Impacts of Continuous Damming on Zooplankton Functional Diversity in Karst Rivers of Southwest China: Different Hydrological Periods and Implications for Karst Reservoir Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods of Analysis
2.3. Statistical Analysis
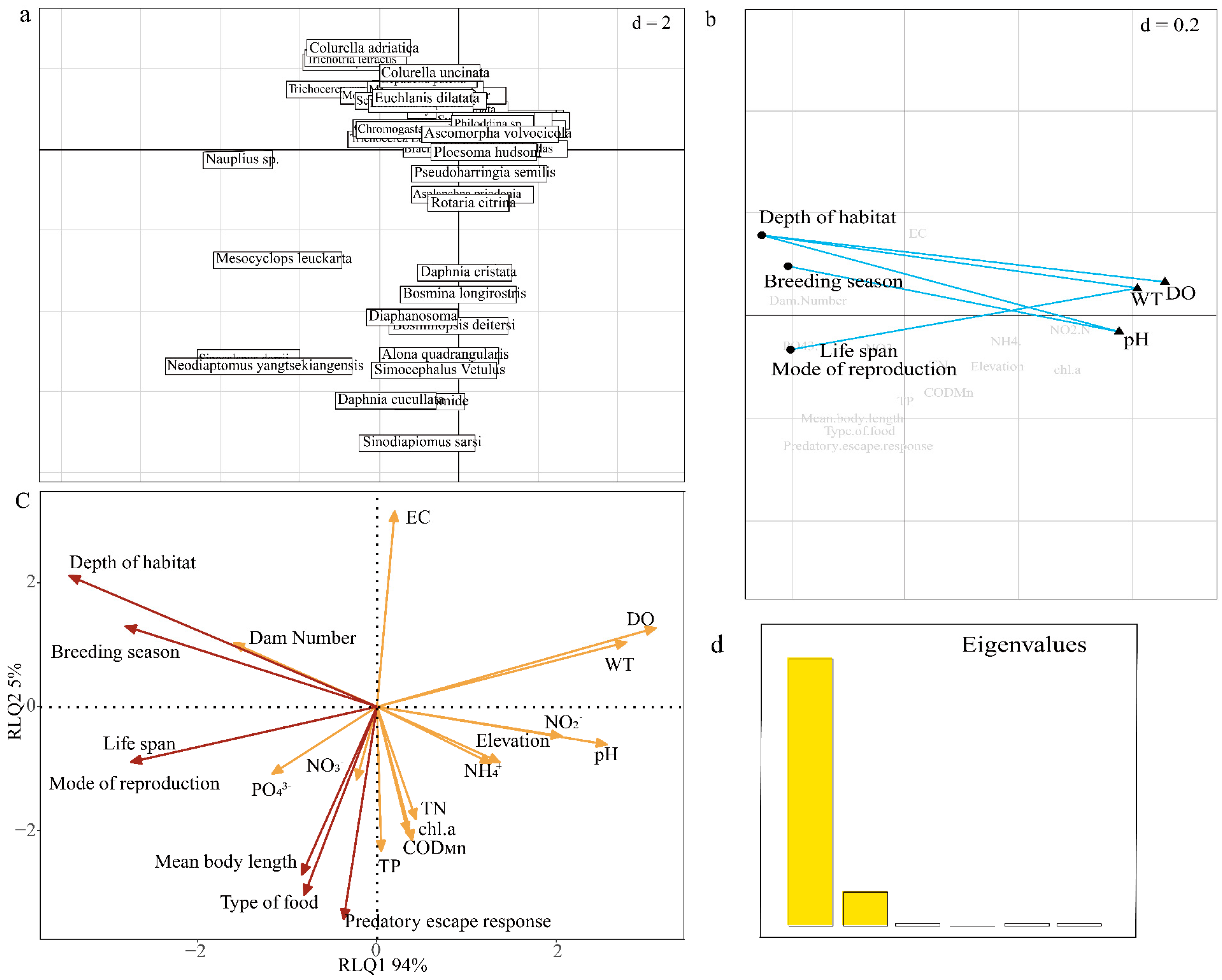
3. Results and Analysis
3.1. The Impact of Continuous Damming on Water Quality Across Different Hydrological Seasons
3.2. The Influence of Environmental and Spatial Factors on Functional Diversity Indices
3.3. Seasonal Regulation by Continuous Dams Constrains Zooplankton Dispersal Between Upstream and Downstream River Reaches
3.4. Relationship Between Environmental and Spatial Factors and Functional Characteristics

4. Discussion
4.1. Seasonal Impacts of Continuous Damming on Water Quality and Zooplankton Functional Diversity Indices
4.2. Seasonally Stepped Damming Alters Upstream and Downstream Zooplankton Community Differences and Functional β-Diversity
4.3. Relationship Between Species, Environmental Factors, and Functional Characteristics
5. Conclusions
6. Recommendations for the Management of Terraced Karst Reservoirs in Different Seasons
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Ripl, W. Water: The bloodstream of the biosphere. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 1921–1934. [Google Scholar] [CrossRef] [PubMed]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
- Ahmad, S.K.; Hossain, F.; Holtgrieve, G.W.; Pavelsky, T.; Galelli, S. Predicting the Likely Thermal Impact of Current and Future Dams Around the World. Earth’s Future 2021, 9, e2020EF001916. [Google Scholar] [CrossRef]
- Maavara, T.; Chen, Q.; Van Meter, K.; Brown, L.E.; Zhang, J.; Ni, J.; Zarfl, C. River dam impacts on biogeochemical cycling. Nat. Rev. Earth Environ. 2020, 1, 103–116. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Huang, J. Hydrologic impacts of cascade dams in a small headwater watershed under climate variability. J. Hydrol. 2020, 590, 125426. [Google Scholar] [CrossRef]
- Bozelli, R.L.; Thomaz, S.M.; Padial, A.A.; Lopes, P.M.; Bini, L.M. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 2015, 753, 233–241. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Xu, J.; Zeng, G.; Sang, L.; Liu, Q.; Yin, Z.; Dai, J.; Yin, D.; Liang, J.; et al. Effects of dam construction on biodiversity: A review. J. Clean. Prod. 2019, 221, 480–489. [Google Scholar] [CrossRef]
- de Souza, C.A.; Beisner, B.E.; Velho, L.F.M.; de Carvalho, P.; Pineda, A.; Vieira, L.C.G. Impoundment, environmental variables and temporal scale predict zooplankton beta diversity patterns in an Amazonian river basin. Sci. Total Environ. 2021, 776, 145948. [Google Scholar] [CrossRef]
- Rose, V.; Rollwagen-Bollens, G.; Bollens, S.M.; Zimmerman, J. The effects of run-of-river dam spill on Columbia River microplankton. River Res. Appl. 2019, 35, 1478–1488. [Google Scholar] [CrossRef]
- Sabo, J.L.; Ruhi, A.; Holtgrieve, G.W.; Elliott, V.; Arias, M.E.; Ngor, P.B.; Räsänen, T.A.; Nam, S. Designing river flows to improve food security futures in the Lower Mekong Basin. Science 2017, 358, eaao1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Mitsch, W.J. Predicting river aquatic productivity and dissolved oxygen before and after dam removal. Ecol. Eng. 2014, 72, 125–137. [Google Scholar] [CrossRef]
- Zhao, K.; Song, K.; Pan, Y.; Wang, L.; Da, L.; Wang, Q. Metacommunity structure of zooplankton in river networks: Roles of environmental and spatial factors. Ecol. Indic. 2017, 73, 96–104. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, J.Q.; Zou, D.W.; Li, J.J.; Qiao, Y.J.; An, S.Q.; Leng, X. Effects of multiple dams on the metacommunity structure of stream macroinvertebrates. Mar. Freshw. Res. 2018, 69, 721. [Google Scholar] [CrossRef]
- Visconti, A.; Caroni, R.; Rawcliffe, R.; Fadda, A.; Piscia, R.; Manca, M. Defining Seasonal Functional Traits of a Freshwater Zooplankton Community Using δ13C and δ15N Stable Isotope Analysis. Water 2018, 10, 108. [Google Scholar] [CrossRef]
- Griffin, J.N.; Méndez, V.; Johnson, A.F.; Jenkins, S.R.; Foggo, A. Functional diversity predicts overyielding effect of species combination on primary productivity. Oikos 2009, 118, 37–44. [Google Scholar] [CrossRef]
- Cardoso, S.J.; Nabout, J.C.; Farjalla, V.F.; Lopes, P.M.; Bozelli, R.L.; Huszar, V.L.M.; Roland, F. Environmental factors driving phytoplankton taxonomic and functional diversity in Amazonian floodplain lakes. Hydrobiologia 2017, 802, 115–130. [Google Scholar] [CrossRef]
- Dunck, B.; Algarte, V.M.; Cianciaruso, M.V.; Rodrigues, L. Functional diversity and trait–environment relationships of periphytic algae in subtropical floodplain lakes. Ecol. Indic. 2016, 67, 257–266. [Google Scholar] [CrossRef]
- Pease, A.A.; González-Díaz, A.A.; Rodiles-Hernández, R.; Winemiller, K.O. Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshw. Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Hong, P.; Schmid, B.; De Laender, F.; Eisenhauer, N.; Zhang, X.; Chen, H.; Craven, D.; De Boeck, H.J.; Hautier, Y.; Petchey, O.L.; et al. Biodiversity promotes ecosystem functioning despite environmental change. Ecol. Lett. 2022, 25, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Qian, X.; Liu, Y.; Li, X.; Gao, H.; An, Y.; Qi, J.; Jiang, L.; Zhang, Y.; Chen, S.; et al. Land conversion to agriculture induces taxonomic homogenization of soil microbial communities globally. Nat. Commun. 2024, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Obertegger, U.; Flaim, G. Taxonomic and functional diversity of rotifers, what do they tell us about community assembly? Hydrobiologia 2018, 823, 79–91. [Google Scholar] [CrossRef]
- Lokko, K.; Virro, T.; Kotta, J. Seasonal variability in the structure and functional diversity of psammic rotifer communities: Role of environmental parameters. Hydrobiologia 2017, 796, 287–307. [Google Scholar] [CrossRef]
- Obertegger, U.; Flaim, G. Community assembly of rotifers based on morphological traits. Hydrobiologia 2015, 753, 31–45. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Špoljar, M.; Zhang, C.; Pronin, M. Zooplankton functional traits as a tool to assess latitudinal variation in the northern-southern temperate European regions during spring and autumn seasons. Ecol. Indic. 2020, 117, 106629. [Google Scholar] [CrossRef]
- Mammola, S.; Carmona, C.P.; Guillerme, T.; Cardoso, P. Concepts and applications in functional diversity. Funct. Ecol. 2021, 35, 1869–1885. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Grenié, M.; Gruson, H. fundiversity: A modular R package to compute functional diversity indices. Ecography 2023, 2023, e06585. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.D.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.A.; et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef]
- Best, J. Anthropogenic stresses on the world’s big rivers. Nat. Geosci. 2019, 12, 7–21. [Google Scholar] [CrossRef]
- Zhao, B.; Zeng, Q.; Wang, J.; Jiang, Y.; Liu, H.; Yan, L.; Yang, Z.; Yang, Q.; Zhang, F.; Tang, J.; et al. Impact of cascade reservoirs on nutrients transported downstream and regulation method based on hydraulic retention time. Water Res. 2024, 252, 121187. [Google Scholar] [CrossRef]
- Haase, P.; Bowler, D.E.; Baker, N.J.; Bonada, N.; Domisch, S.; Marquez, J.R.G.; Heino, J.; Hering, D.; Jähnig, S.C.; Schmidt-Kloiber, A.; et al. The recovery of European freshwater biodiversity has come to a halt. Nature 2023, 620, 582–588. [Google Scholar] [CrossRef]
- Li, Y.; Hou, J.; Xu, L.; Li, M.; Chen, Z.; Zhang, Z.; He, N. Variation in functional trait diversity from tropical to cold-temperate forests and linkage to productivity. Ecol. Indic. 2022, 138, 108864. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; Vergin, K.L. Seasonality in Ocean Microbial Communities. Science 2012, 335, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Ratnarajah, L.; Abu-Alhaija, R.; Atkinson, A.; Batten, S.; Bax, N.J.; Bernard, K.S.; Canonico, G.; Cornils, A.; Everett, J.D.; Grigoratou, M.; et al. Monitoring and modelling marine zooplankton in a changing climate. Nat. Commun. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Jane, S.F.; Hansen, G.J.; Kraemer, B.M.; Leavitt, P.R.; Mincer, J.L.; North, R.L.; Pilla, R.M.; Stetler, J.T.; Williamson, C.E.; Woolway, R.I.; et al. Widespread deoxygenation of temperate lakes. Nature 2021, 594, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, X.; Xu, W.; Sun, Y.; Zhang, L.; Yang, Z. Water acidification weakens the carbon sink capacity of mixotrophic organisms. Sci. Total. Environ. 2023, 865, 161120. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, R.F.; Everett, J.D.; Blanchard, J.L.; Richardson, A.J. Zooplankton Are Not Fish: Improving Zooplankton Realism in Size-Spectrum Models Mediates Energy Transfer in Food Webs. Front. Mar. Sci. 2016, 3, 201. [Google Scholar] [CrossRef]
- Zamarrón-Mieza, I.; Yepes, V.; Moreno-Jiménez, J.M. A systematic review of application of multi-criteria decision analysis for aging-dam management. J. Clean. Prod. 2017, 147, 217–230. [Google Scholar] [CrossRef]
- Michie, L.E.; Hitchcock, J.N.; Thiem, J.D.; Boys, C.A.; Mitrovic, S.M. The effect of varied dam release mechanisms and storage volume on downstream river thermal regimes. Limnologica 2020, 81, 125760. [Google Scholar] [CrossRef]





| Trait | Trait State (Modality) |
|---|---|
| Average body length | Size of body length |
| Habitat depth | Shallow, Intermediate, Deep |
| Feeding type | Filtration-R, Sugador-R, Predator-R, Raptorial-Cop, Filtration-Cop, Filtration-Clad and Scraper-Clad |
| Life span | Short, Long |
| Breeding method | Asexual, Sexual |
| Predatory escape response | Low, Big, Maximum, Medium |
| Breeding season | Warm Season, Cold Season, Extensive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Li, Q.; Long, Y.; Zhang, J.; Wang, H.; Yang, B.; Xiao, J. Impacts of Continuous Damming on Zooplankton Functional Diversity in Karst Rivers of Southwest China: Different Hydrological Periods and Implications for Karst Reservoir Management. Diversity 2025, 17, 478. https://doi.org/10.3390/d17070478
Song X, Li Q, Long Y, Zhang J, Wang H, Yang B, Xiao J. Impacts of Continuous Damming on Zooplankton Functional Diversity in Karst Rivers of Southwest China: Different Hydrological Periods and Implications for Karst Reservoir Management. Diversity. 2025; 17(7):478. https://doi.org/10.3390/d17070478
Chicago/Turabian StyleSong, Xiaochuan, Qiuhua Li, Yue Long, Jingze Zhang, Heng Wang, Bo Yang, and Jing Xiao. 2025. "Impacts of Continuous Damming on Zooplankton Functional Diversity in Karst Rivers of Southwest China: Different Hydrological Periods and Implications for Karst Reservoir Management" Diversity 17, no. 7: 478. https://doi.org/10.3390/d17070478
APA StyleSong, X., Li, Q., Long, Y., Zhang, J., Wang, H., Yang, B., & Xiao, J. (2025). Impacts of Continuous Damming on Zooplankton Functional Diversity in Karst Rivers of Southwest China: Different Hydrological Periods and Implications for Karst Reservoir Management. Diversity, 17(7), 478. https://doi.org/10.3390/d17070478





