Alien Stramenopilous Fungus-like Organisms (Oomycota) Diversity and Distribution in Lithuania
Abstract
1. Introduction
2. Data Collection and Determination of the Current Status of Alien Species
3. Alien Stramenopilous Fungus-like Organisms (Oomycota) Recorded in Lithuania
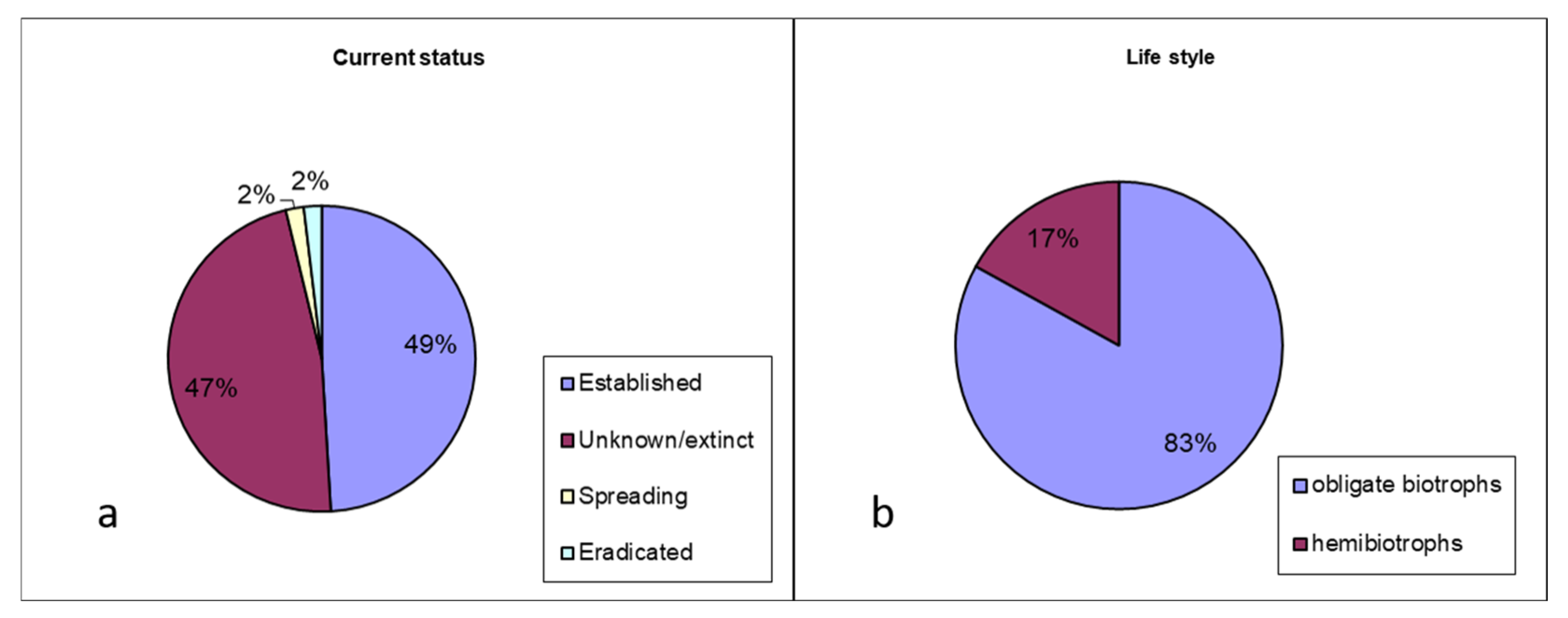
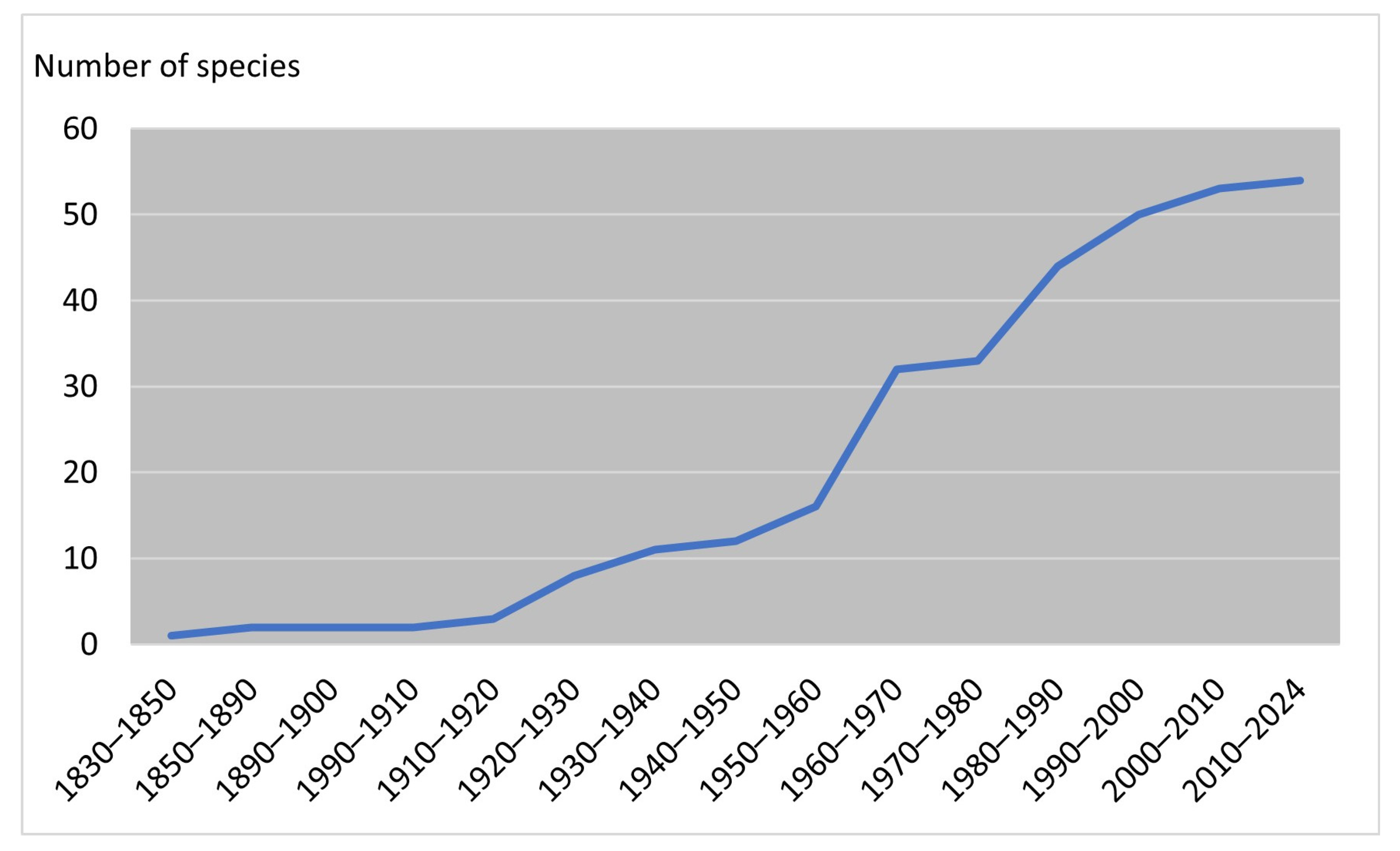
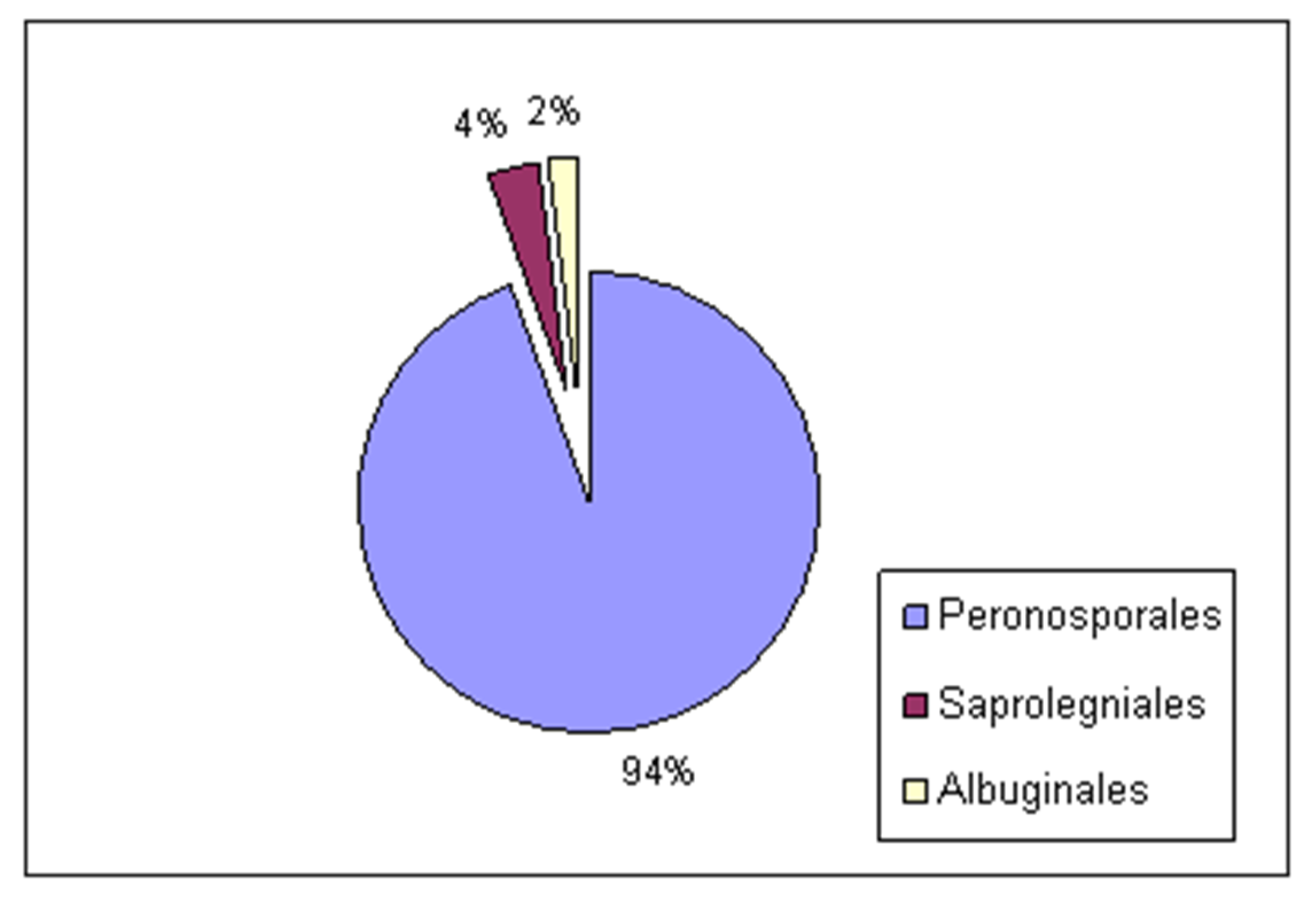
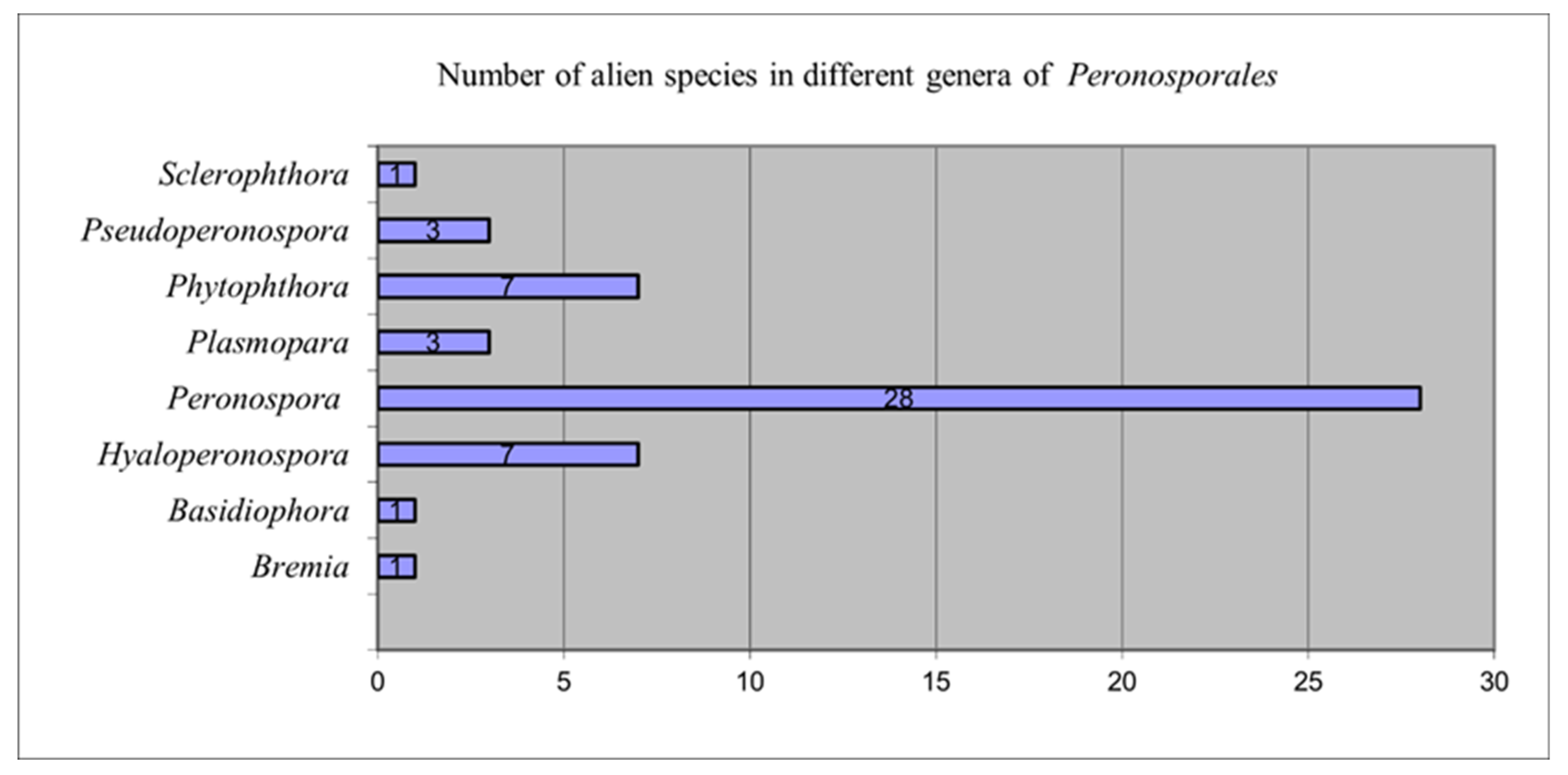
3.1. Diversity and Distribution of Alien Oomycota
3.2. Terrestrial Alien Peronosporales Trophic Preferences, Spread Vectors, and Specificity to Hosts
| Name of Species | Host/Substrate | First Record and Current Status | Reference of First Record |
|---|---|---|---|
| Albugo candida (Pers. ex J.F. Gmel.) Roussel sensu lato species complex (A. candida × A. koreana × A. voglmayrii) (syn. Uredo candida (Pers. ex J.F. Gmel.) Pers.)) | Various plants of Brassicaceae | 1830 established | [53] |
| Aphanomyces astaci Schikora | Astacus astacus L. (crayfish) | 1886 (after 1979 extinct) currently unknown | [37,38] |
| Aphanomyces eusteihes Drechsler | Plant debris in water | 2005 unknown | [44] |
| Basidiophora entospora Roze & Cornu | Conyza | 1964 Unknown | [50] |
| Bremia lactucae Regel | Various plants of Asteraceae | 1926 established | [54] |
| Hyaloperonospora barbareae (Gäum.) Göker, Riethm., Voglmayr, Weiss & Oberw. (syn. Peronospora barbareae Gäum.) | Barbareae | 1962 established | [55] |
| Hyaloperonospora berteroae (Gäum.) Göker, Riethm., Voglmayr, Weiss & Oberw. (syn. P. berteroae Gäum.) | Berteroa | 1962 unknown | [55] |
| Hyaloperonospora camelinae (Gäum.) Göker, Voglmayr, Riethm., Weiss & Oberw. (syn. P. camelinae Gäum.) | Camelina | 1937 established | [56] |
| Hyaloperonospora cochleariae (Gäum.) Göker, Riethm., Voglmayr, Weiss & Oberw. (syn. P. cochleariae Gäum.) | Armoracia | 1927 established | [57] |
| Hyaloperonospora hesperidis (Gäum.) Göker, Riethm., Voglmayr, Weiss & Oberw. (syn. P. hesperidis Gäum. | Hesperis | 1962 unknown | [55] |
| Hyaloperonospora iberidis (Gäum.) C. Salgado & J.A. Crouch (syn. P. iberidis Gäum.) | Iberis | 1984 unknown | [50] |
| Hyaloperonospora parasitica (Pers.) Constant. (syn. P. erysimi Gäum.) | Erysimum | 1962 established | [55] |
| Peronospora aestivalis Syd. | Medicago | 1960 established | [58] |
| P. affinis Rossmann | Fumaria | 1968 unknown | [50] |
| P. arborescens (Berk.) de Bary | Papaver | 1928 established | [57] |
| P. buniadis Gäum. | Bunias | 1962 unknown | [55] |
| P. chorale de Bary | Eustoma | 1993 unknown | [18] |
| P. conglomerata Fuckel | Geranium | 1962 unknown | [55] |
| P. corydalis-intermediae Gäum. | Corydalis | 1984 unknown | [50] |
| P. destructor (Berk.) Casp. Ex Berk. | Allium | 1928 established | [59] |
| P. digitalidis Gäum. | Digitalis | 1962 unknown | [55] |
| P. erodii Fuckel | Erodium | 1962 unknown | [55] |
| P. fagopyri Elenev | Fagopyrum | 1984 established | [50] |
| P. grisea (Unger) Unger | Veronica | 1960 established | [58] |
| Peronospora hyoscyami de Bary (syn.: P. tabacina D. B. Adam., P. nicotianae Speg.) | Nicotiana | 1961 established | [55] |
| P. kochiae-scopariae Kochman & T. Majewski | Kochia | 1984 unknown | [50] |
| P. lamii (A.Braun) Rabenh. | Lamium | 1960 established | [56] |
| P. mathiolae Gäum. | Matthiola | 1962 unknown | [55] |
| P. mayorii Gäum. | Vicia | 1960 established | [58] |
| P. myosotidis de Bary | Myosotis | 1962 established | [55] |
| P. obovata Bonord. | Spergula | 1960 established | [56] |
| P. ornithopi Gäum. | Ornithopus | 1984 Unknown | [50] |
| P. phyteumatis Fuckel | Phyteuma | 1984 unknown | [50] |
| P. scleranthi Rabenh. | Scleranthus | 1962 unknown | [55] |
| P. sisymbrii-officinalis Gäum. | Sisymbrium | 1962 established | [55] |
| P. sparsa Berk. | Rosa | 1984 established | [50] |
| P. spinaceae Laubert | Spinacia | 1962 unknown | [55] |
| P. trifoliorum de Bary | Trifolium | 1936 established | [60] |
| Peronospora viciae (Berk.) Casp. (syn. P. pisi (Syd.) Gäum.) | Pisum, Vicia | 1927 established | [57] |
| P. violae de Bary | Viola | 1928 established | [57] |
| Phytophthora alni Brasier & S.A. Kirk sensu lato species complex (P. × alni and P. uniformis) | Alnus | 1999 spreading | [30,47] |
| Ph. cactorum (Leb. et Cohn) Schröt. | Rhododendron | 2004 unknown | [41] |
| Ph. cinnamomi Rands | Pinus, Quercus | 2000 unknown | [39] |
| Ph. fragariae Hickman | Fragaria | 2000 unknown | [40] |
| Ph. infestans (Mont.) de Bary | Solanum, Lycopersicon, Petunia | 1912 established | [61] |
| Ph. syringae (Kleb.) Kleb. | Syringa, Malus | 1935 unknown | [62] |
| Ph. ramorum Werres, De Cock & Man | Rhododendron | 2007 2019 eradicated | [63] |
| Plasmopara angustiterminalis Novot. | Xanthium | 1984 unknown | [50] |
| Pl. pastinacae Săvul. & O. Săvul. | Pastinaca | 1984 unknown | [50] |
| Pl. viticola (Berk. & M.A. Curtis) Berl. & De Toni | Vitis | 1949 established | [58] |
| Pseudoperonospora cannabina (G.H. Otth) | Cannabis | 1984 established | [50] |
| Pseudoperonospora cubensis (Berk. & M.A. Curtis) Rostovzev | Cucumis | 1985 established | [64] |
| Pseudoperonospora humuli (Miyabe & Takah.) G.W. Wilson (syn. Peronoplasmopara humuli Miyabe & Takah) | Humulus | 1927 established | [57] |
| Sclerophthora macrospora (Sacc.) Thirum., Shaw. et Naras | Triticum | 1989 unknown | [65] |
3.3. Waterborne and Soilborne Alien Pathogens Trophic Preferences and Spread Vectors
4. Historical Data on Invasions of Some Economically Important Pathogens into Lithuania
4.1. The Oldest Obligate Plant Pathogen Albugo candida Sensu Lato
4.2. The Crayfish Plague Pathogen Aphanomyces astaci
4.3. The Potato Blight Pathogen Phytophthora infestans and Grape Pathogen Plasmopara viticola
4.4. The Most Harmful Phytophthora Invaders and Quarantine Species in Lithuania
5. Adaptation and Spreading Ability of Alien Plant Pathogenic Oomycota
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyšek, P.; Richardson, D.M.; Pergi, J.; Jarošík, V.; Sixtová, Z.; Weber, E. Geographical and taxonomic biases in invasion ecology. Trends Ecol. Evol. 2008, 23, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Desprez-Loustau, M.L. Alien fungi of Europe. In DAISIE Handbook of Alien Species in Europe; Drake, J.A., Ed.; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2009; pp. 15–28. ISBN 978-1-4020-8279-5. [Google Scholar]
- Roy, H.E.; Hesketh, H.; Purse, B.V.; Eilenberg, J.; Santini, A.; Scalera, R.; Stentiford, G.D.; Adriaens, T.; Bacela-Spychalska, K.; Bass, D.; et al. Alien Pathogens on the Horizon: Opportunities for Predicting Their Threat to Wildlife. Conserv. Lett. 2017, 10, 477–484. [Google Scholar] [CrossRef]
- Patterson, D.J. Stramenopiles: Chromophytes from a protistan perspective. In The Chromophyte Algae: Problems and Perspectives; Green, J.C., Leadbeater, B.S.C., Diver, W.L., Eds.; Clarendon Press: Oxford, UK, 1990; pp. 357–380. [Google Scholar] [CrossRef]
- Dick, M. Straminipilous Fungi; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2001; p. 670. [Google Scholar]
- Massana, R.; Castresana, J.; Balague, V.; Guillou, L.; Romari, K.; Groisillier, A.; Valentin, K.; Pedros-Alio, C. Phylogenetic and ecological analysis of novel marine stramenopiles. Appl. Environ. Microbiol. 2004, 70, 3528–3534. [Google Scholar] [CrossRef]
- Lévesque, C.A. Fifty years of oomycetes—From consolidation to evolutionary and genomic exploration. Fungal Divers. 2011, 50, 35–46. [Google Scholar] [CrossRef]
- Beakes, G.W.; Glockling, S.L.; Sekimoto, S. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 2012, 249, 3–19. [Google Scholar] [CrossRef]
- Beakes, G.W.; Honda, D.; Thines, M. Systematics of the Straminipila: Labyrinthulomycota, Hyphochytriomycota, and Oomycota. In The Mycota VIII-Part A; McLaughlin, D.J., Spatafora, J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–97. [Google Scholar] [CrossRef]
- Beakes, G.W.; Thines, M. Hyphochytriomycota and Oomycota. In Handbook of the Protists; Archibald, J., Simpson, A., Slamovits, C., Margulis, L., Melkonian, M., Chapman, D., Corliss, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–71. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.B.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The new higher-level classification of the eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukes, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef]
- Baldauf, S.L.; Roger, A.J.; Wenk-Siefert, I.; Doolittle, W.F. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 2000, 290, 972–977. [Google Scholar] [CrossRef]
- Derelle, R.; López-García, P.; Timpano, H.; Moreira, D. A phylogenomic framework to study the diversity and evolution of stramenopiles (= Heterokonts). Mol. Biol. Evol. 2016, 33, 2890–2898. [Google Scholar] [CrossRef]
- McCarthy, C.G.P.; Fitzpatrick, D.A. Phylogenomic reconstruction of the oomycete phylogeny derived from 37 genomes. mSphere 2017, 2, e00095-17. [Google Scholar] [CrossRef]
- Buaya, A.T.; Thines, M. An overview on the biology and phylogeny of the early-diverging oomycetes. Philipp. J. Syst. Biol. 2020, 14, 1–20. [Google Scholar] [CrossRef]
- Larroque, M.; Barriot, R.; Bottin, A.; Barre, A.; Rougé, P.; Dumas, B.; Gaulin, E. The unique architecture and function of cellulose-interacting proteins in oomycetes revealed by genomic and structural analyses. BMC Genom. 2012, 13, 605. [Google Scholar] [CrossRef]
- Mazelaitis, J.; Stanevičienė, S. Gleivūnai (Myxomycota) Peronosporiečiai (Peronosporales). Lietuvos grybai I; Mokslo ir Enciklopedijų leidikla: Vilnius, Lithuania, 1995; p. 292. (In Lithuanian) [Google Scholar]
- Palm, M.E.; Rossman, A. Invasion pathways of terrestrial plant-inhabiting fungi. In Invasive Species: Vectors and Management Strategies; Ruiz, G., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 31–43. [Google Scholar]
- Brasier, C.M. Phytophthora biodiversity: How many Phytophthora species are there? In Phytophthoras in Forests and Natural Ecosystems, Proceedings of the Fourth Meeting of the International Union of Forest Research Organizations (IUFRO); Goheen, E.M., Frankel, S.J., Eds.; USDA Forest Service: Albany, NY, USA, 2009; pp. 101–115. [Google Scholar]
- Santini, A.; Ghelardini, L.; De Pace, C. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef]
- Migliorini, D.; Ghelardini, L.; Tondini, E.; Luchi, N.; Santini, A. The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Divers. Distrib. 2015, 21, 1218–1229. [Google Scholar] [CrossRef]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Brasier, C.; Scanu, B.; Cooke, D.; Jung, T. Phytophthora: An ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 2022, 13, 12. [Google Scholar] [CrossRef]
- Callaghan, S.; Guest, D. Globalisation, the founder effect, hybrid Phytophthora species and rapid evolution: New headaches for biosecurity. Australas. Plant Pathol. 2015, 44, 255–262. [Google Scholar] [CrossRef]
- Depotter, J.R.L.; Seidl, M.F.; Woods, T.A.; Thomma, B.P. Interspecific hybridization impacts host range and pathogenicity of filamentous microbes. Curr. Opin. Microbiol. 2016, 32, 7–13. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Andersson, B. Phytophthora infestans and potato blight in Europe. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI Plant Protection Series, No. 2; CABI: Wallingford, UK, 2013; pp. 59–64. [Google Scholar]
- Goss, E.M.; Cardenas, M.E.; Myers, K.; Forbes, G.A.; Fry, W.E.; Restrepo, S.; Grünwald, N.J. The plant pathogen Phytophthora andina emerged via hybridization of an unknown Phytophthora species and the Irish potato famine pathogen, P. infestans. PLoS ONE 2011, 6, e24543. [Google Scholar] [CrossRef]
- Puidet, B.; Mabon, R.; Guibert, M.; Kiiker, R.; Soonvald, L.; Hong Le, V.; Eikemo, H.; Dewaegeneire, P.; Guillaume, S.; Chatot, C.; et al. Examining phenotypic traits contributing to the spread in Northern European potato crops of EU_41_A2, a new clonal lineage of Phytophthora infestans. Phytopathology 2022, 112, 414–421. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A.; Delcan, J.; Cooke, D.E.L.; Jung, T.; Man In’t Veld, W.A. Phytophthora alni sp. nov. and its variants: Designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol. Res. 2004, 108, 1172–1184. [Google Scholar] [CrossRef]
- Husson, C.; Aguayo, J.; Revellin, C.; Frey, P.; Ioos, R.; Marçais, B. Evidence for homoploid speciation in Phytophthora alni supports taxonomic reclassification in this species complex. Fungal Genet. Biol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Motiejūnaitė, J.; Markovskaja, S.; Kutorga, E.; Iršėnaitė, R.; Kasparavičius, J.; Kačergius, A.; Lygis, V. Alien fungi in Lithuania: List of species, current status and trophic structure. Bot. Lith. 2017, 23, 139–152. [Google Scholar] [CrossRef]
- Jones, D.R.; Baker, R.H.A. Introductions of non-native plant pathogens into Great Britain, 1970–2004. Plant Pathol. 2007, 56, 891–910. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Courtecuisse, R.; Robin, C.; Husson, C.; Moreau, P.-A.; Blancard, D.; Selosse, M.-A.; Lung-Escarmant, B.; Piou, D.; Sache, I. Species diversity and drivers of spread of alien fungi (sensu lato) in Europe with a particular focus on France. Biol. Invasions 2010, 12, 157–172. [Google Scholar] [CrossRef]
- Mułenko, W.; Piątek, M.; Wołczańska, A.; Kozłowska, M.; Ruszkiewicz-Michalska, M. Plant parasitic fungi introduced to Poland in Modern times. Alien and invasive species. Biol. Invasions Pol. 2010, 1, 49–71. [Google Scholar]
- Index Fungorum. 2025. Available online: https://indexfungorum.org/Index.htm (accessed on 25 April 2025).
- Cukerzis, J.M. Biologia Širokopalogo Raka (Astacus astacus L); Mintis: Vilnius, Lithuania, 1970; p. 203. (In Russian) [Google Scholar]
- Mazylis, A.; Grigelis, A. On diseases of Astacus astacus in some Lithuanian lakes. Biol. Rechn. Rakov Vod. Litny. Vilnius 1979, 1979, 121–127. [Google Scholar]
- Jovaišienė, Z. Naujos spygliuočių ligos sukėlėja–cinamoninė fitoftora (Phytophthora cinnamomi Rands). Mūsų Girios 2004, 5, 12. [Google Scholar]
- Jovaišienė, Z. Phytophthora fragariae, a new fungi species in Lithuania. Bot. Lith. 2004, 10, 233–236. [Google Scholar]
- Jovaišiene, Z.; Lane, C. First report of Phytophthora cactorum in Lithuania. Bot. Lith. 2006, 12, 197–199. [Google Scholar]
- Markovskaja, S. Saprolegniaceae (Peronosporomycetes) in Lithuania. I. The genera Achlya and Newbya. Bot. Lith. 2004, 10, 141–160. [Google Scholar]
- Markovskaja, S. Saprolegniaceae (Peronosporomycetes) in Lithuania. II. The genus Saprolegnia. Bot. Lith. 2006, 12, 97–112. [Google Scholar]
- Markovskaja, S. The genus Aphanomyces (Leptolegniaceae, Peronosporomycetes) in Lithuania. Bot. Lith. 2007, 13, 237–244. [Google Scholar]
- Ronis, A.; Rainys, K.; Semaškienė, R.; Lazauskas, S. Potato late blight in Lithuania. In Proceedings of the Tenth Workshop of a European Network for Development of an Integrated Control Strategy of Potato Late Blight, Bologna, Italy, 2–5 May 2007; pp. 303–307. [Google Scholar]
- Norkutė, G. Characterization of Populations of Invasive Pathogens—Causal Agents of Three Forest Tree Diseases: Alder Decline, Dutch Elm Disease and Ash Dieback. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 2018. [Google Scholar]
- Norkutė, G.; Lygis, V. Occurrence and characterization of Phytophthora alni sensu lato populations in Lithuania. In Proceedings of the 11th Conference of the European Foundation for Plant Pathology “Healthy Plants—Healthy People”, Krakow, Poland, 8–13 September 2014; p. 266. [Google Scholar]
- Kutorga, E. Lietuvos grybų įvairovės pažinimas: Dabartis ir perspektyvos. In Mokslas. Gamtos Mokslų Fakultet Trečiosios Mokslinės Konferencijos Pranešimai; Kilkus, K., Ed.; Mokslas: Vilnius, Lithuania, 2004; pp. 102–112. (In Lithuanian) [Google Scholar]
- Voglmayr, H.; Schertler, A.; Essl, F.; Krisai-Greilhuber, I. Alien and cryptogenic fungi and oomycetes in Austria: An annotated checklist (2nd edition). Biol. Invasions 2023, 25, 27–38. [Google Scholar] [CrossRef]
- Stanevičienė, S. Peronosporovyje Griby Pribaltiki; Mokslas: Vilnius, Lithuania, 1984; p. 207. (In Russian) [Google Scholar]
- Bulovienė, V.; Survilienė, E. Effect of onion leaf surface on the infection with Peronospora destructor (Berk.) Casp. and sporulation. Sodininkystė Daržininkystė 2005, 24, 136–142. [Google Scholar]
- Bulovienė, V.; Survilienė, E. Effect of environmental conditions and inoculum concentration on sporulation of Peronospora destructor. Agron. Res. 2006, 4, 147–150. [Google Scholar]
- Jundziłł, J. Opisanie Roślin w Litwie, na Wołyniu, Podolu i Ukrainie Dziko Rosnących, iako i Oswoionych: Podłług Wydania Szesnastego Układu Roślin; Józef Zawadzki Własnym Nakładem: Vilnius, Lithuania, 1830. (In Polish) [Google Scholar]
- Vilkaitis, V. Truputis medžiagos Lietuvos grybų florai. Kosmos 1927, 2–3, 97–102. (In Lithuanian) [Google Scholar]
- Stanevičienė (Jarmalavičiutė), S. Nauja medžiaga Lietuvos TSR peronosporainiams grybams (Peronosporales) pažinti. LTSR MA Darb. Ser. C 1962, 3, 13–22. (In Lithuanian) [Google Scholar]
- Trzebiński, J. Przyczynek do Znajomości Grzybów Pasożytniczych Poludniowo-Zachodniej Części Litwy i Północno-Wschodniej Polski. Prace Tow. Przyjacel Nauk w Wilnie; Wydział nauk Matem. I Przyrodn: Vilnius, Lithuania, 1937; Volume 11, pp. 163–170. (In Polish) [Google Scholar]
- Vilkaitis, V. Apie du peronosporainių grybelius. Kosmos 1928, 5–6, 249–251. (In Lithuanian) [Google Scholar]
- Stanevičienė (Jarmalavičiutė), S.; Minkevičius, A. Medžiaga Lietuvos TSR Peronosporainiams Grybams (Peronosporales) Pažinti; Vilniaus Universiteto Mokslo Darbai. Biologija, Geografija, Geologija: Vilnius, Lithuania, 1960; Volume 7, pp. 25–39. (In Lithuanian) [Google Scholar]
- Vilkaitis, V. Augalų Apsaugos Stoties 1927–1932 m. Darbų Apyskaita; Spindulys: Kaunas, Lithuania, 1933; pp. 37–55. (In Lithuanian) [Google Scholar]
- Michalski, A. Grzyby pasożytnicze, zaobserwowane na roślinach dziko rosnąncych, oraz uprawnych na terenie powiatu Wileńsko-Trockiego. Kosmos 1936, 61, 239–279. (In Polish) [Google Scholar]
- Petruscinski, Z.F. Rezultaty Trudov i Opytov Proizvedennyh na Opytnoj Stancii v Baisiogale v 1912; Spindulys: Vilnius, Lithuania, 1914; p. 28. (In Russian) [Google Scholar]
- Brundza, K. Parazitnye Griby Kul’tiviruemyh Rastenij Litovskoj SSR; AN Litovskoj SSR: Vilnius, Lithuania, 1961. (In Russian) [Google Scholar]
- EPPO Reporting Service. Phytophthora ramorum (PHYTRA). Distribution in Lithuania. 2019. Available online: https://gd.eppo.int/taxon/PHYTRA/distribution/LT (accessed on 25 April 2025).
- Stanevičienė, S. Svogūnų Veislių Atsparumas Peronosporozei Lietuvos Sąlygomis. Augalų Apsaugos Naujovės; Mokslas: Vilnius, Lithuania, 1988; pp. 45–46. (In Lithuanian) [Google Scholar]
- Špokauskienė, O. Varpinių Augalų Mikromicetai Lietuvoje. Augalų Apsaugos Naujoves; Mokslas: Vilnius, Lithuania, 1989; p. 228. (In Lithuanian) [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA; American Phytopathological Society: St. Paul, MN, USA, 1996; p. 592. [Google Scholar]
- Willoughby, L.G. Fungi and Fish Diseases; Pisces Press in Association with the Institute of Aquaculture, University of Stirling, Pisces Press: Stirling, UK, 1994; p. 57. [Google Scholar]
- Wicker, E.; Hulle, M.; Rouxel, F. Pathogenic characteristics of isolates of Aphanomyces eusteiches from pea in France. Plant Pathol. 2001, 50, 433–442. [Google Scholar] [CrossRef]
- Van West, P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: New challenges for an old problem. Mycologist 2006, 20, 99–104. [Google Scholar] [CrossRef]
- Adomas, V.; Oszako, T.; Nowakowska, J.; Stankevičienė, A. First records of Phytophthora spp. based on DNA analysis in Lithuania. Folia For. Pol. A 2012, 54, 25–31. [Google Scholar] [CrossRef]
- Bjelke, U.; Boberg, J.; Oliva, J.; Tattersdill, K.; McKie, B.G. Dieback of riparian alder caused by the Phytophthora alni complex: Projected consequences for stream ecosystems. Freshw. Biol. 2016, 61, 565–813. [Google Scholar] [CrossRef]
- Biga, M.L.B. Riesaminazione delle specie del genere Albugo in base alla morfologia dei conidi. Sydowia 1955, 9, 339–358. (In Italian) [Google Scholar]
- Rouppert, K.; Namyslowski, B. Żmujdzkie Grzyby, Zebrine Przez Prof. dr. E. Janczewskiego; Sprawozdanie Komisji fiziograficznej: Kraków, Poland, 1909; Volume 43, pp. 161–165. (In Polish) [Google Scholar]
- Voglmayr, H.; Riethmüller, A. Phylogenetic relationship of Albugo species (white blister rusts) based on LSU rDNA sequence and oospore data. Mycol. Res. 2006, 110, 75–85. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Hong, S.B.; Shin, H.D. A reconsideration of Pseudoperonospora cubensis and P. humuli based on molecular and morphological data. Mycol. Res. 2005, 109, 841–848. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Shin, H.-D.; Ploch, S.; Thines, M. Evidence for uncharted biodiversity in the Albugo candida complex, with the description of a new species. Mycol. Res. 2008, 112, 1327–1334. [Google Scholar] [CrossRef]
- Alderman, D.J. Geographical spread of bacterial and fungal diseases of crustaceans. Rev. Sci. Tech. (Int. Off. Epizoot.) 1996, 15, 603–632. [Google Scholar] [CrossRef]
- Filipová, L.; Petrusek, A.; Matasová, K.; Delaunay, C.; Grandjean, F. Prevalence of the Crayfish Plague Pathogen Aphanomyces astaci in Populations of the Signal Crayfish Pacifastacus eniusculus in France: Evaluating the Threat to Native Crayfish. PLoS ONE 2013, 8, e70157. [Google Scholar] [CrossRef]
- Maguire, I.; Jelić, M.; Klobučar, G.; Delpy, M.; Delaunay, C.; Grandjean, F. Prevalence of the pathogen Aphanomyces astaci in freshwater crayfish populations in Croatia. Dis. Aquat. Org. 2016, 118, 45–53. [Google Scholar] [CrossRef]
- Kaldre, K.; Paaver, T.; Hurt, M.; Grandjeanet, F. First records of the non-indigenous signal crayfish (Pacifastacus leniusculus) and its threat to noble crayfish (Astacus astacus) populations in Estonia. Biol. Invasions 2017, 19, 2771. [Google Scholar] [CrossRef]
- Mrugała, A.; Kozubíková-Balcarová, E.; Chucholl, C.; Cabanillas Resino, S.; Viljamaa-Dirks, S.; Vukić, J.; Petrusek, A. Trade of ornamental crayfish in Europe as a possible introduction pathway for important crustacean diseases: Crayfish plague and white spot syndrome. Biol. Invasions 2015, 17, 1313–1326. [Google Scholar] [CrossRef]
- Panteleit, J.; Keller, N.S.; Kokko, H.; Jussila, J.; Makkonen, J.; Theissinger, K.; Schrimpf, A. Investigation of ornamental crayfish reveals new carrier species of the crayfish plague pathogen (Aphanomyces astaci). Aquat. Invasions 2017, 12, 77–83. [Google Scholar] [CrossRef]
- Cukerzis, J.M. On acclimatization of Pacifastacius leniusculus Dana in an isolated lake. Freshw. Crayfish 1979, 4, 445–450. [Google Scholar]
- Cukerzis, J.M. Astacus astacus in Europe. In Freshwater Crayfish—Biology, Managament and Explotation; Holdich, D.M., Lowerly, R.S., Eds.; Croom Helm: London, UK, 1989; pp. 309–340. [Google Scholar]
- Koreiva, Č. Orconectes limosus in Lithuania. Crayfish news. IAA Newsl. 1994, 16, 7. [Google Scholar]
- Kozubíková, E.; Viljamaa-Dirks, S.; Heinikainen, S.; Petrusek, A. Spiny-cheek crayfish Orconectes limosus carry a novel genotype of the crayfish plague pathogen Aphanomyces astaci. J. Invertebr. Pathol. 2011, 108, 214–216. [Google Scholar] [CrossRef]
- Svoboda, J.; Mrugała, A.; Kozubíková-Balcarová, E.; Petrusek, A. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. J. Fish. Dis. 2017, 40, 127–140. [Google Scholar] [CrossRef]
- Burba, A. Assessment of crayfish situation in Lithuania and prognosis for the future. Žuvininkystė Liet. 2008, 8, 218–230. (In Lithuanian) [Google Scholar]
- Arbačiauskas, K.; Višinskienė, G.; Smilgevičienė, S.; Rakauskas, V. Non-indigenous macroinvertebrate species in Lithuanian fresh waters, Part 1: Distributions, dispersal and future. Knowl. Manag. Aquat. Ecosyst. 2011, 402, 12. [Google Scholar] [CrossRef]
- Schrimpf, A.; Maiwald, T.; Vrålstad, T.; Schultz, H.K.; Śmietana, P.; Schultz, R. Absence of the crayfish plague pathogen (Aphanomyces astaci) facilitates coexistence of European and American crayfish in central Europe. Freshw. Biol. 2013, 58, 1116–1125. [Google Scholar] [CrossRef]
- Bourke, A. Emergence of Potato Blight, 1843–46. Nature 1964, 203, 805–808. [Google Scholar] [CrossRef]
- Andrivon, D. The origin of Phytophthora infestans populations present in Europe in the 1840s: A critical review of historical and scientific evidence. Plant Pathol. 1996, 54, 1027–1035. [Google Scholar] [CrossRef]
- Siemaszko, W. Zapiski Grzyboznawcze z Gubernii Wilenskej. Sprawozdania z Posiedzeń Tow. Przuj. Naukow. Warszawskiego; Wydzial nauk Matem i Przyrodn: Vilnius, Lithuania, 1914; Volume 7, pp. 124–128. (In Polish) [Google Scholar]
- Valskyte, A. The resistance of Phytophthora infestans population to metalaxyl in Lithuania. Transactions of the Estonian Agricultural University. Agronomy 2000, 209, 224–226. [Google Scholar]
- Asakavičiūtė, R.; Ražukas, A.; Jundulas, J. Susceptibility of new potato varieties to the potato late blight oomycete Phytophthora infestans (Mont.) de Bary in Lithuania. Agrociencia 2009, 43, 625–633. [Google Scholar]
- Kiiker, R.; Skrabule, I.; Ronis, A.; Cooke, D.E.L.; Hansen, J.G.; Williams, I.H.; Mänd, M.; Runno-Paurson, E. Diversity of populations of Phytophthora infestans in relation to patterns of potato crop management in Latvia and Lithuania. Plant Pathol. 2019, 68, 1207–1214. [Google Scholar] [CrossRef]
- Diliautas, A.; Vynmedžių Grybinės Ligos ir Apsauga nuo jų. Vynmedžių Ligos ir Kenkėjai. 2016. (In Lithuanian). Available online: http://www.vynuoges.lt/ligos-ir-kenkejai/grybines-vynmedziu- (accessed on 16 December 2024).
- Nentwig, W.; Bacher, S.; Kumschick, S.; Pyšek, P.; Vila, M. More than “100 worst” alien species in Europe. Biol. Invasions 2018, 20, 1611–1621. [Google Scholar] [CrossRef]
- Ioos, R.; Andrieux, A.; Marçais, B.; Frey, P. Genetic characterization of the natural hybrid species Phytophthora alni as inferred from nuclear and mitochondrial DNA analyses. Fungal Genet. Biol. 2006, 43, 511–529. [Google Scholar] [CrossRef]
- Oszako, T. Alder decline in Europe. Leśne Pr. Badaw. 2005, 1 (Suppl. 1), 53–63. [Google Scholar]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef]
- Hantula, J.; Lilja, A.; Nuorteva, H.; Parikka, P.; Werres, S. Pathogenicity, morphology, and genetic variation of Phytophthora cactorum from strawberry, apple, rhododendron, and silver birch. Mycol. Res. 2000, 104, 1062–1068. [Google Scholar] [CrossRef]
- Hudler, G.W. Phytophthora cactorum. For. Phytophthoras 2013, 3, 20153142254. [Google Scholar] [CrossRef]
- EPPO Reporting Service. Phytophthora fragariae (PHYTFR). Distribution in Lithuania. 2018. Available online: https://gd.eppo.int/taxon/PHYTFR/distribution/LT (accessed on 25 April 2025).
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Miyabe, K.; Takahashi, Y. A new disease of hopvine caused by Peronoplasmopara humuli n. sp. Trans. Sapporo Nat. Hist. Soc. 1906, 1, 149–157. [Google Scholar]
- Purayannur, S.; Gent, D.H.; Miles, T.D.; Radišek, S.; Quesada-Ocampo, L.M. The hop downy mildew pathogen Pseudoperonospora humuli. Mol. Plant Pathol. 2021, 22, 755–768. [Google Scholar] [CrossRef]
- Lebeda, A.; Schwinn, F.J. The downy mildews—An overview of recent research progress. J. Plant Dis. Prot. 1994, 101, 225–254. [Google Scholar]
- Lebeda, A.; Gadasová, V. Pathogenic variation of Pseudoperonospora cubensis in the Czech Republic and some other European countries. Acta Hortic. 2002, 588, 137–141. [Google Scholar] [CrossRef]
- Cappelli, C.; Buonaurio, R.; Stravato, V.M. Occurrence of Pseudoperonospora cubensis pathotype 5 on squash in Italy. Plant Dis. 2003, 87, 449. [Google Scholar] [CrossRef]
- Urban, J.; Lebeda, A. Variation for fungicide resistance in Czech populations of Pseudoperonospora cubensis. J. Phytopathol. 2007, 155, 143–151. [Google Scholar] [CrossRef]
- Polat, İ.; Baysal, Ö.; Mercati, F.; Kitner, M.; Cohen, Y.; Lebeda, A.; Carimi, F. Characterization of Pseudoperonospora cubensis isolates from Europe and Asia using ISSR and SRAP molecular markers. Eur. J. Plant Pathol. 2014, 139, 641–653. [Google Scholar] [CrossRef]
- Voglmayr, H.; Piątek, M.; Mossebo, D.C. Pseudoperonospora cubensis causing downy mildew disease on Impatiens irvingii in Cameroon: A new host for the pathogen. Plant Pathol. 2009, 58, 394. [Google Scholar] [CrossRef]
- Voglmayr, H. Progress and challenges in systematics of downy mildews and white blister rusts: New insights from genes and morphology. Eur. J. Plant Pathol. 2008, 122, 3–18. [Google Scholar] [CrossRef]
- Runge, F.; Choi, Y.-J.; Thines, M. Phylogenetic investigations in the genus Pseudoperonospora reveal overlooked species and cryptic diversity in the P. cubensis species cluster. Eur. J. Plant Pathol. 2011, 129, 135–146. [Google Scholar] [CrossRef]
- Liatukienė, A.; Liatukas, Ž.; Ruzgas, V. Netikrosios miltligės tyrimai liucernų pasėlyje. Žemės Ūkio Moksl. 2010, 17, 87–92. (In Lithuanian) [Google Scholar]
- Liatukienė, A.; Liatukas, Ž. Downy Mildew Reaction of Alfalfa Accessions of Different Geographical Origin under Lithuanian Conditions. Int. J. Agric. Biol. 2014, 16, 905–910. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markovskaja, S. Alien Stramenopilous Fungus-like Organisms (Oomycota) Diversity and Distribution in Lithuania. Diversity 2025, 17, 426. https://doi.org/10.3390/d17060426
Markovskaja S. Alien Stramenopilous Fungus-like Organisms (Oomycota) Diversity and Distribution in Lithuania. Diversity. 2025; 17(6):426. https://doi.org/10.3390/d17060426
Chicago/Turabian StyleMarkovskaja, Svetlana. 2025. "Alien Stramenopilous Fungus-like Organisms (Oomycota) Diversity and Distribution in Lithuania" Diversity 17, no. 6: 426. https://doi.org/10.3390/d17060426
APA StyleMarkovskaja, S. (2025). Alien Stramenopilous Fungus-like Organisms (Oomycota) Diversity and Distribution in Lithuania. Diversity, 17(6), 426. https://doi.org/10.3390/d17060426






