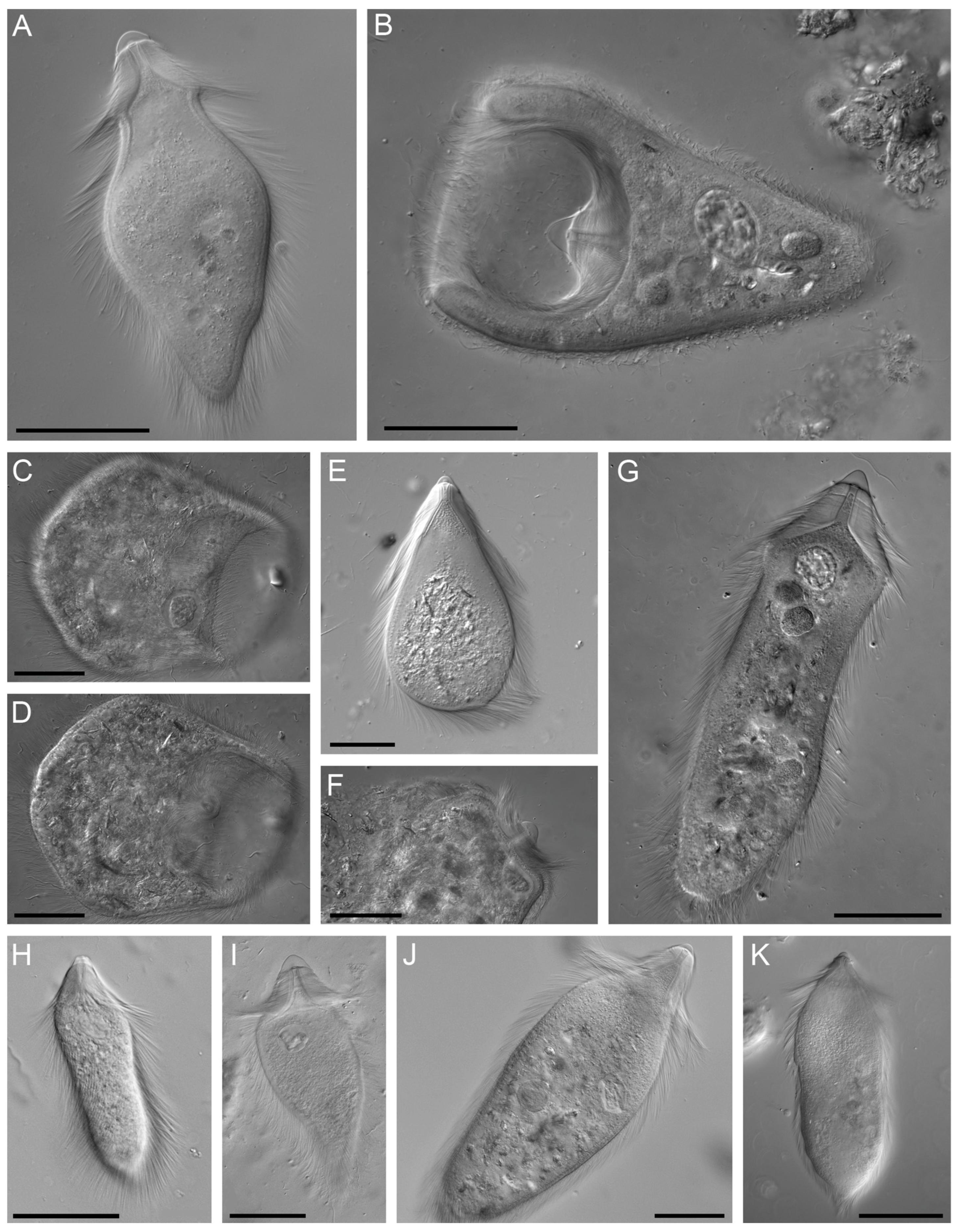
3.2.1. Pseudotrichonympha
3.2.2. Holomastigotoides
3.2.3. Cononympha
3.2.4. Cthulhu
4. Discussion
4.1. Symbiont Community Composition Across Neoisoptera
4.2. Distinct Evolutionary Trajectories Among Neoisoptera Symbionts
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellemans, S.; Roch, M.M.; Wang, M.; Romero Arias, J.; Aanen, D.K.; Bagnères, A.G.; Buček, A.; Carrijo, T.F.; Chouvenc, T.; Cuezzo, C.; et al. Genomic data provide insights into the classification of extant termites. Nat. Commun. 2024, 15, 6724. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, L.R. The effects of oxygenation and starvation on the symbiosis between the termite, Termopsis, and its intestinal flagellates. Biol. Bull. 1925, 48, 309–327. [Google Scholar] [CrossRef]
- Čepička, I.; Dolan, M.F.; Gile, G.H. Parabasalia. In Handbook of the Protist; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1175–1218. [Google Scholar]
- Ohkuma, M.; Noda, S.; Hongoh, Y.; Nalepa, C.A.; Inoue, T. Inheritance and diversification of symbiotic trichonymphid flagellates from a common ancestor of termites and the cockroach Cryptocercus. Proc. R. Soc. B Biol. Sci. 2009, 276, 239–245. [Google Scholar] [CrossRef]
- Bourguignon, T.; Lo, N.; Cameron, S.L.; Šobotník, J.; Hayashi, Y.; Shigenobu, S.; Watanabe, D.; Roisin, Y.; Miura, T.; Evans, T.A. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol. Biol. Evol. 2015, 32, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Chouvenc, T.; Šobotník, J.; Engel, M.S.; Bourguignon, T. Termite evolution: Mutualistic associations, key innovations, and the rise of Termitidae. Cell Mol. Life Sci. 2021, 78, 2749–2769. [Google Scholar] [CrossRef]
- Gile, G.H. Protist symbionts of termites: Diversity, distribution, and coevolution. Biol. Rev. 2024, 99, 622–652. [Google Scholar] [CrossRef]
- Kirby, H. Systematic differentiation and evolution of flagellates in termites. Rev. Soc. Mex. Hist. Nat. 1949, 10, 57–79. [Google Scholar]
- Nguyen, L.; Taerum, S.J.; Jasso-Selles, D.E.; Slamovits, C.H.; Silberman, J.D.; Gile, G.H. True molecular phylogenetic position of the cockroach gut commensal Lophomonas blattarum (Lophomonadida, Parabasalia). J. Eukaryot. Microbiol. 2023, 70, e12988. [Google Scholar] [CrossRef]
- Boscaro, V.; James, E.R.; Fiorito, R.; del Campo, J.; Scheffrahn, R.H.; Keeling, P.J. Updated classification of the phylum Parabasalia. J. Eukaryot. Microbiol. 2024, 71, e13035. [Google Scholar] [CrossRef]
- Hampl, V. Preaxostyla. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1139–1174. [Google Scholar]
- Nalepa, C.A. Origin of termite eusociality: Trophallaxis integrates the social, nutritional, and microbial environments. Ecol. Entomol. 2015, 40, 323–335. [Google Scholar] [CrossRef]
- Nalepa, C.A. What kills the hindgut flagellates of lower termites during the host molting cycle? Microorganisms 2017, 5, 82. [Google Scholar] [CrossRef]
- Tokuda, G.; Tsuboi, Y.; Kihara, K.; Saitou, S.; Moriya, S.; Lo, N.; Kikuchi, J. Metabolomic profiling of 13C-labelled cellulose digestion in a lower termite: Insights into gut symbiont function. Proc. R. Soc. B Biol. Sci. 2014, 281, 1987. [Google Scholar]
- Evangelista, D.A.; Wipfler, B.; Béthoux, O.; Donath, A.; Fujita, M.; Kohli, M.K.; Legendre, F.; Liu, S.; Machida, R.; Misof, B.; et al. An integrative phylogenomic approach illuminates the evolutionary history of cockroaches and termites (Blattodea). Proc. R. Soc. B Biol. Sci. 2019, 286, 20182076. [Google Scholar] [CrossRef]
- Buček, A.; Šobotník, J.; He, S.; Shi, M.; McMahon, D.P.; Holmes, E.C.; Roisin, Y.; Lo, N.; Bourguignon, Y. Evolution of termite symbiosis informed by transcriptome-based phylogenies. Curr. Biol. 2019, 29, 3728–3734.e4. [Google Scholar] [CrossRef]
- Honigberg, B.M. Protozoa associated with termites and their role in digestion. In Biology of Termites; Krishna, K., Wheesner, F.M., Eds.; Academic Press: New York, NY, USA, 1970; pp. 1–36. [Google Scholar]
- Harper, J.T.; Gile, G.H.; James, E.R.; Carpenter, K.J.; Keeling, P.J. The inadequacy of morphology for species and genus delineation in microbial eukaryotes: An example from the parabasalian termite symbiont Coronympha. PLoS ONE 2009, 4, e6577. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Kitade, O.; Inoue, T.; Kawai, M.; Kanuka, M.; Hiroshima, K.; Hongoh, Y.; Constantino, R.; Uys, V.; Zhong, J.; et al. Cospeciation in the triplex symbiosis of termite gut protists (Pseudotrichonympha spp.), their hosts, and their bacterial endosymbionts. Mol. Ecol. 2007, 16, 1257–1266. [Google Scholar] [CrossRef]
- Taerum, S.J.; De Martini, F.; Liebig, J.; Gile, G.H. Incomplete co-cladogenesis between Zootermopsis termites and their associated protists. Environ. Entomol. 2018, 47, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Kitade, O.; Matsumoto, T. Symbiotic protistan faunae of Reticulitermes (Isoptera: Rhinotermitidae) in the Japan archipelago. Sociobiology 1993, 23, 135–153. [Google Scholar]
- Radek, R.; Meuser, K.; Strassert, J.F.H.; Arslan, O.; Teßmer, A.; Šobotník, J.; Sillam-Dussès, D.; Nink, R.A.; Brune, A. Exclusive gut flagellates of Serritermitidae suggest a major transfaunation event in lower termites: Description of Heliconympha glossotermitis gen. nov. spec. nov. J. Eukaryot. Microbiol. 2018, 65, 77–92. [Google Scholar] [CrossRef]
- Kitade, O. Comparison of symbiotic flagellate faunae between termites and a wood-feeding cockroach of the genus Cryptocercus. Microbes Environ. 2004, 19, 215–220. [Google Scholar] [CrossRef]
- Coots, N.L.; Jasso-Selles, D.E.; Swichtenberg, K.L.; Aguilar, S.G.; Nguyen, L.A.; Sidles, P.G.; Woo, C.; Smith, S.M.; Bresee, B.J.; Abboud, A.A.; et al. The protist symbionts of Reticulitermes tibialis: Unexpected diversity enables a new taxonomic framework. Protist 2025, 176, 126087. [Google Scholar] [CrossRef]
- Song, Y.Q.; Zhang, D.; Chen, W.; Dang, X.X.; Yang, H. Phylogenetic identification of symbiotic protists of five Chinese Reticulitermes species indicates a cospeciation of gut microfauna with host termites. J. Eukaryot. Microbiol. 2021, 68, e12862. [Google Scholar] [CrossRef]
- Igai, K.; Kitade, O.; Fu, J.; Omata, K.; Yonezawa, T.; Ohkuma, M.; Hongoh, Y. Fine-scale genetic diversity and putative ecotypes of oxymonad protists coinhabiting the hindgut of Reticulitermes speratus. Mol. Ecol. 2022, 31, 1317–1331. [Google Scholar] [CrossRef]
- Radek, R.; Oztas, D.; Meuser, K.; Konrad, F.; Stiblík, P.; Sillam-Dussès, D.; Brune, A. Retractinympha glossotermitis gen. nov. sp. nov.—new insights into the phylogeny of termite gut flagellates (Parabasalia: Trichonymphida). Front. Ecol. Evol. 2023, 11, 1111484. [Google Scholar] [CrossRef]
- del Campo, J.; James, E.R.; Hirakawa, Y.; Fiorito, R.; Kolisko, M.; Irwin, N.A.T.; Mathur, V.; Boscaro, V.; Hehenberger, E.; Karnkowska, A.; et al. Pseudotrichonympha leei, Pseudotrichonympha lifesoni, and Pseudotrichonympha pearti, new species of parabasalian flagellates and the description of a rotating subcellular structure. Sci. Rep. 2017, 7, 16349. [Google Scholar] [CrossRef]
- Kitade, O.; Matsumoto, T. Characteristics of the symbiotic flagellate composition within the termite family Rhinotermitidae (Isoptera). Symbiosis 1998, 25, 271–278. [Google Scholar]
- James, E.R.; Okamoto, N.; Burki, F.; Scheffrahn, R.H.; Keeling, P.J. Cthulhu macrofasciculumque ng, n. sp and Cthylla microfasciculumque n. g., n. sp., a newly identified lineage of parabasalian termite symbionts. PLoS ONE 2013, 8, e58509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gile, G.H.; James, E.R.; Tai, V.; Harper, J.T.; Merrell, T.L.; Boscaro, V.; Husník, F.; Scheffrahn, R.H.; Keeling, P.J. New species of Spirotrichonympha from Reticulitermes and the relationships among genera in Spirotrichonymphea (Parabasalia). J. Eukaryot. Microbiol. 2018, 65, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Selles, D.E.; De Martini, F.; Freeman, K.D.; Garcia, M.D.; Merrell, T.L.; Scheffrahn, R.H.; Gile, G.H. The parabasalid symbiont community of Heterotermes aureus: Molecular and morphological characterization of four new species and reestablishment of the genus Cononympha. Eur. J. Protistol. 2017, 61, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Frati, F.F.F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Kambhampati, S.; Smith, P.T. PCR primers for the amplification of four insect mitochondrial gene fragments. Insect Mol. Biol. 1995, 4, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Gile, G.H.; Taerum, S.J.; Jasso-Selles, D.E.; Sillam-Dussès, D.; Ohkuma, M.; Kitade, O.; Noda, S. Molecular phylogenetic position of Microjoenia (Parabasalia: Spirotrichonymphea) from Reticulitermes and Hodotermopsis termite hosts. Protist 2021, 172, 125836. [Google Scholar] [CrossRef]
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the World. Bull. Am. Museum Nat. Hist. 2013, 377, 623–973. [Google Scholar] [CrossRef]
- Wang, M.; Hellemans, S.; Buček, A.; Kanao, T.; Arora, J.; Clitheroe, C.; Rafanomezantsoa, J.J.; Fisher, B.L.; Scheffrahn, R.; Sillam-Dussès, D.; et al. Neoisoptera repeatedly colonised Madagascar after the Middle Miocene climatic optimum. Ecography 2023, 7, e06463. [Google Scholar] [CrossRef]
- Wang, M.; Buček, A.; Šobotník, J.; Sillam-Dussès, D.; Evans, T.A.; Roisin, Y.; Lo, N.; Bourguignon, T. Historical biogeography of the termite clade Rhinotermitinae (Blattodea: Isoptera). Mol. Phylogenet. Evol. 2019, 132, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Koidzumi, M. Studies on the intestinal protozoa found in the termites of Japan. Parasitology 1921, 13, 235–309. [Google Scholar] [CrossRef]
- Jasso-Selles, D.E.; De Martini, F.; Velenovsky, J.F.; Mee, E.D.; Montoya, S.J.; Hileman, J.T.; Garcia, M.D.; Su, N.Y.; Chouvenc, T.; Gile, G.H. The complete protist symbiont communities of Coptotermes formosanus and Coptotermes gestroi: Morphological and molecular characterization of five new species. J. Eukaryot. Microbiol. 2020, 67, 626–641. [Google Scholar] [CrossRef]
- De Martini, F.; Coots, N.L.; Jasso-Selles, D.E.; Shevat, J.; Ravenscraft, A.; Stiblík, P.; Šobotník, J.; Sillam-Dusses, D.; Scheffrahn, R.H.; Carrijo, T.F.; et al. Biogeography and independent diversification in the protist symbiont community of Heterotermes tenuis. Front. Ecol. Evol. 2021, 9, 640625. [Google Scholar] [CrossRef]
- Noda, S.; Kitade, O.; Radek, R.; Takayanagi, M.; Jasso-Selles, D.E.; Taerum, S.J.; Lo, N.; Ohkuma, M.; Gile, G.H. Molecular phylogeny of Spirotrichonymphea (Parabasalia) with emphasis on Spironympha, Spirotrichonympha, and three new genera: Pseudospironympha, Nanospironympha, and Brugerollina. J. Eukaryot. Microbiol. 2023, 70, e12967. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Gile, G.H.; James, E.R.; Horák, A.; Scheffrahn, R.H.; Keeling, P.J. Morphology and molecular phylogeny of Pseudotrichonympha hertwigi and Pseudotrichonympha paulistana (Trichonymphea, Parabasalia) from Neotropical rhinotermitids. J. Eukaryot. Microbiol. 2011, 58, 487–496. [Google Scholar] [CrossRef]
- Nishimura, Y.; Otagiri, M.; Yuki, M.; Shimizu, M.; Inoue, J.; Moriya, S.; Ohkuma, M. Division of functional roles for termite gut protists revealed by single-cell transcriptomes. ISME J. 2020, 14, 2449–2460. [Google Scholar] [CrossRef]
- Mannesmann, R. Vergleichende Untersuchungen über den Einfluß der Temperatur auf die Darm-Symbionten von Termiten und über die regulatorischen Mechanismen bei der Symbiose. Angew. Zool. 1969, 4, 385–440. [Google Scholar]
- Grassi, B. Flagelatti viventi nei termiti. Mem. R. Accad. Lincei Ser. 5 1917, 12, 331–394. [Google Scholar]
- Dini, W.; Cesar, H.C. Métodos para estudo de protozoários de térmita. Rev. Bras. Biol. 1960, 20, 403–407. [Google Scholar]
- Karandikar, K.; Vittal, M. Flagellates in the termites from Dharwar. J. Univ. Bombay 1954, 23, 1–24. [Google Scholar]
- de Mello, I.F. Sur des trichonymphides nouveau des termites indienes. C. R. XIIe Congr. Int. Zool. 1937, 2, 1353–1380. [Google Scholar]
- Koidzumi, M. Studies on the protozoa harboured by the termites of Japan. Rep. Investig. Termit. 1917, 6, 93–175. [Google Scholar]
- Kitade, O.; Hayashi, Y.; Noda, S. Symbiotic protist communities in the termite Coptotermes formosanus in Japan and a comparison of community structures between workers and soldiers. Jpn. J. Protozool. 2013, 46, 21–29. [Google Scholar]
- Hollande, A.; Carruette-Valentin, J. Les atractophores, l’induction du fuseau, et la division cellulaire chez les hypermastigines: Étude infrastructurale et révision systématique des trichonymphines et des spirotrichonymphines. Protistologica 1971, 7, 5–100. [Google Scholar]
- Grasse, P.-P.; Hollande, A. Les flagellés des genres Holomastigotoides et Rostronympha. Structure et cycle de spiralisation des chromosomes chez Holomastigotoides psammotermitidis. Ann. Sci. Nat. Zool. Paris 1963, 12, 749–792. [Google Scholar]
- Ghidini, G.M. Trichonympha scortecci nuova specie di flagellato vivente in Psammotermes hybostoma Desn. della Libia. Boll. Zool. 1942, 13, 1–8. [Google Scholar]
- Sutherland, J.L. Protozoa from Australian termites. Q. J. Microsc. Sci. 1933, 76, 145–178. [Google Scholar] [CrossRef]
- Dunkerly, J.S. A new structure in the flagellate, Pseudotrichonympha sphaerophora sp. n. Parasitology 1923, 15, 211–213. [Google Scholar] [CrossRef]
- Brugerolle, G. Morphological characters of Spirotrichonymphids: Microjoenia, Spirotrichonymphella and Spirotrichonympha symbionts of the Australian termite Porotermes grandis. Eur. J. Protistol. 2001, 37, 103–117. [Google Scholar] [CrossRef]
- Tai, V.; James, E.R.; Nalepa, C.A.; Scheffrahn, R.H.; Perlman, S.J.; Keeling, P.J. The role of host phylogeny varies in shaping microbial diversity in the hindguts of lower termites. Appl. Environ. Microbiol. 2015, 81, 1059–1070. [Google Scholar] [CrossRef]
- Yoshimura, T. Contribution of the protozoan fauna to nutritional physiology of the lower termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Wood Res. 1995, 82, 68–129. [Google Scholar]
- Hongoh, Y.; Sharma, V.K.; Prakash, T.; Noda, S.; Toh, H.; Taylor, T.D.; Kudo, T.; Sakaki, Y.; Toyoda, A.; Hattori, M.; et al. Genome of an endosymbiont coupling N2 fixation to cellulolysis within protist cells in termite gut. Science 2008, 322, 1108–1109. [Google Scholar] [CrossRef] [PubMed]




| Termite Family | Termite Species | Collection Location | P | H | Con | Cth |
|---|---|---|---|---|---|---|
| Rhinotermitidae | Rhinotermes marginalis | Puerto Maldonado, Peru | X | X * | ||
| Schedorhinotermes putorius | Ebogo, Mbalmayo, Cameroon | X | X * | |||
| Dolichorhinotermes longilabius | Petit Saut, French Guiana | X | X † | |||
| Psammotermitidae | Psammotermes allocerus | Rundu, Namibia | X | X | X | |
| Prorhinotermes canalifrons | Réunion Island | X | X | X | ||
| Prorhinotermes inopinatus | Baitabag, Papua New Guinea | X | X | X | ||
| Prorhinotermes simplex | Omar Torrijos Nt. Park, Panama | X | X | X | ||
| Prorhinotermes simplex | Piñar del Rio, Soroa, Cuba | X | X | X | ||
| Heterotermitidae | Heterotermes cardini | Panama City, Panama | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, S.G.; Shevat, J.; Jasso-Selles, D.E.; Swichtenberg, K.L.; Vecco-Giove, C.D.; Šobotník, J.; Sillam-Dussès, D.; De Martini, F.; Gile, G.H. Distinct Patterns of Co-Evolution Among Protist Symbionts of Neoisoptera Termites. Diversity 2025, 17, 537. https://doi.org/10.3390/d17080537
Aguilar SG, Shevat J, Jasso-Selles DE, Swichtenberg KL, Vecco-Giove CD, Šobotník J, Sillam-Dussès D, De Martini F, Gile GH. Distinct Patterns of Co-Evolution Among Protist Symbionts of Neoisoptera Termites. Diversity. 2025; 17(8):537. https://doi.org/10.3390/d17080537
Chicago/Turabian StyleAguilar, Serena G., Jordyn Shevat, Daniel E. Jasso-Selles, Kali L. Swichtenberg, Carlos D. Vecco-Giove, Jan Šobotník, David Sillam-Dussès, Francesca De Martini, and Gillian H. Gile. 2025. "Distinct Patterns of Co-Evolution Among Protist Symbionts of Neoisoptera Termites" Diversity 17, no. 8: 537. https://doi.org/10.3390/d17080537
APA StyleAguilar, S. G., Shevat, J., Jasso-Selles, D. E., Swichtenberg, K. L., Vecco-Giove, C. D., Šobotník, J., Sillam-Dussès, D., De Martini, F., & Gile, G. H. (2025). Distinct Patterns of Co-Evolution Among Protist Symbionts of Neoisoptera Termites. Diversity, 17(8), 537. https://doi.org/10.3390/d17080537






