Benthic Infauna in the Shallow-Water Hydrothermal System of Banderas Bay, Mexico: A Two-Period Comparison
Abstract
1. Introduction
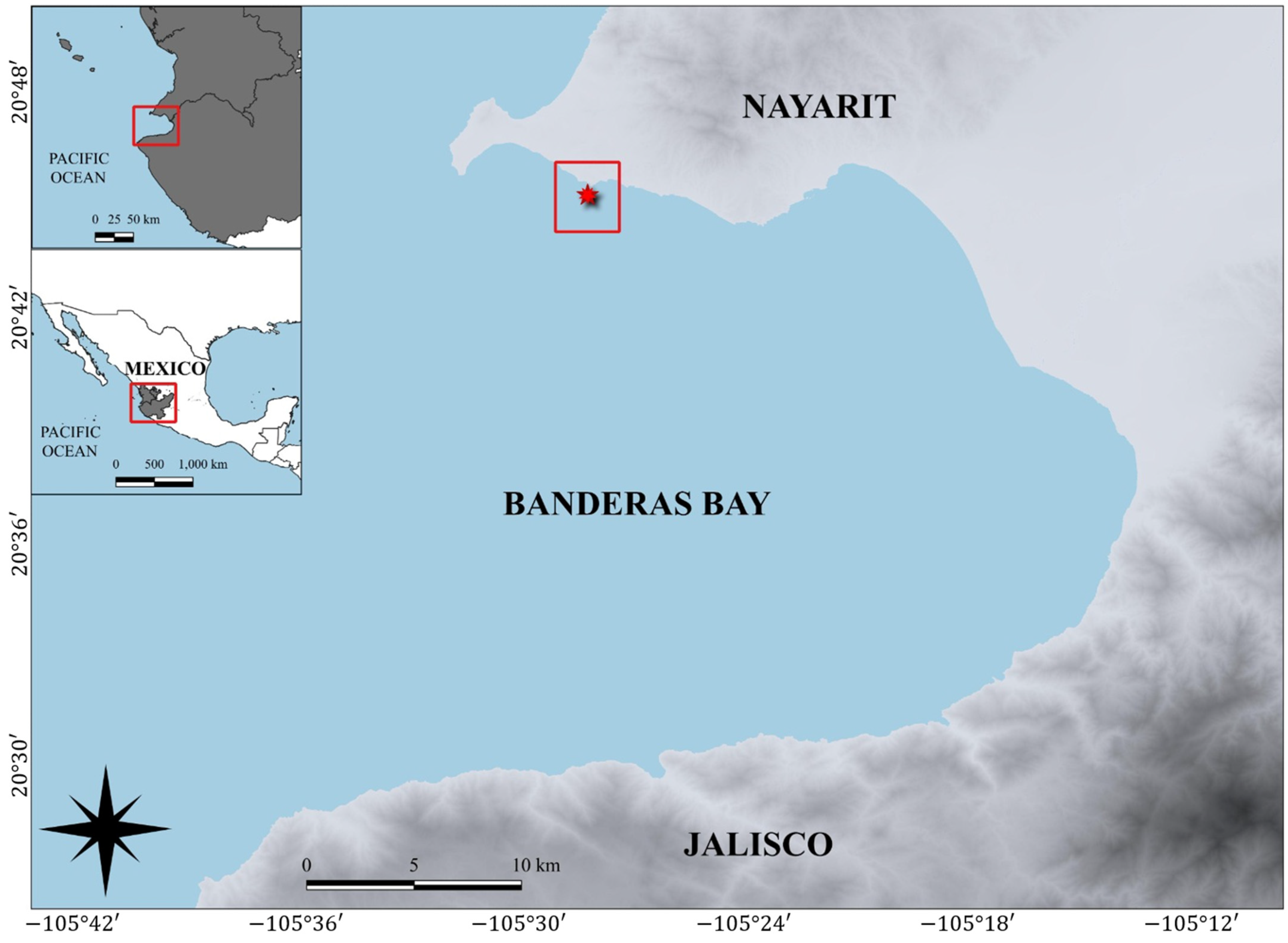
2. Materials and Methods
3. Results
3.1. Physicochemical Parameters
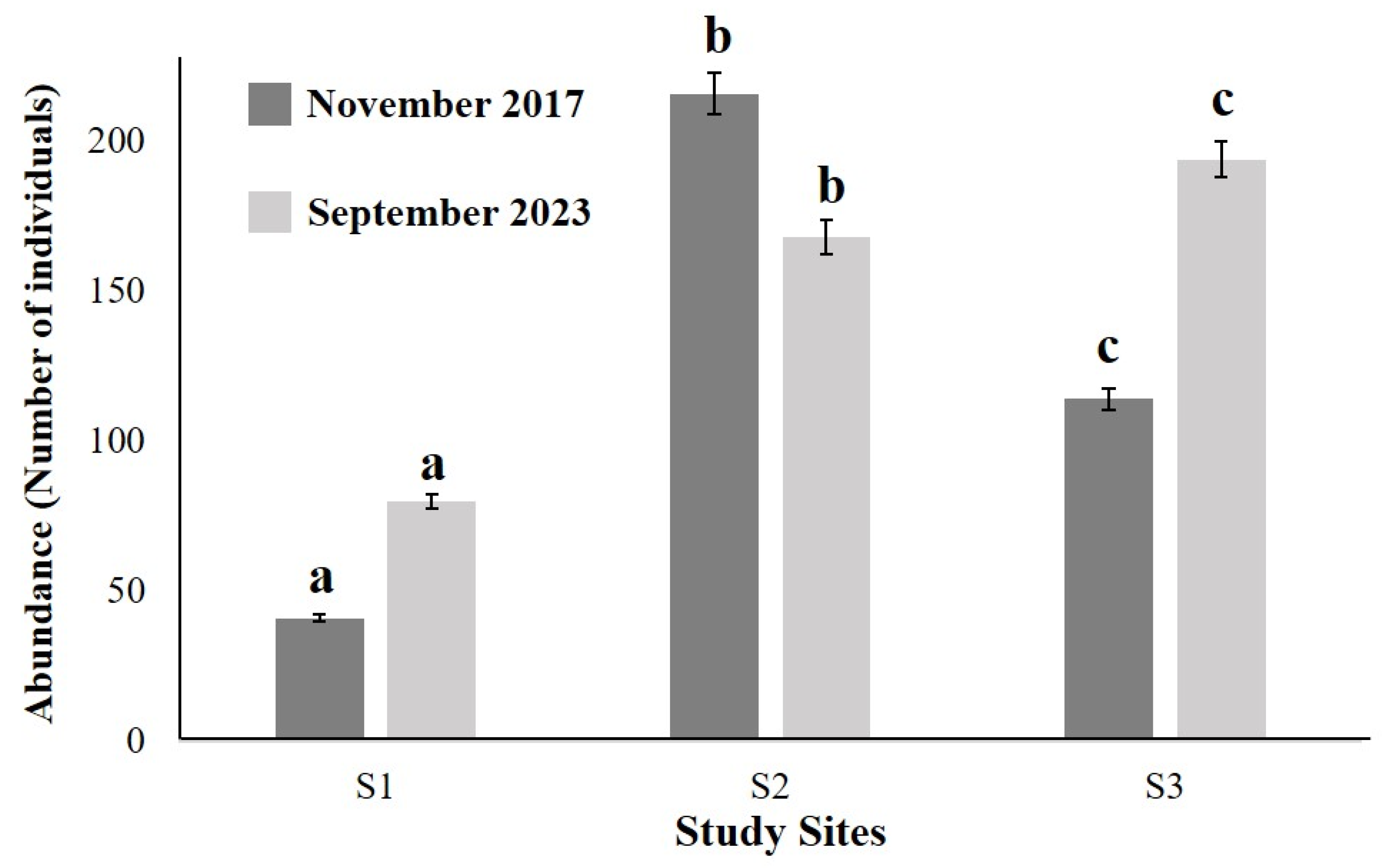
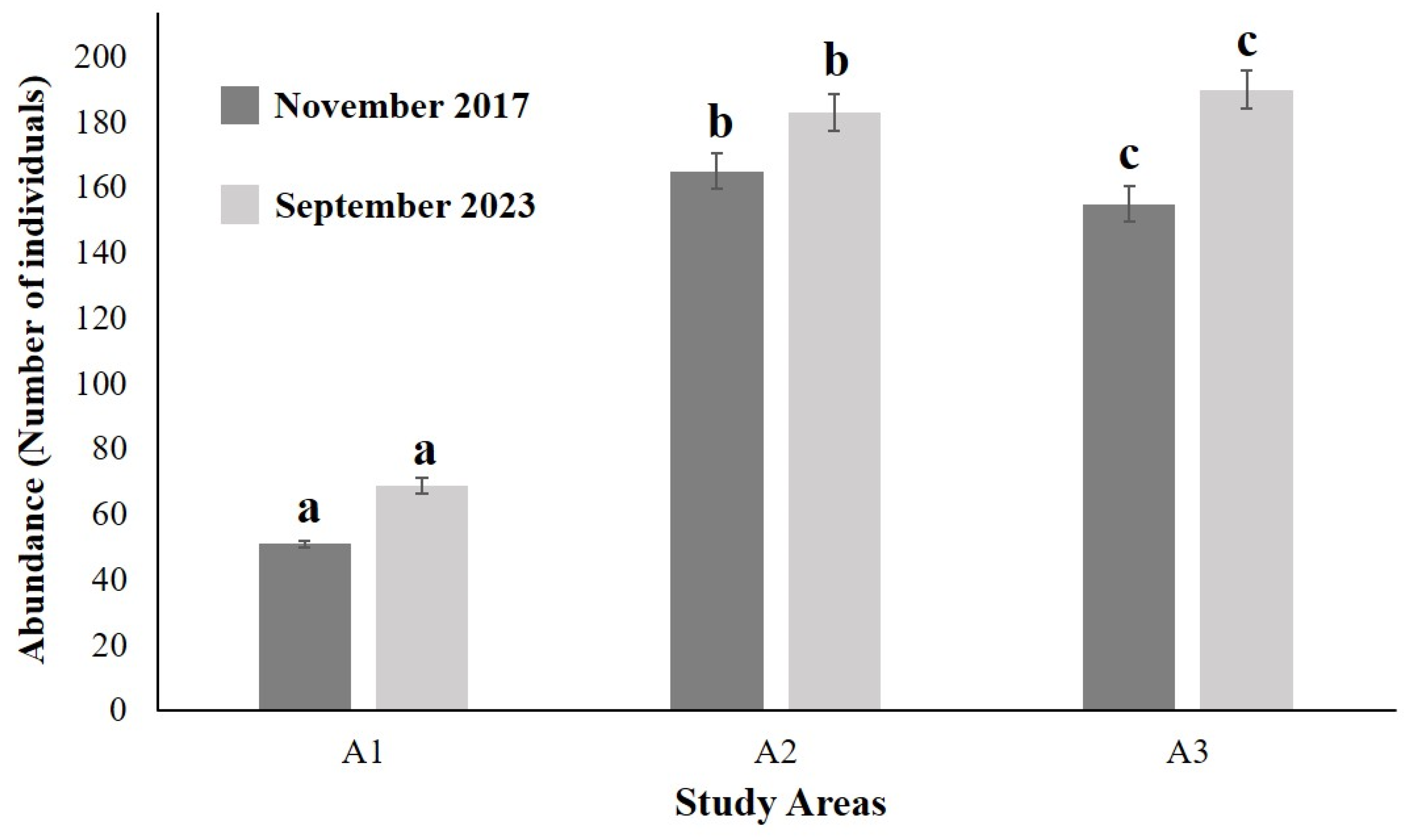
3.2. Benthic Infauna
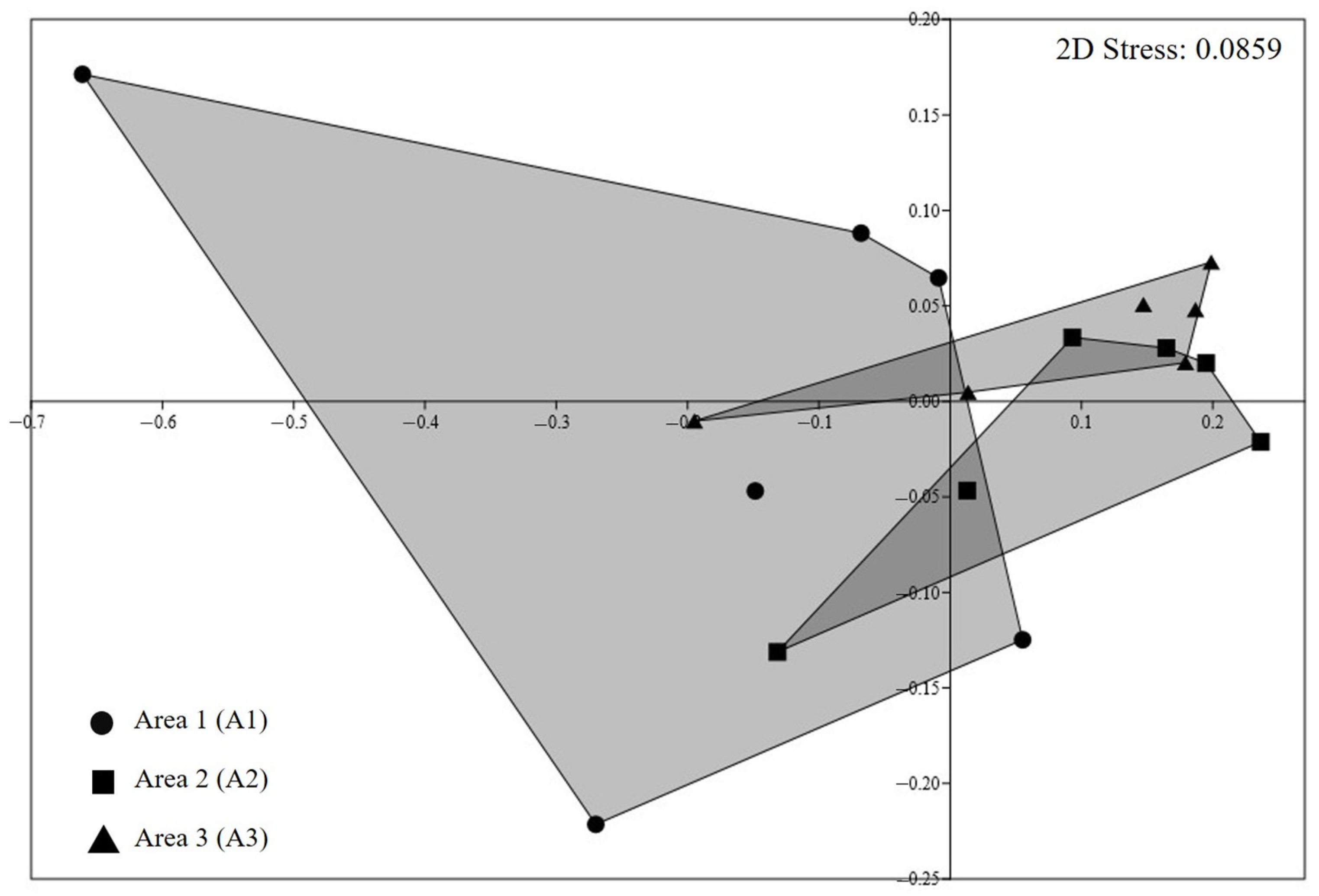
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarasov, V.G.; Gebruk, A.V.; Mironov, A.N.; Moskalev, L.I. Deep-Sea and Shallow-Water Hydrothermal Vent Communities: Two Different Phenomena? Chem. Geol. 2005, 224, 5–39. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Canet, C. Evaluación y explotación de los recursos geotérmicos del océano. In La Frontera Final: El Océano Profundo; Low-Pfeng, A., Peters-Recagno, E.M., Eds.; Secretaría de Medio Ambiente y Recursos Naturales & Instituto Nacional de Ecología y Cambio Climático: Ciudad de México, Mexico, 2014; pp. 11–30. [Google Scholar]
- Gugliandolo, C.; Maugeri, T.L. Chemolithotrophic, Sulfur-Oxidizing Bacteria from a Marine, Shallow Hydrothermal Vent of Vulcano (Italy). Geomicrobiol. J. 1993, 11, 109–120. [Google Scholar] [CrossRef]
- Dando, P.R.; Varnavas, S.P. Hydrothermalism in the Mediterranean Sea. Prog. Oceanogr. 1999, 44, 333–367. [Google Scholar] [CrossRef]
- Price, R.E.; Savov, I.; Planer-Friedrich, B.; Bühring, S.I.; Amend, J.; Pichler, T. Processes Influencing Extreme as Enrichment in Shallow-Sea Hydrothermal Fluids of Milos Island, Greece. Chem. Geol. 2013, 348, 15–26. [Google Scholar] [CrossRef]
- Tarasov, V.G. Effects of Shallow-Water Hydrothermal Venting on Biological Communities of Coastal Marine Ecosystems of the Western Pacific. Adv. Mar. Biol. 2006, 50, 267–421. [Google Scholar] [CrossRef] [PubMed]
- Dando, P.R.; Hughes, J.A.; Leahy, Y.; Niven, S.S.J.; Taylor, L.J.; Smiths, C. Gas Venting Rates from Submarine Hydrothermal Areas around the Island of Milos, Hellenic Volcanic Arc. Cont. Shelf Res. 1995, 15, 913–929. [Google Scholar] [CrossRef]
- Botz, R.; Stüben, D.; Winckler, G.; Bayer, R.; Schmitt, M.; Faber, E. Hydrothermal Gases Offshore Milos Island, Greece. Chem. Geol. 1996, 130, 161–173. [Google Scholar] [CrossRef]
- Fitzsimons, M.F.; Dando, P.R.; Hughes, J.A.; Thiermann, F.; Akoumianaki, I.; Pratt, S.M. Submarine Hydrothermal Brine Seeps off Milos, Greece: Observations and Geochemistry. Mar. Chem. 1997, 57, 325–340. [Google Scholar] [CrossRef]
- Tarasov, V.G. The Coastal Ecosystems and Shallow-Water Hydrothermal Venting; Dalnauka, V., Ed.; Dalnauka Press: Vladivostok, Russia, 1999. [Google Scholar]
- Rodríguez-Uribe, M.C.; Núñez-Cornú, F.J.; Prol-Ledesma, R.M.; Salazar-Silva, P. Benthic Infauna Associated with a Shallow-Water Hydrothermal System of Punta Mita (Mexico). J. Mar. Biol. Assoc. UK 2023, 103, e26. [Google Scholar] [CrossRef]
- Núñez-Cornú, F.J.; Prol-Ledesma, R.M.; Cupul-Magaña, A.; Suárez-Plascencia, C. Near Shore Submarine Hydrothermal Activity in Bahia Banderas Western Mexico. West. Mex. Geofísica Int. 2000, 39, 171–178. [Google Scholar] [CrossRef]
- Vidal, V.M.; Vidal, F.V.; Isaacs, J.D.; Young, D.R. Coastal Submarine Hydrothermal Activity off Northern Baja California. J. Geophys. Res. 1978, 83, 1757–1774. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Canet, C.; Torres-Vera, M.A.; Forrest, M.J.; Armienta, M.A. Vent Fluid Chemistry in Bahía Concepción Coastal Submarine Hydrothermal System, Baja California Sur, Mexico. J. Volcanol. Geotherm. Res. 2004, 137, 311–328. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Báncora-Alsina, C.; Prol-Ledesma, R.M.; Hiriart, G. A New Geothermal Resource in Los Cabos, Baja California Sur, Mexico. In Proceedings of the 28th New Zealand Geothermal Workshop, Auckland, New Zealand, 15–17 November 2006; pp. 15–17. [Google Scholar]
- Arellano-Ramirez, Y.; Kretzschmar, T.G.; Hernandez-Martinez, R. Water-Rock-Microbial Interactions in the Hydrothermal Spring of Puertecitos, Baja California, Mexico. Procedia Earth Planet. Sci. 2017, 17, 865–868. [Google Scholar] [CrossRef]
- Estradas-Romero, A.; María Prol-Ledesma, R. Effect of Shallow Hydrothermal Venting on the Richness of Benthic Diatom Species. Cah. Biol. Mar. 2014, 55, 399–408. [Google Scholar]
- Dávila-Ramos, S.; Estradas-Romero, A.; Prol-Ledesma, R.M.; Juárez-López, K. Bacterial Populations (First Record) at Two Shallow Hydrothermal Vents of the Mexican Pacific West Coast. Geomicrobiol. J. 2015, 32, 657–665. [Google Scholar] [CrossRef]
- Rodríguez-Uribe, M.C.; Chávez-Dagostino, R.M.; Del Moral-Flores, L.F.; Bravo-Olivas, M.L. First Record of Amphioxus Branchiostoma Californiense (Amphioxiformes: Branchiostomatidae) Adjacent to a Shallow Submarine Hydrothermal System at Banderas Bay (Mexico). Diversity 2019, 11, 227. [Google Scholar] [CrossRef]
- Rodríguez-Uribe, M.C.; Jarquín-González, J.; Salazar-Silva, P.; Chávez-Dagostino, R.M.; Merino, N.B. Cumaceans (Crustacea, Peracarida) Associated with Shallow-Water Hydrothermal Vents at Banderas Bay, Mexico. Biodivers. Data J. 2024, 12, e139801. [Google Scholar] [CrossRef]
- De León-González, J.A.; Bastida-Zavala, J.R.; Carrera-Parra, F.; García-Garza, M.E.; Peña-Rivera, A.; Salazar-Vallejo, S.I.; Solis-Weiss, V. Poliquetos (Annelida: Polychaeta) de México y América Tropical; Universidad Autonoma de Nuevo León, Dirección de Publicaciones: San Nicolás de los Garza, Nuevo León, México, 2009. [Google Scholar]
- Brusca, R.C.; Moore, W.; Shuster, S.M. Invertebrates, 3rd ed.; Oxford University Press: Oxford, UK, 2016; ISBN 978-1605353753. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Kruskal, J.B.; Wish, M. Multidimensional Scaling; Sage University Paper Series on Quantitative Applications in the Social Science; No. 07-011; Sage Publications: Newbury Park, CA, USA, 1978. [Google Scholar]
- Chapman, M.G.; Underwood, A.J. Ecological Patterns in Multivariate Assemblages: Information and Interpretation of Negative Values in ANOSIM Tests. Mar. Ecol. Prog. Ser. 1999, 180, 257–265. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Clarke, K.R.; Gorley, R.N. Analysis of Similarities (ANOSIM) for 3-Way Designs. Austral Ecol. 2021, 46, 927–941. [Google Scholar] [CrossRef]
- Velázquez Ruiz, A.; Manuel Martínez, L.R.; Maciel Carrillo González, F. Caracterización Climática Para La Región de Bahía de Banderas Mediante El Sistema de Köppen, Modificado Por García, y Técnicas de Sistemas de Información Geográfica. Investig. Geográficas 2012, 79, 7–19. [Google Scholar] [CrossRef]
- Tovilla Hernández, C.; de la Lanza Espino, G. Balance Hidrológico y de Nutrientes En Un Humedal Costero Del Pacífico Sur de México. Hidrobiológica 2001, 11, 133–140. [Google Scholar]
- Mateus, L.O.; Saavedra, D.M.Q. Variación Espacio-Temporal de La Calidad Del Agua Del Golfo de Morrosquillo Durante El Año 2013 Spatial and Temporal Variability of Quality Water in Morrosquillo Gulf during 2013. Bol. Cient. CIOH 2015, 33, 19–38. [Google Scholar]
- Cardigos, F.; Colaço, A.; Dando, P.R.; Ávila, S.P.; Sarradin, P.M.; Tempera, F.; Conceição, P.; Pascoal, A.; Santos, R.S. Shallow Water Hydrothermal Vent Field Fluids and Communities of the D. João de Castro Seamount (Azores). Chem. Geol. 2005, 224, 153–168. [Google Scholar] [CrossRef]
- Marques Mendes, R. Influência Das Fontes Hidrotermais Marinhas de Baixa Profundidade na Composição das Comunidades de Meiofauna. Secção d Relatório de Estágio D de Anatomia e Taxonomia Zoológica; Universidade dos Açores: Ponta Delgada, Portugal, 2008. [Google Scholar]
- Melwani, A.R.; Kim, S.L. Benthic Infaunal Distributions in Shallow Hydrothermal Vent Sediments. Acta Oecologica 2008, 33, 162–175. [Google Scholar] [CrossRef]
- Couto, R.P.; Rodrigues, A.S.; Neto, A.I. Shallow-Water Hydrothermal Vents in the Azores (Portugal). J. Integr. Coast. Zone Manag. 2015, 15, 495–505. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Canet, C.; Melgarejo, J.C.; Tolson, G.; Rubio-Ramos, M.A.; Cruz-Ocampo, J.C.; Ortega-Osorio, A.; Torres-Vera, M.A.; Reyes, A. Cinnabar Deposition in Submarine Coastal Hydrothermal Vents, Pacific Margin of Central Mexico. Econ. Geol. 2002, 97, 1331–1340. [Google Scholar] [CrossRef]
| Parameter | S1 | S2 | S3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A1 | A2 | A3 | A1 | A2 | A3 | |
| November 2017 | |||||||||
| pH | 7.67 | 7.93 | 8.05 | 7.71 | 8.00 | 8.03 | 7.66 | 7.91 | 8.08 |
| Temperature (°C) | 87.0 | 31.0 | 27.4 | 85.0 | 30.0 | 27.3 | 88.0 | 30.5 | 27.8 |
| September 2023 | |||||||||
| pH | 7.72 | 7.90 | 8.06 | 7.76 | 7.95 | 8.08 | 7.79 | 7.98 | 8.07 |
| Temperature (°C) | 78.0 | 32.0 | 28.3 | 82.0 | 31.0 | 28.2 | 79.0 | 31.0 | 28.1 |
| Taxonomic Class | Invertebrate Group | November 2017 | September 2023 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sites | Number of Individuals | Abundance (%) | Sites | Number of Individuals | Abundance (%) | ||||||
| S1 | S2 | S3 | S1 | S2 | S3 | ||||||
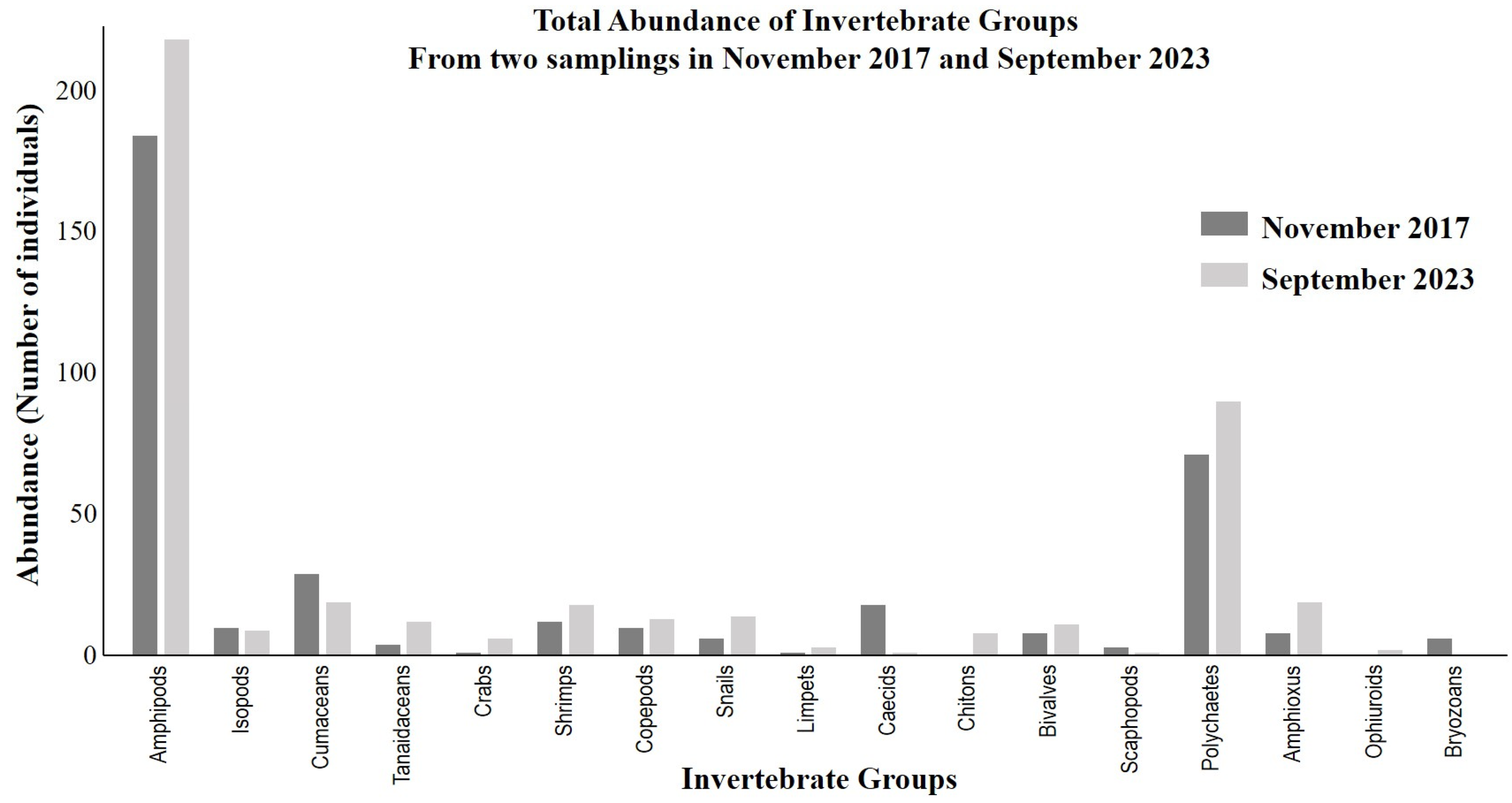
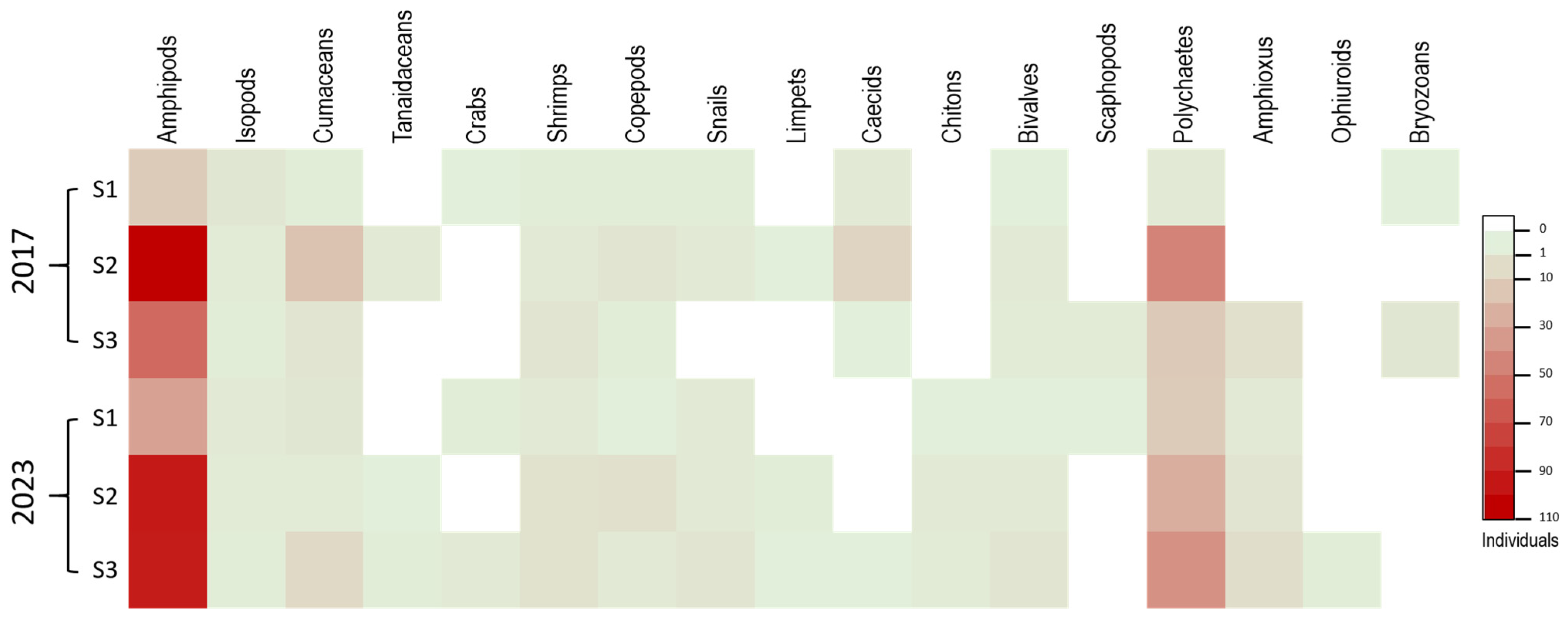
| Malacostraca | Amphipods | 17 | 107 | 60 | 184 | 49.60 | 36 | 96 | 95 | 227 | 51.13 |
| Isopods | 5 | 3 | 2 | 10 | 2.70 | 4 | 3 | 2 | 9 | 2.03 | |
| Cumaceans | 2 | 21 | 6 | 29 | 7.82 | 5 | 3 | 11 | 19 | 4.28 | |
| Tanaidaceans | 0 | 4 | 0 | 4 | 1.08 | 0 | 1 | 2 | 3 | 0.68 | |
| Crabs | 1 | 0 | 0 | 1 | 0.27 | 2 | 0 | 4 | 6 | 1.35 | |
| Shrimps | 2 | 4 | 6 | 12 | 3.23 | 4 | 7 | 7 | 18 | 4.05 | |
| Maxillopoda | Copepods | 2 | 6 | 2 | 10 | 2.70 | 1 | 8 | 4 | 13 | 2.93 |
| Gastropoda | Snails | 2 | 4 | 0 | 6 | 1.62 | 4 | 4 | 6 | 14 | 3.15 |
| Limpets | 0 | 1 | 0 | 1 | 0.27 | 0 | 2 | 1 | 3 | 0.68 | |
| Caecids | 4 | 13 | 1 | 18 | 4.85 | 0 | 0 | 1 | 1 | 0.23 | |
| Polyplacophora | Chitons | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 3 | 8 | 1.80 |
| Bivalvia | Bivalves | 1 | 4 | 3 | 8 | 2.16 | 1 | 4 | 6 | 11 | 2.48 |
| Scaphopoda | Scaphopods | 0 | 0 | 3 | 3 | 0.81 | 1 | 0 | 0 | 1 | 0.23 |
| Polychaeta | Polychaetes | 4 | 49 | 18 | 71 | 19.14 | 17 | 30 | 43 | 90 | 20.27 |
| Leptocardii | Amphioxus | 0 | 0 | 8 | 8 | 2.16 | 4 | 6 | 9 | 19 | 4.28 |
| Ophiuroidea | Ophiuroids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0.45 |
| Stenolaemata | Bryozoans | 1 | 0 | 5 | 6 | 1.62 | 0 | 0 | 0 | 0 | 0 |
| Abundance | 41 | 216 | 114 | 371 | 100 | 80 | 168 | 196 | 444 | 100 | |
| Taxonomic richness | 11 | 11 | 11 | 12 | 12 | 15 | |||||
| Taxonomic Class | Invertebrate Group | November 2017 | September 2023 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Number of Individuals | Abundance (%) | Area | Number of Individuals | Abundance (%) | ||||||
| A1 | A2 | A3 | A1 | A2 | A3 | ||||||
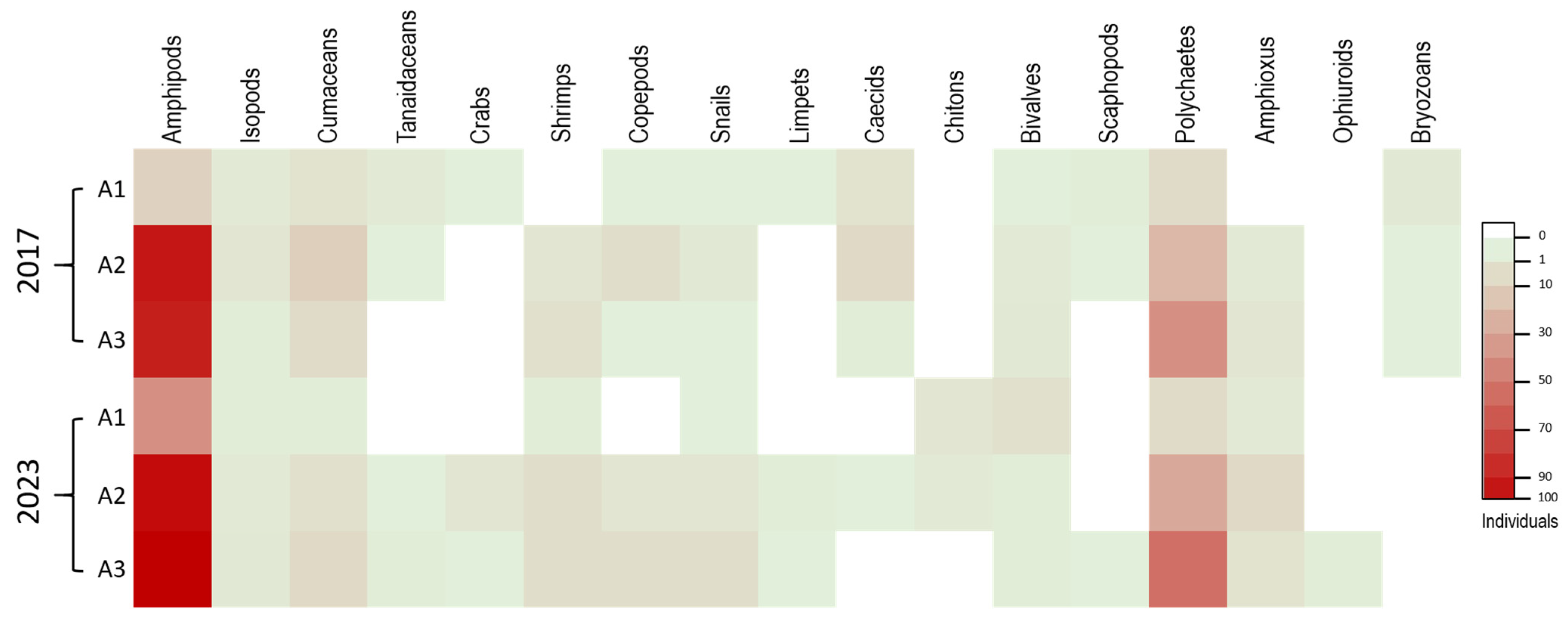
| Malacostraca | Amphipods | 13 | 87 | 84 | 184 | 49.60 | 39 | 92 | 96 | 227 | 51.13 |
| Isopods | 3 | 5 | 2 | 10 | 2.70 | 2 | 3 | 4 | 9 | 2.03 | |
| Cumaceans | 6 | 14 | 9 | 29 | 7.82 | 2 | 7 | 10 | 19 | 4.28 | |
| Tanaidaceans | 3 | 1 | 0 | 4 | 1.08 | 0 | 1 | 2 | 3 | 0.68 | |
| Crabs | 1 | 0 | 0 | 1 | 0.27 | 0 | 5 | 1 | 6 | 1.35 | |
| Shrimps | 0 | 5 | 7 | 12 | 3.23 | 2 | 8 | 8 | 18 | 4.05 | |
| Maxillopoda | Copepods | 1 | 8 | 1 | 10 | 2.70 | 0 | 5 | 8 | 13 | 2.93 |
| Gastropoda | Snails | 1 | 4 | 1 | 6 | 1.62 | 1 | 5 | 8 | 14 | 3.15 |
| Limpets | 1 | 0 | 0 | 1 | 0.27 | 0 | 2 | 1 | 3 | 0.68 | |
| Caecids | 6 | 10 | 2 | 18 | 4.85 | 0 | 1 | 0 | 1 | 0.23 | |
| Polyplacophora | Chitons | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 0 | 8 | 1.80 |
| Bivalvia | Bivalves | 1 | 3 | 4 | 8 | 2.16 | 7 | 2 | 2 | 11 | 2.48 |
| Scaphopoda | Scaphopods | 2 | 1 | 0 | 3 | 0.81 | 0 | 0 | 1 | 1 | 0.23 |
| Polychaeta | Polychaetes | 9 | 23 | 39 | 71 | 19.14 | 9 | 29 | 52 | 90 | 20.27 |
| Leptocardii | Amphioxus | 0 | 3 | 5 | 8 | 2.16 | 3 | 10 | 6 | 19 | 4.28 |
| Ophiuroidea | Ophiuroids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0.45 |
| Stenolaemata | Bryozoans | 4 | 1 | 1 | 6 | 1.62 | 0 | 0 | 0 | 0 | 0 |
| Abundance | 51 | 165 | 155 | 371 | 100 | 70 | 173 | 201 | 444 | 100 | |
| Taxonomic richness | 13 | 13 | 11 | 9 | 14 | 14 | |||||
| Taxonomic Class | Invertebrate Group | Present in | |
|---|---|---|---|
| 2017 | 2023 | ||
| Malacostraca | Amphipods | ✓ | ✓ |
| Isopods | ✓ | ✓ | |
| Cumaceans | ✓ | ✓ | |
| Tanaidaceans | ✓ | ✓ | |
| Crabs | ✓ | ✓ | |
| Shrimps | ✓ | ✓ | |
| Maxillopoda | Copepods | ✓ | ✓ |
| Gastropoda | Snails | ✓ | ✓ |
| Limpets | ✓ | ✓ | |
| Caecids | ✓ | ✓ | |
| Polyplacophora | Chitons | ✗ | ✓ |
| Bivalvia | Bivalves | ✓ | ✓ |
| Scaphopoda | Scaphopods | ✓ | ✓ |
| Polychaeta | Polychaetes | ✓ | ✓ |
| Leptocardii | Amphioxus | ✓ | ✓ |
| Ophiuroidea | Ophiuroids | ✗ | ✓ |
| Stenolaemata | Bryozoans | ✓ | ✗ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Uribe, M.C.; Chávez-Dagostino, R.M.; Salazar-Silva, P.; Jarquín-González, J.; Raymundo-Huizar, A.R.; Carrillo-González, F.M. Benthic Infauna in the Shallow-Water Hydrothermal System of Banderas Bay, Mexico: A Two-Period Comparison. Diversity 2025, 17, 440. https://doi.org/10.3390/d17070440
Rodríguez-Uribe MC, Chávez-Dagostino RM, Salazar-Silva P, Jarquín-González J, Raymundo-Huizar AR, Carrillo-González FM. Benthic Infauna in the Shallow-Water Hydrothermal System of Banderas Bay, Mexico: A Two-Period Comparison. Diversity. 2025; 17(7):440. https://doi.org/10.3390/d17070440
Chicago/Turabian StyleRodríguez-Uribe, María Carolina, Rosa María Chávez-Dagostino, Patricia Salazar-Silva, Jani Jarquín-González, Alma Rosa Raymundo-Huizar, and Fátima Maciel Carrillo-González. 2025. "Benthic Infauna in the Shallow-Water Hydrothermal System of Banderas Bay, Mexico: A Two-Period Comparison" Diversity 17, no. 7: 440. https://doi.org/10.3390/d17070440
APA StyleRodríguez-Uribe, M. C., Chávez-Dagostino, R. M., Salazar-Silva, P., Jarquín-González, J., Raymundo-Huizar, A. R., & Carrillo-González, F. M. (2025). Benthic Infauna in the Shallow-Water Hydrothermal System of Banderas Bay, Mexico: A Two-Period Comparison. Diversity, 17(7), 440. https://doi.org/10.3390/d17070440






