An Overview of Post-Fertilization Parental Care in Gobiidae
Abstract
1. Introduction
Defining Parental Care
2. Methods
3. Results
3.1. Behavioral Parental Care in Gobies
3.2. Parental Care Is Not Just Behavior: Non-Behavioral Parental Care in Gobies
3.3. Form of Parental Care
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, G.R.; Robertson, D.R. Fishes of the Tropical Eastern Pacific; University of Hawaii Press: Hong Kong, China, 1994; ISBN 0-8248-1675-7. [Google Scholar]
- Carpenter, K.E.; Niem, V.H. (Eds.) FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. In Bony Fishes Part 4 (Labridae to Latimeriidae), Estuarine Crocodiles, Sea Turtles, Sea Snakes and Marine Mammals; FAO: Rome, Italy, 2001; Volume 6. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781118342336. [Google Scholar]
- Balon, E.K. Epigenesis of an Epigeneticist: The Development of Some Alternative Concepts on the Early Ontogeny and Evolution of Fishes. Guelph Ichthyol. Rev. 1990, 1, 1–48. [Google Scholar]
- Balon, E.K. Reproductive Guilds of Fishes: A Proposal and Definition. J. Fish. Board Can. 1975, 32, 821–864. [Google Scholar] [CrossRef]
- Patzner, R.A.; van Tassell, J.L.; Kovačić, M.; Kapoor, B.G. (Eds.) The Biology of Gobies; Science Publishers: Enfield, CT, USA, 2011; ISBN 9781578084364 1578084369. [Google Scholar]
- Skolbekken, R.; Utne-Palm, A.C. Parental Investment of Male Two-Spotted Goby, Gobiusculus flavescens (Fabricius). J. Exp. Mar. Bio. Ecol. 2001, 261, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Reynolds, J.D. Oxygen and the Trade-off between Egg Ventilation and Brood Protection in the Common Goby. Behaviour 1999, 136, 819–832. [Google Scholar] [CrossRef]
- Lissåker, M.; Kvarnemo, C. Ventilation or Nest Defense: Parental Care Trade-Offs in a Fish with Male Care. Behav. Ecol. Sociobiol. 2006, 60, 864–873. [Google Scholar] [CrossRef]
- Forsgren, E. Female Sand Gobies Prefer Good Fathers over Dominant Males. Proc. Biol. Sci. 1997, 264, 1283–1286. [Google Scholar] [CrossRef]
- Bjelvenmark, J.; Forsgren, E. Effects of Mate Attraction and Male-Male Competition on Parental Care in a Goby. Behaviour 2003, 140, 55–69. [Google Scholar] [CrossRef]
- Trivers, R. Parental Investment and Sexual Selection. In Sexual Selection and the Descent of Man; Campbell, B., Ed.; Aldine: Chicago, IL, USA, 1972; pp. 52–95. ISBN 0-202-02005-3. [Google Scholar]
- Clutton-Brock, T. The Evolution of Parental Care; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Tavolga, W.N. Reproductive Behavior in the Gobiid Fish Bathygobius soporator. Bull. Am. Mus. Nat. Hist. 1954, 104, 427–460. [Google Scholar]
- Faria, C.; Almada, V.C.; Gonçalves, E.J.; Gil, M.F.; Baptista, C.; Carreiro, H. Notes on the Social Behaviour of Gobius cobitis (Pisces, Gobiidae). Acta Ethol. 1998, 1, 49–56. [Google Scholar]
- Faria, C.; Almada, V.C. Some Aspects of the Breeding Ecology of Gobius cobitis Pallas and Gobius paganellus L. in the West Coast of Portugal. Arq. Mus. Bocage (Nova Série) 1995, 29, 463–471. [Google Scholar]
- Torricelli, P.; Lugli, M.; Gandolfi, G. A Quantitative Analysis of the Fanning Activity in the Male Padogobius martensi (Pisces: Gobiidae). Behaviour 1985, 92, 288–301. [Google Scholar]
- Zerunian, S.; D’onofrio, E.; Gibertini, G. The Biology of Gobius nigricans (Osteichthyes, Gobiidae). I. Observations on the Reproductive Behaviour. Bollet. Zool. 1988, 55, 293–298. [Google Scholar] [CrossRef]
- Gordon, J.C.D. Some Notes on Small Kelp Forest Fish Collected from Saccorhiza polyschides Bulbs on the Isle of Cumbrae Scotland. Ophelia 1983, 22, 173–183. [Google Scholar] [CrossRef]
- Trujillo-García, M.; Klug, H.; Balart, E.F.; Ceballos-Vázquez, B.P. Paternal Care in the Redhead Goby, Elacatinus puncticulatus. J. Ethol. 2025, 43, 69–76. [Google Scholar] [CrossRef]
- Smith, C.; Wootton, R.J. The Costs of Parental Care in Teleost Fishes. Rev. Fish Biol. Fish. 1995, 5, 7–22. [Google Scholar] [CrossRef]
- Smiseth, P.T.; Kölliker, M.; Royle, N.J. What Is Parental Care? In The Evolution of Parental Care; Royle, N.J., Smiseth, P.T., Kölliker, M., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 1–17. [Google Scholar]
- Klug, H.; Alonzo, S.H.; Bonsall, M.B. Theoretical Foundations of Parental Care. In The Evolution of Parental Care; Royle, N.J., Smiseth, P.T., Kölliker, M., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 21–39. [Google Scholar]
- Blumer, L.S. A Bibliography and Categorization of Bony Fishes Exhibiting Parental Care. Zool. J. Linn. Soc. 1982, 75, 1–22. [Google Scholar] [CrossRef]
- Blumer, L.S. Male Parental Care in the Bony Fishes. Q. Rev. Biol. 1979, 54, 149–161. [Google Scholar] [CrossRef]
- Goiran, C.; Shine, R. Parental Defence on the Reef: Antipredator Tactics of Coral-Reef Fishes against Egg-Eating Seasnakes. Biol. J. Linn. Soc. 2015, 114, 415–425. [Google Scholar] [CrossRef]
- Meunier, B.; Yavno, S.; Ahmed, S.; Corkum, L.D. First Documentation of Spawning and Nest Guarding in the Laboratory by the Invasive Fish, the Round Goby (Neogobius melanostomus). J. Great Lakes Res. 2009, 35, 608–612. [Google Scholar] [CrossRef]
- Takegaki, T.; Nakazono, A. Responses of the Egg-Tending Gobiid Fish Valenciennea longipinnis to the Fluctuation of Dissolved Oxygen in the Burrow. Bull. Mar. Sci. 1999, 65, 815–823. [Google Scholar]
- Keenleyside, M.H.A. Diversity and Adaptation in Fish Behaviour; Hoar, W.S., Hoelldobler, B., Johansen, K., Langer, H., Lindauer, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Sunobe, T.; Nakazono, A. Alternative Mating Tactics in the Gobiid Fish, Eviota prasina. Ichthyol. Res. 1999, 46, 212–215. [Google Scholar] [CrossRef]
- Majoris, J.E.; Francisco, F.A.; Burns, C.M.; Brandl, S.J.; Warkentin, K.M.; Buston, P.M. Paternal Care Regulates the Timing, Synchrony and Success of Hatching in a Coral Reef Fish. Proc. R. Soc. B 2022, 289, 20221466. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Marri, L.; Marchini, D.; Mazzoldi, C.; Rasotto, M.B. Sperm-Duct Gland Secretion of the Grass Goby Zosterisessor ophiocephalus Exhibits Antimicrobial Activity. J. Fish Biol. 2008, 73, 1823–1828. [Google Scholar] [CrossRef]
- Pizzolon, M.; Giacomello, E.; Marri, L.; Marchini, D.; Pascoli, F.; Mazzoldi, C.; Rasotto, M.B. When Fathers Make the Difference: Efficacy of Male Sexually Selected Antimicrobial Glands in Enhancing Fish Hatching Success. Funct. Ecol. 2010, 24, 141–148. [Google Scholar] [CrossRef]
- Takegaki, T.; Nakazono, A. The Role of Mounds in Promoting Water-Exchange in the Egg-Tending Burrows of Monogamous Goby, Valenciennea longipinnis (Lay et Bennett). J. Exp. Mar. Biol. Ecol. 2000, 253, 149–163. [Google Scholar] [CrossRef]
- Takegaki, T. Monogamous Mating System and Spawning Cycle in the Gobiid Fish, Amblygobius phalaena (Gobiidae). Environ. Biol. Fishes 2000, 59, 61–67. [Google Scholar] [CrossRef]
- Takegaki, T. Female Egg Care Subsequent to Removal of Egg-Tending Male in a Monogamous Goby, Amblygobius phalaena (Gobiidae): A Preliminary Observation. J. Mar. Biol. Assoc. 2005, 85, 189–190. [Google Scholar] [CrossRef]
- Chiu, P.-S.; Ho, S.-W.; Huang, C.-H.; Lee, Y.-C.; Lin, Y.-H. Captive Reproductive Behavior, Spawning, and Early Development of White-Barred Goby Amblygobius phalaena (Valenciennes, 1837) and Examined Larval Survival and Viability at Different Water Temperatures and Salinities. Fishes 2023, 8, 364. [Google Scholar] [CrossRef]
- Hishida, Y. Egg Consumption by the Female in the Paternal Brooding Goby Bathygobius fuscus. Fish. Sci. 2002, 68, 449–451. [Google Scholar] [CrossRef]
- Nakanishi, A.; Takegaki, T. Tactic-Specific Sperm Traits in the Dusky Frillgoby (Bathygobius fuscus). J. Zool. 2019, 307, 71–77. [Google Scholar] [CrossRef]
- Takegaki, T.; Nakanishi, A.; Kanatani, Y.; Kawase, S.; Yoshida, M.; Sato, N. Evidence of Sperm Removal Behaviour in an Externally Fertilizing Species and Compensatory Behaviour for the Risk of Self-Sperm Removal. Proc. R. Soc. B 2020, 287, 20202004. [Google Scholar] [CrossRef] [PubMed]
- Kawase, S.; Hayashi, T.; Matsumoto, Y.; Takegaki, T. Testis Size Variation within Sneaker Males of the Dusky Frillgoby Bathygobius fuscus (Gobiidae): Effects of within-Tactic Competition. Biol. J. Linn. Soc. 2017, 122, 394–399. [Google Scholar] [CrossRef]
- Takegaki, T.; Kaneko, T.; Matsumoto, Y. Large- and Small-Size Advantages in Sneaking Behaviour in the Dusky Frillgoby Bathygobius fuscus. Naturwissenschaften 2012, 99, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Breeder, C.M., Jr. The Eggs of Bathygobius soporator (Cuvier and Valenciennes) with a Discussion of Other Non-Spherical Teleost Eggs. Bull. Bingham Oceanogr. Collect. 1943, 8, 1–49. [Google Scholar]
- Peters, K.M. Larval and Early Juvenile Development of the Frillfin Goby, Bathygobius soporator (Perciformes: Gobiidae). Northeast Gulf Sci. 1983, 6, 137–153. [Google Scholar] [CrossRef]
- Symons, N.; Svensson, P.A.; Wong, B.B.M. Do Male Desert Gobies Compromise Offspring Care to Attract Additional Mating Opportunities? PLoS ONE 2011, 6, e20576. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, T.K.; Svensson, P.A.; Wong, B.B.M. The Influence of Recent Social Experience and Physical Environment on Courtship and Male Aggression. BMC Evol. Biol. 2016, 16, 18. [Google Scholar] [CrossRef]
- Svensson, P.A.; Lehtonen, T.K.; Wong, B.B.M. The Interval between Sexual Encounters Affects Male Courtship Tactics in a Desert-Dwelling Fish. Behav. Ecol. Sociobiol. 2010, 64, 1967–1970. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Svensson, P.A. Strategic Male Signalling Effort in a Desert-Dwelling Fish. Behav. Ecol. Sociobiol. 2009, 63, 543–549. [Google Scholar] [CrossRef]
- van Lieshout, E.; Svensson, P.A.; Wong, B.B.M. Consequences of Paternal Care on Pectoral Fin Allometry in a Desert-Dwelling Fish. Behav. Ecol. Sociobiol. 2013, 67, 513–518. [Google Scholar] [CrossRef]
- Caputo, V.; Mesa, M.L.; Candi, G.; Cerioni, P.N. The Reproductive Biology of the Crystal Goby with a Comparison to That of the Transparent Goby. J. Fish Biol. 2003, 62, 375–385. [Google Scholar] [CrossRef]
- Majoris, J.E.; Francisco, F.A.; Atema, J.; Buston, P.M. Reproduction, Early Development, and Larval Rearing Strategies for Two Sponge-Dwelling Neon Gobies, Elacatinus lori and E. colini. Aquaculture 2018, 483, 286–295. [Google Scholar] [CrossRef]
- Harding, J.A.; Almany, G.R.; Houck, L.D.; Hixon, M.A. Experimental Analysis of Monogamy in the Caribbean Cleaner Goby, Gobiosoma evelynae. Anim. Behav. 2003, 65, 865–874. [Google Scholar] [CrossRef]
- Olivotto, I.; Zenobi, A.; Rollo, A.; Migliarini, B.; Avella, M.; Carnevali, O. Breeding, Rearing and Feeding Studies in the Cleaner Goby Gobiosoma evelynae. Aquaculture 2005, 250, 175–182. [Google Scholar] [CrossRef]
- Whiteman, E.A.; Côté, I.M. Social Monogamy in the Cleaning Goby Elacatinus evelynae: Ecological Constraints or Net Benefit? Anim. Behav. 2003, 66, 281–291. [Google Scholar] [CrossRef]
- Whittey, K.E.; Dunkley, K.; Young, G.C.; Cable, J.; Perkins, S.E. Microhabitats of Sharknose Goby (Elacatinus evelynae) Cleaning Stations and Their Links with Cleaning Behaviour. Coral Reefs 2021, 40, 1069–1080. [Google Scholar] [CrossRef]
- Colin, P. The Neon Gobies. In The Comparative Biology of the Gobies of the Genus Gobiosoma, Subgenus Elacatinus, (Pisces: Gobiidae) in the Tropical Western North Atlantic Ocean; T.F.H. Publications: Neptune, NJ, USA, 1975. [Google Scholar]
- Meirelles, M.E.; Tsuzuki, M.Y.; Ribeiro, F.F.; Medeiros, R.C.; Silva, I.D. Reproduction, Early Development and Larviculture of the Barber Goby, Elacatinus figaro (Sazima, Moura & Rosa 1997). Aquac. Res. 2009, 41, 11–18. [Google Scholar] [CrossRef]
- Shei, M.R.P.; Miranda-Filho, K.C.; Rodrigues, R.V.; Sampaio, L.A. Production of Juvenile Barber Goby Elacatinus figaro in Captivity: Developing Technology to Reduce Fishing Pressure on an Endangered Species. Mar. Biodivers. Rec. 2010, 3, e57. [Google Scholar] [CrossRef]
- Feddern, H.A. Larval Development of the Neon Goby, Elacatinus oceanops, in Florida. Bull. Mar. Sci. 1967, 17, 367–375. [Google Scholar]
- Valenti, R.J. The Embryology of the Neon Goby, Gobiosoma oceanops. Thalassas 1972, 3, 477–482. [Google Scholar] [CrossRef]
- Swenson, R.O. The Reproductive Behavior and Ecology of the Tidewater Goby Eucyclogobius newberryi (Pisces: Gobiidae). Ph.D. Thesis, University of California, Berkeley, CA, USA, 1995. [Google Scholar]
- Swenson, R.O. The Ecology, Behavior, and Conservation of the Tidewater Goby, Eucyclogobius newberry. Environ. Biol. Fishes 1999, 55, 99–114. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: http://fishbase.org (accessed on 2 February 2025).
- Swift, C.C.; Nelson, J.L.; Maslow, C.; Stein, T. Biology and Distribution of the Tidewater Goby, Eucyclogobius newberryi (Pisces: Gobiidae) of California. Contrib. Sci. 1989, 404, 1–19. [Google Scholar] [CrossRef]
- Taru, M.; Sunobe, T. Notes on Reproductive Ecology of the Gobiid Fish Eviota abax at Kominato, Japan. Bull. Mar. Sci. 2000, 66, 507–512. [Google Scholar]
- Sunobe, T. Reproductive Behavior in Six Species of Eviota (Gobiidae) in Aquaria. Ichthyol. Res. 1998, 45, 409–412. [Google Scholar] [CrossRef]
- Karino, K.; Arai, R. Effect of Clutch Size on Male Egg-Fanning Behavior and Hatching Success in the Goby, Eviota prasina (Klunzinger). J. Exp. Mar. Bio. Ecol. 2006, 334, 43–50. [Google Scholar] [CrossRef]
- Gibson, R.N. Observations on the Biology of the Giant Goby Gobius cobitis Pallas. J. Fish Biol. 1970, 2, 281–288. [Google Scholar] [CrossRef]
- Gil, M.F.; Gonçalves, E.J.; Faria, C.; Almada, V.C.; Baptista, C.; Carreiro, H. Embryonic and Larval Development of the Giant Goby Gobius cobitis (Pisces: Gobiidae). J. Nat. Hist. 1997, 31, 799–804. [Google Scholar] [CrossRef]
- Gil, F.; Borges, R.; Faria, C.; Gonçalves, E.J. Early Development of the Red Mouthed Goby, Gobius cruentatus (Pisces: Gobiidae). J. Mar. Biol. Assoc. 2002, 82, 161–163. [Google Scholar] [CrossRef]
- Torricelli, P.; Malavasi, S.; Novarini, N.; Pranovi, F.; Mainardi, D. Elongation of Fin Rays in Parental Males of Zosterisessor ophiocephalus (Pisces, Gobiidae). Environ. Biol. Fishes 2000, 58, 105–108. [Google Scholar] [CrossRef]
- Ota, D.; Marchesan, M.; Ferrero, E.A. Sperm Release Behaviour and Fertilization in the Grass Goby. J. Fish Biol. 1996, 49, 246–256. [Google Scholar] [CrossRef]
- Scaggiante, M.; Mazzoldi, C.; Petersen, C.W.; Rasotto, M.B. Sperm Competition and Mode of Fertilization in the Grass Goby Zosterisessor ophiocephalus (Teleostei: Gobiidae). J. Exp. Zool. 1999, 283, 81–90. [Google Scholar] [CrossRef]
- Immler, S.; Mazzoldi, C.; Rasotto, M.B. From Sneaker to Parental Male: Change of Reproductive Traits in the Black Goby, Gobius niger (Teleostei, Gobiidae). J. Exp. Zool. A 2004, 301A, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Magnhagen, C. Reproduction under Predation Risk in the Sand Goby, Pomatoschistus minutus, and the Black Goby, Gobius niger: The Effect of Age and Longevity. Behav. Ecol. Sociobiol. 1990, 26, 331–335. [Google Scholar] [CrossRef]
- Rasotto, M.B.; Mazzoldi, C. Male Traits Associated with Alternative Reproductive Tactics in Gobius niger. J. Fish Biol. 2002, 61, 173–184. [Google Scholar] [CrossRef]
- Morimoto, Y.; Shibata, J.; Takahata, M.; Myint, O.; Kohda, M. Male Isaza (Gymnogobius isaza, Gobiidae) Prefer Large Mates: A Counterstrategy against Brood Parasitism by Conspecific Females. J. Ethol. 2010, 28, 429–436. [Google Scholar] [CrossRef]
- Takahashi, D.; Asada, H.; Takeyama, T.; Takahata, M.; Katoh, R.; Awata, S.; Kohda, M. Why Egg-Caring Males of Isaza (Gymnogobius isaza, Gobiidae) Refuse Additional Females: Preliminary Field Observations. J. Ethol. 2004, 22, 153–159. [Google Scholar] [CrossRef]
- Hidaka, T.; Takahashi, S. Reproductive Strategy and Interspecific Competition in the Lake-Living Gobiid Fish Isaza, Chaenogobius isaza. J. Ethol. 1987, 5, 185–196. [Google Scholar] [CrossRef]
- Massironi, M.; Rasotto, M.B.; Mazzoldi, C. A Reliable Indicator of Female Fecundity: The Case of the Yellow Belly in Knipowitschia panizzae (Teleostei: Gobiidae). Mar. Biol. 2005, 147, 71–76. [Google Scholar] [CrossRef]
- Nonnis Marzano, F.; Gandolfi, G. Active Cannibalism among Adults of Knipowitschia panizzae (Pisces Gobiidae) Induced by Starvation and Reproduction. Ethol. Ecol. Evol. 2001, 13, 385–391. [Google Scholar] [CrossRef]
- Zerunian, S. Pesci delle Acque Interne d’Italia; Quaderni Conservazione Natura, N. 20; Ministero dell’Ambiente—Istituto Nazionale per la Fauna Selvatica: Rome, Italy, 2004. (In Italian)
- Pradhan, D.S.; Willis, M.C.; Solomon-Lane, T.K.; Thonkulpitak, K.; Grober, M.S. Simultaneous Courtship and Parenting in Males and Sex Role Reversal in Females of the Haremic Bluebanded Goby, Lythrypnus dalli. Behaviour 2015, 152, 917–940. [Google Scholar] [CrossRef]
- Solomon-Lane, T.K.; Pradhan, D.S.; Willis, M.C.; Grober, M.S. Agonistic Reciprocity Is Associated with Reduced Male Reproductive Success within Haremic Social Networks. Proc. R. Soc. B 2015, 282, 20150914. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.W. Life Histories and Systematics of the Western North American Gobies Lythrypnus dalli (Gilbert) and Lythrypnus zebra (Gilbert). Trans. San Diego Soc. Nat. Hist. 1976, 18, 169–183. [Google Scholar] [CrossRef]
- Archambeault, S.; Ng, E.; Rapp, L.; Cerino, D.; Bourque, B.; Solomon-Lane, T.; Grober, M.S.; Rhyne, A.; Crow, K. Reproduction, Larviculture and Early Development of the Bluebanded Goby, Lythrypnus dalli, an Emerging Model Organism for Studies in Evolutionary Developmental Biology and Sexual Plasticity. Aquac. Res. 2016, 47, 1899–1916. [Google Scholar] [CrossRef]
- Muñoz-Arroyo, S.; Martínez-Rincón, R.O.; Findley, L.T.; Hernández-Olalde, L.; Balart, E.F. Reproductive Behaviors and Sex Roles during a Diurnal Cycle of the Goby, Lythrypnus pulchellus (Teleostei: Gobiidae). J. Ethol. 2020, 38, 79–98. [Google Scholar] [CrossRef]
- Gaisiner, A. Parental Care and Reproductive Behavior of the Clown Goby, Microgobius gulosus, with Observations on Predator Interactions. Environ. Biol. Fishes 2005, 73, 341–356. [Google Scholar] [CrossRef]
- Bisazza, A.; Marconato, A.; Marin, G. Male Competition and Female Choice in Padogobius martensi (Pisces, Gobiidae). Anim. Behav. 1989, 38, 406–413. [Google Scholar] [CrossRef]
- Marconato, A.; Bisazza, A.; Marin, G. Correlates of Male Reproductive Success in Padogobius martensi (Gobiidae). J. Fish Biol. 1989, 34, 889–899. [Google Scholar] [CrossRef]
- Pompei, L.; Giannetto, D.; Lorenzoni, M. Reproductive Parameters in Native and Non-Native Areas of Padogobius bonelli and Comparison with P. nigricans (Actynopterigii, Gobiidae). Hydrobiologia 2016, 779, 173–182. [Google Scholar] [CrossRef]
- Kuwamura, T.; Yogo, Y.; Nakashima, Y. Size-Assortative Monogamy and Parental Egg Care in a Coral Goby Paragobiodon echinocephalus. Ethology 1993, 95, 65–75. [Google Scholar] [CrossRef]
- Kuwamura, T.; Nakashima, Y.; Yogo, Y. Sex Change in Either Direction by Growth-Rate Advantage in the Monogamous Coral Goby, Paragobiodon echinocephalus. Behav. Ecol. 1994, 5, 434–438. [Google Scholar] [CrossRef]
- Collins, S.P. Littoral and Benthic Investigations on the West Coast of Ireland—XIII. The Biology of Gobiusculus flavescens (Fabricius) on the Connemara Coast. Proc. R. Ir. Acad. Sect. B Biol. Geol. Chem. Sci. 1981, 81B, 63–87. [Google Scholar]
- Pélabon, C.; Borg, Å.A.; Bjelvenmark, J.; Forsgren, E.; Barber, I.; Amundsen, T. Do Male Two-Spotted Gobies Prefer Large Fecund Females? Behav. Ecol. 2003, 14, 787–792. [Google Scholar] [CrossRef]
- Svensson, P.A.; Pélabon, C.; Blount, J.D.; Surai, P.F.; Amundsen, T. Does Female Nuptial Coloration Reflect Egg Carotenoids and Clutch Quality in the Two-Spotted Goby (Gobiusculus flavescens, Gobiidae)? Funct. Ecol. 2006, 20, 689–698. [Google Scholar] [CrossRef]
- Mazzoldi, C.; Poltronieri, C.; Rasotto, M.B. Egg Size Variability and Mating System in the Marbled Goby Pomatoschistus marmoratus (Pisces: Gobiidae). Mar. Ecol. Prog. Ser. 2002, 233, 231–239. [Google Scholar] [CrossRef]
- Koutrakis, E.T.; Tsikliras, A.C. Reproductive Biology of the Marbled Goby, Pomatoschistus marmoratus (Pisces, Gobiidae), in a Northern Aegean Estuarine System (Greece). Folia Zool. 2009, 58, 447–456. [Google Scholar]
- Järvi-Laturi, M.; Lehtonen, T.K.; Pampoulie, C.; Lindström, K. Paternal Care Behaviour of Sand Gobies Is Determined by Habitat Related Nest Structure. Behaviour 2008, 145, 39–50. [Google Scholar]
- Forsgren, E.; Karlsson, A.; Kvarnemo, C. Female Sand Gobies Gain Direct Benefits by Choosing Males with Eggs in Their Nests. Behav. Ecol. Sociobiol. 1996, 39, 91–96. [Google Scholar] [CrossRef]
- Lindström, K.; Kangas, N. Egg Presence, Egg Loss, and Female Mate Preferences in the Sand Goby (Pomatoschistus minutus). Behav. Ecol. 1996, 7, 213–217. [Google Scholar] [CrossRef]
- Lindström, K.; Hellström, M. Male Size and Parental Care in the Sand Goby, Pomatoschistus minutus. Ethol. Ecol. Evol. 1993, 5, 97–106. [Google Scholar] [CrossRef]
- Pampoulie, C.; Lindström, K.; St. Mary, C.M. Have Your Cake and Eat It Too: Male Sand Gobies Show More Parental Care in the Presence of Female Partners. Behav. Ecol. 2004, 15, 199–204. [Google Scholar] [CrossRef]
- Lindström, K.; St. Mary, C.M.; Pampoulie, C. Sexual Selection for Male Parental Care in the Sand Goby, Pomatoschistus minutus. Behav. Ecol. Sociobiol. 2006, 60, 46–51. [Google Scholar] [CrossRef]
- Lindström, K. Female Spawning Patterns and Male Mating Success in the Sand Goby Pomatoschistus minutus. Mar. Biol. 1992, 113, 475–480. [Google Scholar] [CrossRef]
- Sunobe, T.; Nakazono, A. Embryonic Development and Pre-Larva of a Gobiid Fish Priolepsis naraharae. Jpn. J. Ichthyol. 1989, 35, 484–487. [Google Scholar] [CrossRef]
- Sunobe, T.; Nakazono, A. Mating System and Hermaphroditism in the Gobiid Fish, Priolepis cincta, at Kagoshima, Japan. Ichthyol. Res. 1999, 46, 103–105. [Google Scholar] [CrossRef]
- Kroon, F.J.; de Graaf, M.; Liley, N.R. Social Organisation and Competition for Refuges and Nest Sites in Coryphopterus nicholsii (Gobiidae), a Temperate Protogynous Reef Fish. Environ. Biol. Fishes 2000, 57, 401–411. [Google Scholar] [CrossRef]
- Cole, K.S. Male Reproductive Behaviour and Spawning Success in a Temperate Zone Goby, Coryphopterus nicholsi. Can. J. Zool. 1982, 60, 2309–2316. [Google Scholar] [CrossRef]
- Ebert, E.E.; Turner, C.H. The Nesting Behavior, Eggs and Larvae of the Bluespot Goby. Calif. Fish Game 1962, 48, 249–252. [Google Scholar]
- Teichert, N.; Keith, P.; Valade, P.; Richarson, M.; Metzger, M.; Gaudin, P. Breeding Pattern and Nest Guarding in Sicyopterus lagocephalus, a Widespread Amphidromous Gobiidae. J. Ethol. 2013, 31, 239–247. [Google Scholar] [CrossRef]
- Teichert, N.; Valade, P.; Fostier, A.; Lagarde, R.; Gaudin, P. Reproductive Biology of an Amphidromous Goby, Sicyopterus lagocephalus, in La Réunion Island. Hydrobiologia 2014, 726, 123–141. [Google Scholar] [CrossRef]
- Delacroix, P.; Champeau, A. Ponte En Eau Douce de Sicyopterus lagocephalus (Pallas) Poisson Gobiidae Amphibionte Des Rivières de La Réunion. Hydroécologie Appliquée 1992, 4, 49–63. [Google Scholar] [CrossRef]
- Hudson, R.C.L. Preliminary Observations on the Behaviour of the Gobiid Fish Signigobius biocellatus Hoese and Allen, with Particular Reference to Its Burrowing Behaviour. Z. Tierpsychol. 1977, 43, 214–220. [Google Scholar] [CrossRef]
- Oyama, T.; Tomatsu, S.; Manabe, H.; Sakurai, M.; Matsuoka, M.; Shinomiya, A.; Dewa, S.; Sunobe, T. Monogamous Mating System and Protandrous-like Sexuality in the Goby Trimma taylori. Ichthyol. Res. 2023, 70, 287–292. [Google Scholar] [CrossRef]
- MacGinitie, G.E. The Natural History of the Blind Goby, Typhlogobius californiensis Steindachner. Am. Midl. Nat. 1939, 21, 489–505. [Google Scholar] [CrossRef]
- Takegaki, T.; Nakazono, A. Reproductive Behavior and Mate Fidelity in the Monogamous Goby, Valenciannea longipinnis. Ichthyol. Res. 1999, 46, 115–123. [Google Scholar] [CrossRef]
- Takegaki, T. Factors Affecting Female Parental Investment in the Monogamous Goby, Valenciennea longipinnis. Hydrobiologia 2003, 510, 147–152. [Google Scholar] [CrossRef]
- Reavis, R.H. The Natural History of a Monogamous Coral-Reef Fish, Valenciennea strigata (Gobiidae): 2. Behavior, Mate Fidelity and Reproductive Success. Environ. Biol. Fishes 1997, 49, 247–257. [Google Scholar] [CrossRef]
- Reavis, R.H.; Barlow, G.W. Why Is the Coral-Reef Fish Valenciennea strigata (Gobiidae) Monogamous? Behav. Ecol. Sociobiol. 1998, 43, 229–237. [Google Scholar] [CrossRef]
- Gross, M.R.; Sargent, R.C. The Evolution of Male and Female Parental Care in Fishes. Am. Zool. 1985, 25, 807–822. [Google Scholar] [CrossRef]
- Green, B.S.; McCormick, M.I. O2 Replenishment to Fish Nests: Males Adjust Brood Care to Ambient Conditions and Brood Development. Behav. Ecol. 2005, 16, 389–397. [Google Scholar] [CrossRef]
- Jones, J.C.; Reynolds, J.D. Costs of Egg Ventilation for Male Common Gobies Breeding in Conditions of Low Dissolved Oxygen. Anim. Behav. 1999, 57, 181–188. [Google Scholar] [CrossRef]
- Walsh, W.A.; Swanson, C.; Lee, C.-S.; Banno, J.E.; Eda, H. Oxygen Consumption by Eggs and Larvae of Striped Mullet, Mugil cephalus, in Relation to Development, Salinity and Temperature. J. Fish Biol. 1989, 35, 347–358. [Google Scholar] [CrossRef]
- Vallon, M.; Anthes, N.; Heubel, K.U. Water Mold Infection but Not Paternity Induces Selective Filial Cannibalism in a Goby. Ecol. Evol. 2016, 6, 7221–7229. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.P.H. Parent–Offspring Cannibalism throughout the Animal Kingdom: A Review of Adaptive Hypotheses. Biol. Rev. 2022, 97, 1868–1885. [Google Scholar] [CrossRef]
- Davenport, M.E.; Bonsall, M.B.; Klug, H. Unconventional Care: Offspring Abandonment and Filial Cannibalism Can Function as Forms of Parental Care. Front. Ecol. Evol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Goulet, D.; Green, J.M.; Shears, T.H. Courtship, Spawning, and Parental Care Behavior of the Lumpfish, Cyclopterus lumpus L., in Newfoundland. Can. J. Zool. 1986, 64, 1320–1325. [Google Scholar] [CrossRef]
- Thomson, J.M.; Bennett, A.E. Parental Care of the Eggs and the Early Larvae of the Oyster Blenny, Omobranchus anolius (Valenciennes) (Blenniidae). Aust. J. Mar. Freshw. Res. 1953, 4, 227–233. [Google Scholar] [CrossRef]
- Wirtz, P. The Behaviour of the Mediterranean Tripterygion Species (Pisces, Blennioidei). Z. Tierpsychol. 1978, 48, 142–174. [Google Scholar] [CrossRef]
- Rupp, H.-G. Paternal Help in Larval Release of Three Mudskipper Species. Mar. Freshw. Behav. Physiol. 2023, 56, 57–72. [Google Scholar] [CrossRef]
- Trujillo-García, M.; Klug, H.; Ceballos-Vázquez, B.P. Female Filial Cannibalism in the Redhead Goby (Elacatinus puncticulatus) in Captivity. Diversity 2025, 17, 365. [Google Scholar] [CrossRef]
- Giacomello, E.; Marchini, D.; Rasotto, M.B. A Male Sexually Dimorphic Trait Provides Antimicrobials to Eggs in Blenny Fish. Biol. Lett. 2006, 2, 330–333. [Google Scholar] [CrossRef]
- Little, T.J.; Perutz, M.; Palmer, M.; Crossan, C.; Braithwaite, V.A. Male Three-Spined Sticklebacks Gasterosteus aculeatus Make Antibiotic Nests: A Novel Form of Parental Protection? J. Fish Biol. 2008, 73, 2380–2389. [Google Scholar] [CrossRef]
- Knouft, J.H.; Page, L.M.; Plewa, M.J. Antimicrobial Egg Cleaning by the Fringed Darter (Perciformes: Percidae: Etheostoma crossopterum): Implications of a Novel Component of Parental Care in Fishes. Proc. R. Soc. Lond. B 2003, 270, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J. The Tokology of Gobioid Fishes. In Fish Reproduction: Strategies and Tactics; Potts, G.W., Wootton, R.J., Eds.; Academic Press: London, UK, 1984; pp. 119–153. [Google Scholar]
- Fishelson, L. Comparative Cytology and Morphology of Seminal Vesicles in Male Gobiid Fishes. Jpn. J. Ichthyol. 1991, 38, 17–30. [Google Scholar] [CrossRef]
- Eckert, L.; Miller, J.S.; Fitzpatrick, J.L.; Balshine, S.; Bolker, B.M. Parental Care Drives the Evolution of Male Reproductive Accessory Glands across Ray-Finned Fishes. Evolution 2025, qpaf062. [Google Scholar] [CrossRef]
- Wootton, R.J.; Smith, C. Reproductive Biology of Teleost Fishes; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 9781118891360. [Google Scholar]
- Sargent, R.C.; Gross, M.R. Parental Investment Decision Rules and the Concorde Fallacy. Behav. Ecol. Sociobiol. 1985, 17, 43–45. [Google Scholar] [CrossRef]
- Whittingham, L.A.; Taylor, P.D.; Robertson, R.J. Confidence of Paternity and Male Parental Care. Am. Nat. 1992, 139, 1115–1125. [Google Scholar] [CrossRef]
- Perrone, M., Jr.; Zaret, T.M. Parental Care Patterns of Fishes. Am. Nat. 1979, 113, 351–361. [Google Scholar] [CrossRef]
- Kappeler, P.M.; Benhaiem, S.; Fichtel, C.; Fromhage, L.; Höner, O.P.; Jennions, M.D.; Kaiser, S.; Krüger, O.; Schneider, J.M.; Tuni, C.; et al. Sex Roles and Sex Ratios in Animals. Biol. Rev. 2023, 98, 462–480. [Google Scholar] [CrossRef]
- Klug, H.; Bonsall, M.B.; Alonzo, S.H. The Origin of Parental Care in Relation to Male and Female Life History. Ecol. Evol. 2013, 3, 779–791. [Google Scholar] [CrossRef]
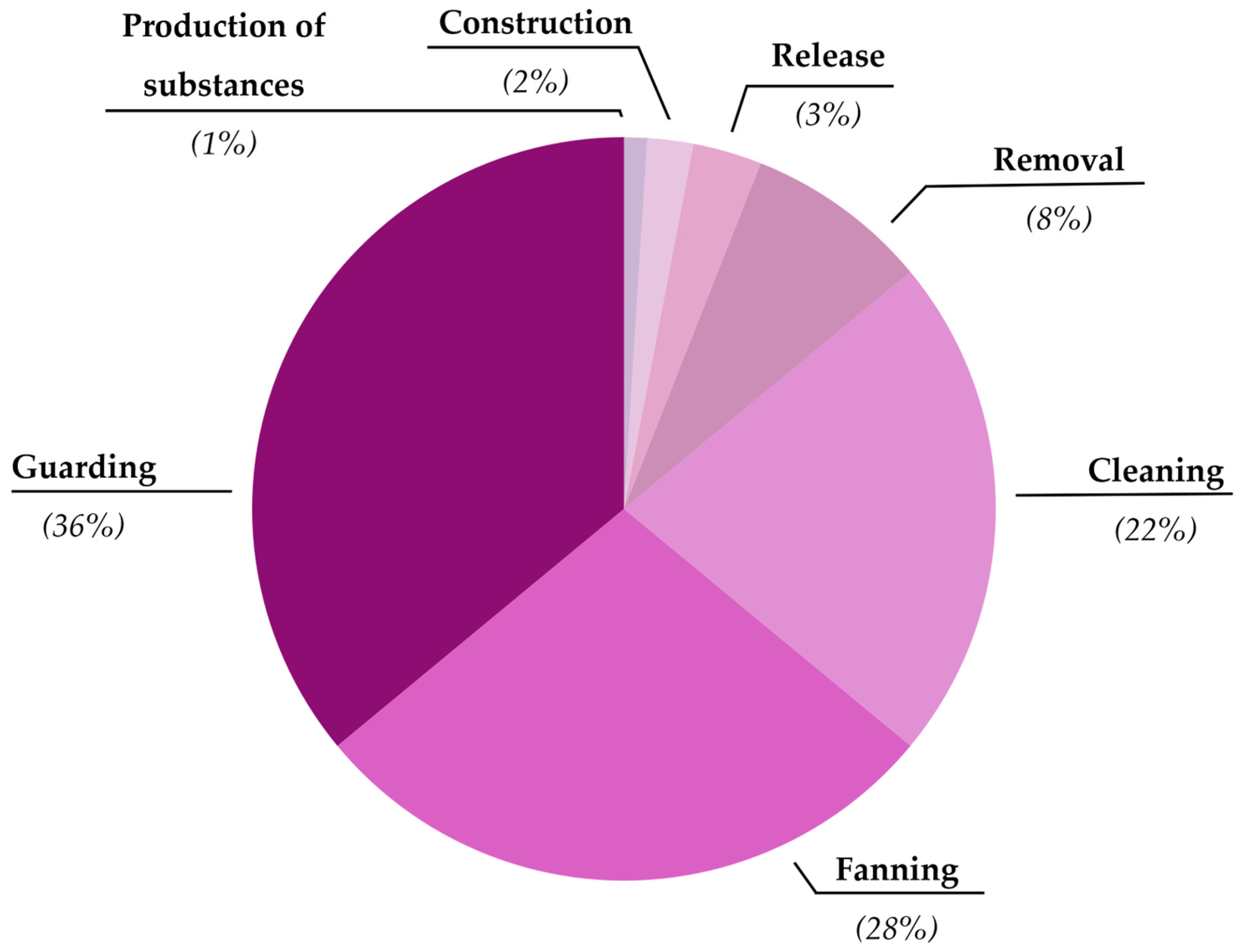
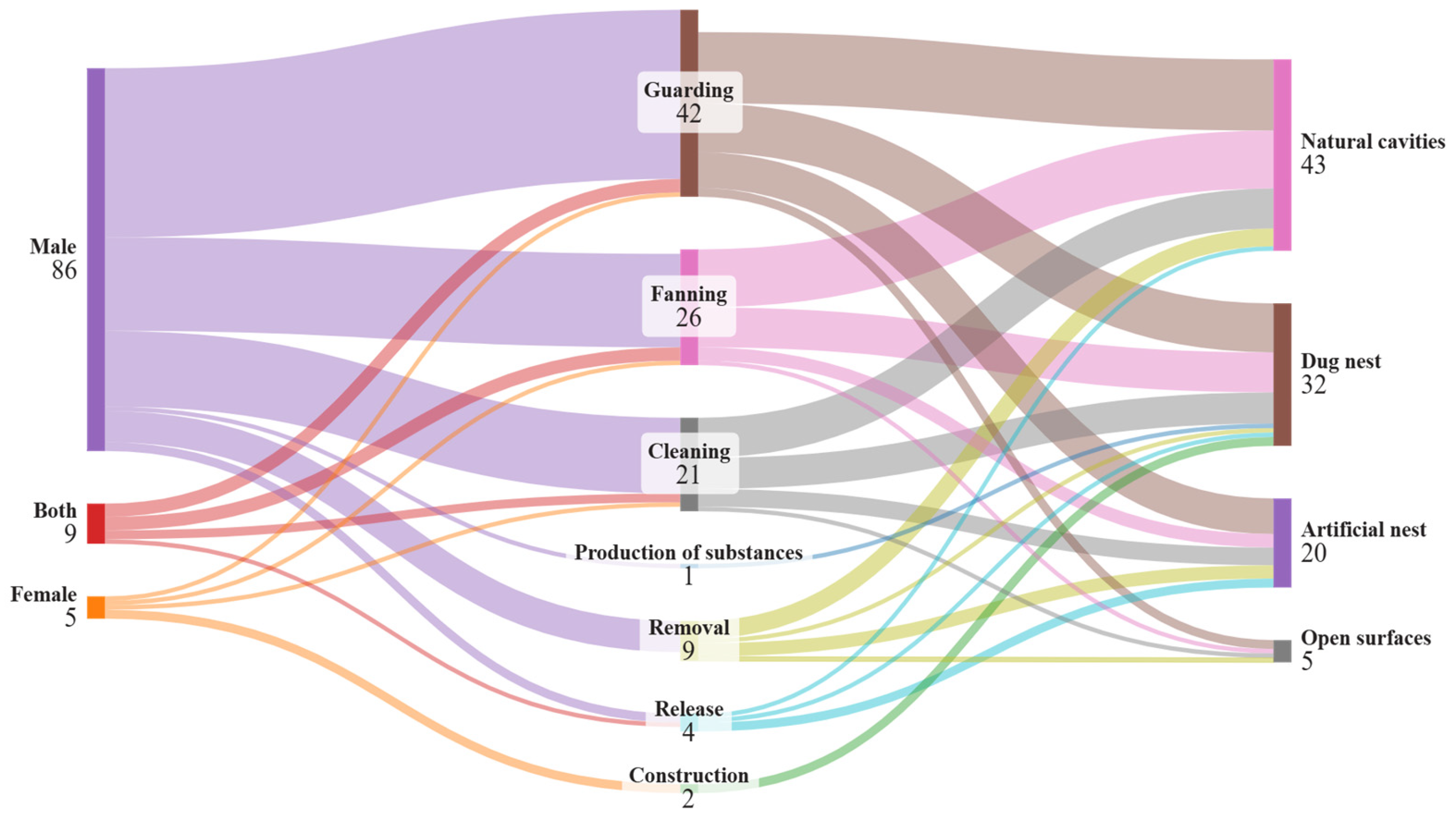

| Type of Care | Description | References |
|---|---|---|
| Protection/guarding | Displays toward and/or directed at conspecifics or heterospecifics, presumably to drive potential predators away from eggs and/or the nest. Parents blocking the nest entrance in a vigilant posture. Parents bite or sometimes throw sand towards possible predators. This behavior is assumed to increase offspring survival by reducing predation on offspring. | [20,24,25,26] |
| Fanning/aeration of eggs | Parent creates a flow of water over eggs by rhythmically moving their pectoral fins, pelvic fins, caudal fin, or tail over the egg mass. This behavior is assumed to eliminate sedimentation from eggs and/or to increase oxygenation of eggs. | [24,25,27,28,29] |
| Cleaning | Taking eggs from the nest into mouth and manipulating the eggs without consuming them. Actions such as touching the eggs with fins were also considered as cleaning. Cleaning is assumed to increase egg survival or quality by improving the health and viability of eggs through preventative mechanisms. | [24,25] |
| Egg/organism removal | Dead, sick, or non-viable eggs are removed from the egg mass with the mouth to prevent further spread of egg diseases. Parents can remove other organisms present in the nest such as gastropods. This type of behavior may be accompanied by inspection of eggs that may serve to detect non-viable eggs. Removal behavior is assumed to increase remaining egg survival and quality by improving the health and viability of eggs following infection or the presence of other living organisms that could harm the eggs. | [20,24,25,30] |
| Larval release * | Parents help fully developed larvae to hatch and leave the nest. This behavior is assumed to increase offspring survival by increasing hatching success and allowing larvae to be placed in a location that increases survival (e.g., a location away from potential predators). | [20,31] |
| Production of antimicrobial substances * | Parents produce substances that inhibit the proliferation of pathogens such as bacteria or fungi that can cause disease among eggs. Production of antimicrobial substances is assumed to increase egg survival or quality by improving the health and viability of eggs. | [32,33] |
| Construction of elevated mound | Using substrates to create a mound after closing the entrance of the burrow. This behavior is assumed to increase offspring survival by influencing oxygenation inside the burrow. | [24,25,34] |
| Species | Spawning Site | Clutch Size (Number of Eggs) | Egg Size (mm) | Form of Care | Type Of Care Performed By Each Sex | Care Duration (days) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | F | C | R | L | O | M | |||||||
| Amblygobius phalaena ✹ | Males construct a burrow under coral pavement | 11,022–95,858 | 1.64 | Uniparental 1 | ♂ or ♀ | ♂ or ♀ | ♂ or ♀ 2 | 3–4 | [35,36,37] | ||||
| Bathygobius fuscus 🟉★✹ | Holes or cracks in the rocks or empty shells of bivalves | 72,467 | Uniparental | ♂ | ♂ | 4–5 | [38,39,40,41,42] | ||||||
| Bathygobius soporator 🟉★✹ | Shells (underside) | 15,000–18,000 per female | 2.25–2.42 | Uniparental | ♂ | ♂ | ♂ | 5 | [14,43,44] | ||||
| Chlamydogobius eremius 🟉 | Under rocks crevices | Uniparental | ♂ | ♂ | ♂ | [45,46,47,48,49] | |||||||
| Crystallogobius linearis ✹ | Empty tubes of polychaetes | Uniparental | ♂ | [50] | |||||||||
| Elacatinus colini ✹ | Inner sponge lumen; PVC pipes | 168 | Uniparental | ♂ | ♂ | ♂ | 6.8 | [31,51] | |||||
| Elacatinus evelynae ✹ | Cracks or holes between/in live coral or dead coral debris | 200–250 | 1.1–3.3 | Potentially Biparental 3 | ♂ | ♂ | 5–6 | [52,53,54,55,56] | |||||
| Elacatinus figaro ✹ | Underside of shells 4 | 430–1020 | 1.81 | Uniparental | ♂ | ♂ | 6.8 | [57,58] | |||||
| Elacatinus oceanops ✹ | Small shell of Tridacna with concave surface downwards | 300–450 | 2.3–3.3 | Uniparental | ♂ | [56,59,60] | |||||||
| Elacatinus puncticulatus ✹ | PVC pipes 4 | 277–418 | 1.71–2.64 | Uniparental | ♂ | ♂ | ♂ | ♂ | ♂ | 6–8 | [20] | ||
| Eucyclogobius newberryi ★✹ | Burrows constructed in sand or mud by males; PVC pipes | 100–1000 | 5–6 | Uniparental | ♂ | ? 5 | 9–11 | [61,62,63,64] | |||||
| Eviota abax ✹ | PVC pipes 4 | Uniparental | ♂ | ♂ | ♂ | 5 | [65,66] | ||||||
| Eviota prasina ✹ | Holes or crack in substrate | Uniparental | ♂ | ♂ | ♂ | 4–5 | [30,67] | ||||||
| Gobius cobitis ★✹ | Under rocks with flattened lower side | 3000–24,000 | 3.44–4.3 | Uniparental | ♂ | ♂ | ♂ 6 | [15,16,68,69] | |||||
| Gobius cruentatus ✹ | In the lower side of a horizontal rock 4 | 21,296 | 1.90–2.10 | Uniparental | ♂ | ♂ 6 | [70] | ||||||
| Gobius ophiocephalus ★✹ | Males excavate cavity in soft bottom of seagrass meadows | Uniparental | ♂ | ♂ | ♂ | ♂ | [32,71,72,73] | ||||||
| Gobius niger ★✹ | Excavation of small cavities under rocks or artificial nests or shells | Uniparental | ♂ | ♂ | [74,75,76] | ||||||||
| Gymnogobius isaza 🟉 | Under a stone or a large rock | 5000 | Biparental 7 | ♂ & ♀ 8 | ♂ & ♀ | ♂ & ♀ 6 | 10–21 | [77,78,79] | |||||
| Knipowitschia panizzae 🟉★ | Empty shells of bivalves, preferably Cerastoderma lamarcki; the male excavates under the shell and covers it with sand | Uniparental | ♂ | 7 | [80,81,82] | ||||||||
| Lythrypnus dalli ✹ | PVC pipes; 4 natural nesting sites can include empty abalone shells, under the surface of rocks, or small crevices | 319–2333 | 1.88 | Uniparental | ♂ | ♂ | ♂ 6 | [83,84,85,86] | |||||
| Lythrypnus pulchellus ✹ | PVC pipes 4 | Uniparental | ♂ | [87] | |||||||||
| Microgobius gulosus 🟉★✹ | Construction of burrows in loose sand, under the roots of aquatics plants; after spawning, males cover burrow entrance. | 340–442 | Biparental 3,9 | ♂ | ? 10 | 4 | [88] | ||||||
| Padogobius bonelli 🟉 | Underside of a rock | 100–300 per female | Uniparental | ♂ | ♂ | ♂ 6 | 12–22 | [17,89,90,91] | |||||
| Padogobius nigricans 🟉 | Under rocks | 70–100 per female | Uniparental | ♂ | ♂ | ♂ | ♂ | 15–17 | [18,91] | ||||
| Paragobiodon echinocephalus ✹ | Males crop coral tissue from a branch | 245–1118 | 1–1.1 | Uniparental | ♂ | ♂ | ♂ 6 | 3–5 | [92,93] | ||||
| Pomatoschistus flavescens ★✹ | Empty mussel shells or on algae | 1234–16,514 | Uniparental | ♂ | ♂ | ♂ | ♂ | 10 | [7,11,19,94,95,96] | ||||
| Pomatoschistus marmoratus 🟉★✹ | Inside of empty bivalve shells that are covered with sand | 412–2904 | 0.66–1.05 | Uniparental | ♂ | ♂ | [97,98] | ||||||
| Pomatoschistus minutus ★✹ | Male digs a nest under a suitable site (usually a shell of bivalve Mya arenaria or Mytilus edulis or a rock) and covers it with sand, leaving a single narrow opening | Uniparental | ♂ | ♂ | ♂ | ♂ | 7–21 | [75,99,100,101,102,103,104,105] | |||||
| Priolepis cincta ★✹ | PVC pipes 4 | 0.76–1.12 | Uniparental | ♂ | ♂ | 3–4 | [106,107] | ||||||
| Rhinogobiops nicholsii ✹ | Male excavates a nest under a rock | 1700 | 2.1 | Uniparental | ♂ | [108,109,110] | |||||||
| Sicyopterus lagocephalus 🟉★✹ | Male constructs a nest on or under stones or on plant stems | 5424–112,000 | Uniparental | ♂ 11 | 1–2 | [111,112,113] | |||||||
| Signigobius biocellatus ✹ | Both parents construct several burrows | Biparental | ♂ & ♀ 12 | ♂ & ♀ | ? 10 | [63,114] | |||||||
| Trimma taylori ✹ | PVC pipes 4 | 190–1233 | Biparental | ♂ 13 & ♀ 13 | ♂ | [115] | |||||||
| Typhlogobius californiensis ✹ | Burrow in the sand | 5040 | 2.7–2.85 | Biparental | ♂ & ♀ | ♂ & ♀ | ♂ & ♀ 14 | 10–12 | [116] | ||||
| Valenciennea longipinnis ✹ | Excavate burrows under coral pavement and rubble; after spawning, female closes the entrance and constructs a conspicuous mound | 128,873 | 1.1 | Biparental | ♂ | ♂ | ♀ | 3–5 | [34,117,118] | ||||
| Valenciennea strigata ✹ | A mating pair construct burrows under coral pavement and rubble; after spawning, female deposits coral rubble and algae over the burrow entrance | Biparental 15 | ♀ 16 | 1–4 | [119,120] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo-García, M.; Ceballos-Vázquez, B.P.; Guevara-Fiore, P.; Klug, H. An Overview of Post-Fertilization Parental Care in Gobiidae. Diversity 2025, 17, 446. https://doi.org/10.3390/d17070446
Trujillo-García M, Ceballos-Vázquez BP, Guevara-Fiore P, Klug H. An Overview of Post-Fertilization Parental Care in Gobiidae. Diversity. 2025; 17(7):446. https://doi.org/10.3390/d17070446
Chicago/Turabian StyleTrujillo-García, Miguel, Bertha Patricia Ceballos-Vázquez, Palestina Guevara-Fiore, and Hope Klug. 2025. "An Overview of Post-Fertilization Parental Care in Gobiidae" Diversity 17, no. 7: 446. https://doi.org/10.3390/d17070446
APA StyleTrujillo-García, M., Ceballos-Vázquez, B. P., Guevara-Fiore, P., & Klug, H. (2025). An Overview of Post-Fertilization Parental Care in Gobiidae. Diversity, 17(7), 446. https://doi.org/10.3390/d17070446








