Fungal Diversity in the Dry Forest and Salt Flat Ecosystems of Reserva Ecologica Arenillas, El Oro, Ecuador
Abstract
:1. Introduction
2. Materials and Methods
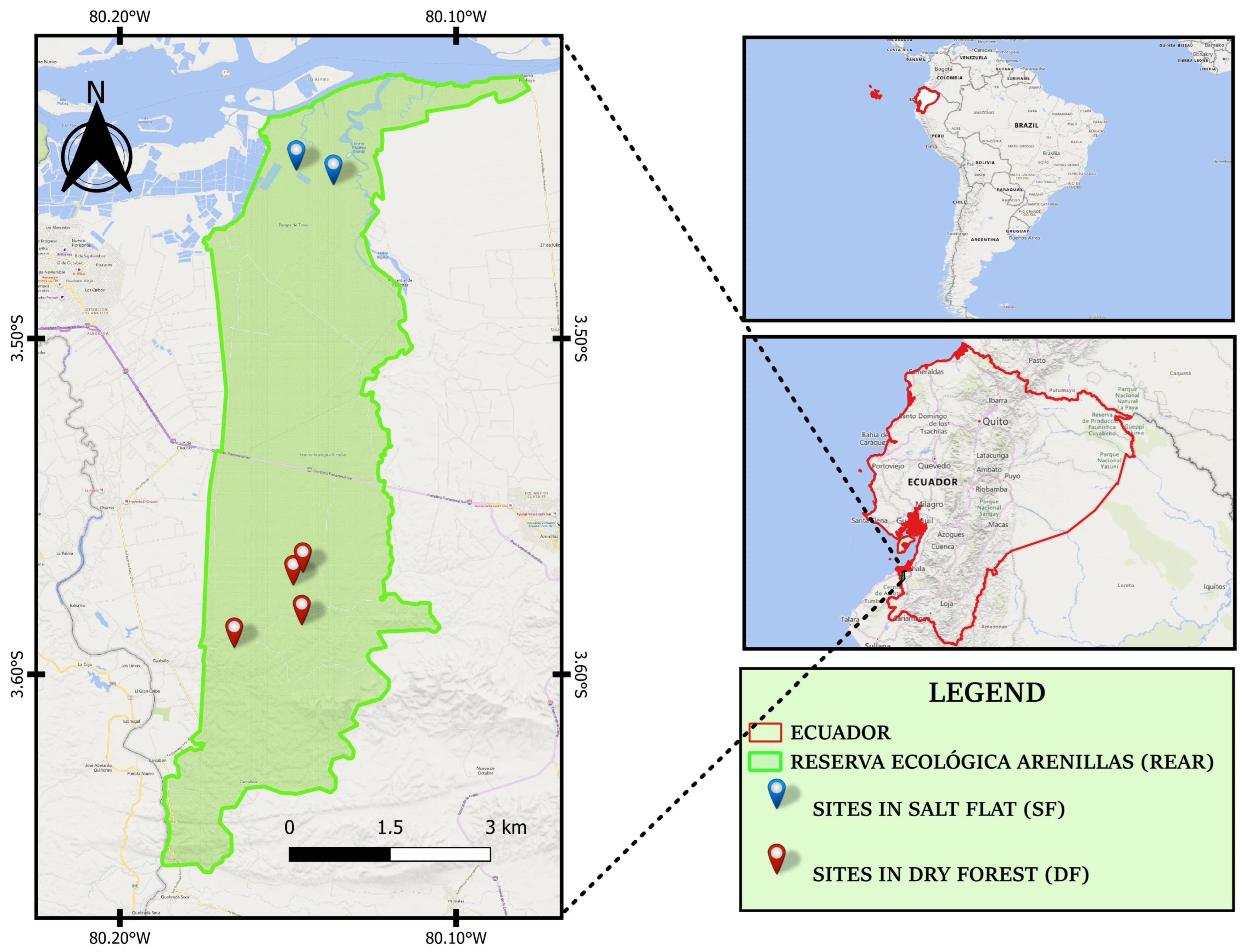
2.1. Sampling Area
2.2. Specimen Identification
2.3. Diversity Analysis
3. Results and Discussion
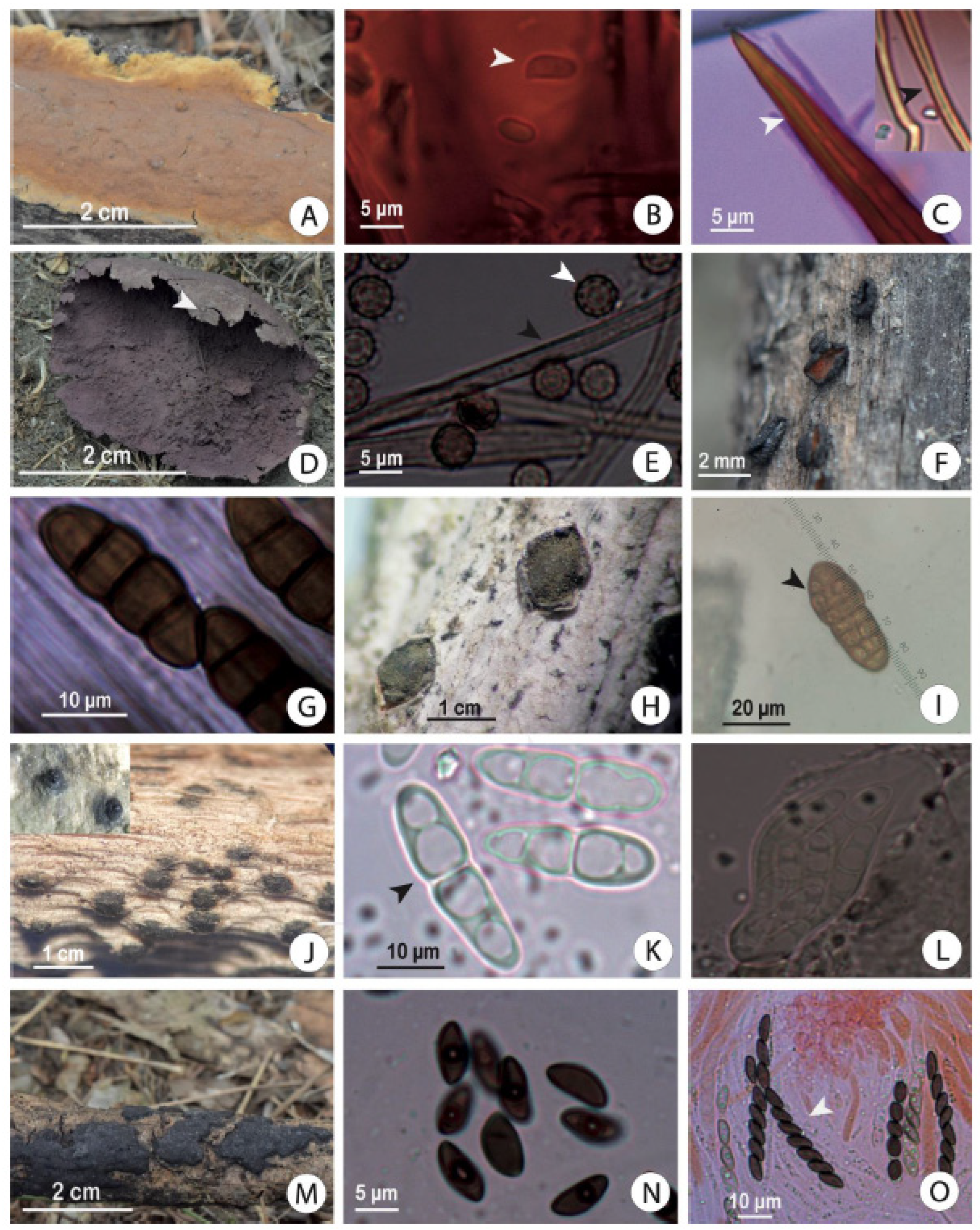
3.1. Morphological and Descriptive Diversity Recorded in the Dry Forest and Salt Flat
3.2. Alpha Diversity
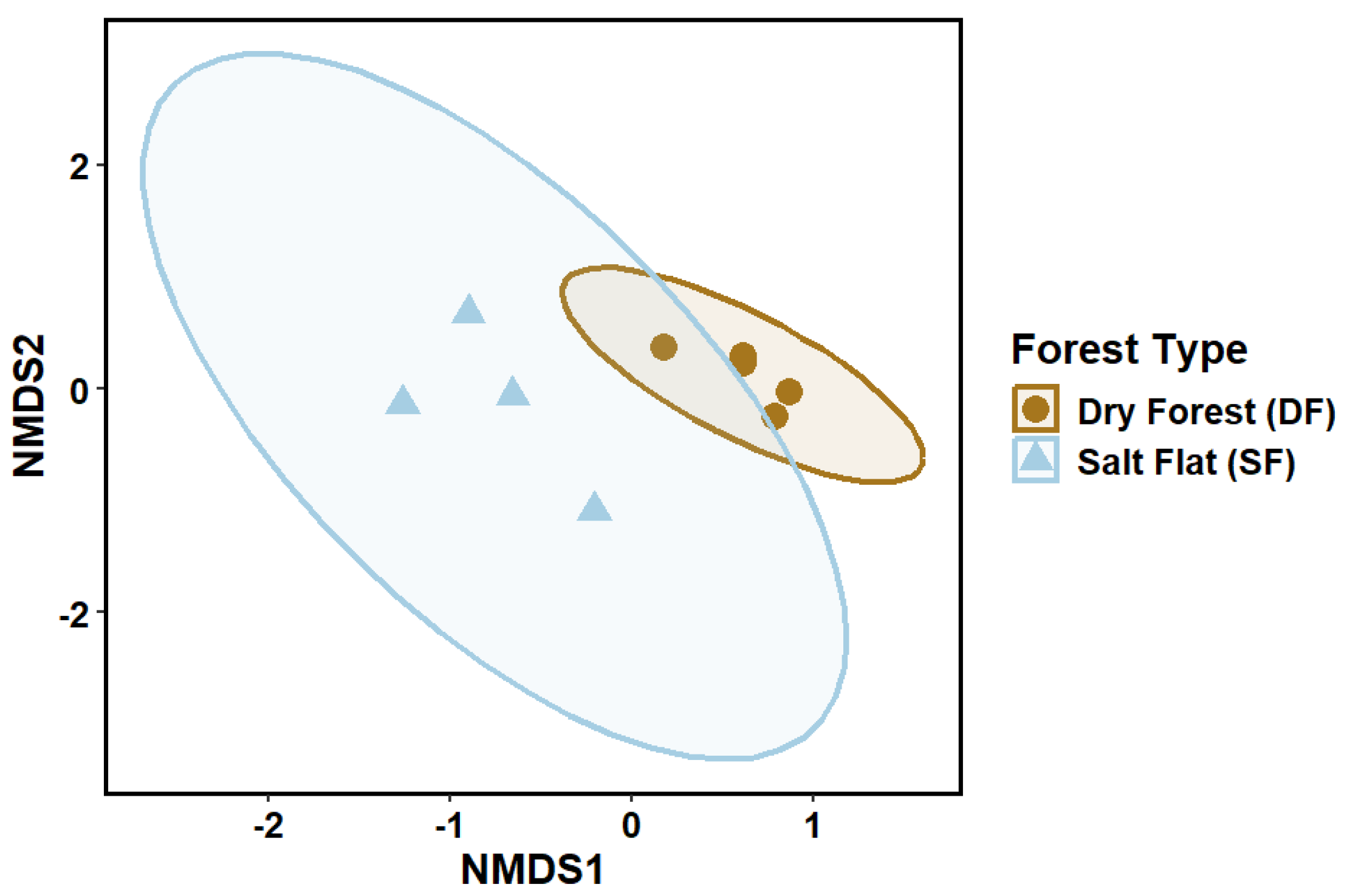
3.3. Ordination and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Best, B.J.; Kessler, M. Biodiversity and Conservation in Tumbesian Ecuador and Peru; BirdLife International: Cambridge, UK, 1995. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Rivas, C.A.; Guerrero-Casado, J.; Navarro-Cerillo, R.M. Deforestation and fragmentation trends of seasonal dry tropical forest in Ecuador: Impact on conservation. For. Ecosyst. 2021, 8, 46. [Google Scholar] [CrossRef]
- Luna-Florin, A.D.; Nole-Nole, D.A.; Rodríguez-Caballero, E.; Molina-Pardo, J.L.; Giménez-Luque, E. Ecological Characterization of the Flora in Reserva Ecológica Arenillas, Ecuador. Appl. Sci. 2022, 12, 8656. [Google Scholar] [CrossRef]
- Cerón, C.; Reyes, C. Características botánicas de la Reserva Militar y Ecológica Arenillas, El Oro-Ecuador. Cinchonia 2006, 7, 115–130. [Google Scholar]
- Espinosa, C.I.; Jara-Guerrero, A.; Cisneros, R.; Sotomayor, J.-D.; Escribano-Ávila, G. Reserva Ecológica Arenillas; ¿un refugio de diversidad biológica o una isla de extinción? Ecosistemas 2016, 25, 5–12. [Google Scholar] [CrossRef]
- Tamayo-Cevallos, R. Lignicolous marine fungi in mangrove ecosystem: State of knowledge and research perspective in Ecuador. Rev. Ecuat. De Med. Y Cienc. Biol. 2020, 41, 95–106. [Google Scholar]
- Cruz, D.; Masache, D. Listado y guía visual preliminar de macrohongos del Bosque Petrificado de Puyango-Ecuador. ACI Av. En Cienc. E Ing. 2023, 15, 2939. [Google Scholar] [CrossRef]
- Piepenbring, M.; López, F.; Cáceres, O. La importancia de los Hongos en los Ecosistemas. Puente Biológico 2016, 8, 57–91. [Google Scholar]
- Ghate, S.D.; Sridhar, K.R. Contribution to the knowledge on macrofungi in mangroves of the Southwest India. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2016, 150, 977–986. [Google Scholar] [CrossRef]
- Dutta, A.K.; Pradhan, P.; Basu, S.K.; Acharya, K. Macrofungal diversity and ecology of the mangrove ecosystem in the Indian part of Sundarbans. Biodiversity 2013, 14, 196–206. [Google Scholar] [CrossRef]
- Luna-Fontalvo, J.A.; Abaunza, C.; Barrios, A.; Ramírez-Roncallo, K.; Guerrero, R.J.; Negritto, M.A. New records of agaricoid macrofungi (Agaricales, Basidiomycota) in an urban fragment of tropical dry forest from Colombian Caribbean Region. Check List 2023, 19, 371–379. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, M.T.; Troche-Souza, C.; Vázquez-Lule, A.D.; Márquez-Mendoza, J.D.; Vázquez-Balderas, B.; Valderrama-Landeros, L.; Velázquez-Salazar, S.; Cruz-López, M.I.; Ressl, R.A.; Uribe-Martínez, A.; et al. Manglares de México/Extensión, Distribución y Monitoreo; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: México City, Mexico, 2013; p. 128. [Google Scholar]
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.M.; Chacón, N.V.; Flor, J.P.; Tamayo, J.L.; de María Valverde, F. Composición Florística y Nuevos Registros para la Reserva Ecológica Arenillas, El Oro-Ecuador. Investigatio 2016, 8, 111–132. [Google Scholar] [CrossRef]
- Maza Maza, J.E.; Sánchez Asanza, A.W.; Añazco Loaiza, H.E.; García Ochoa, J.A. Estudio de las carac-terísticas físico-químicas del suelo en áreas prioritarias de la reserva ecológica Arenillas. Rev. Científica Agro-Ecosistemas 2021, 9, 30–40. [Google Scholar]
- Ministerio del Ambiente. Plan de Manejo de la Reserva Ecológica Arenillas; Ministerio del Ambiente: Quito, Ecuador, 2015; 68p.
- Largent, D. How to Identify Mushrooms to Genus, I. Macroscopic Features; Mad River Press: Eureka, CA, USA, 1973; 125p. [Google Scholar]
- Hanlin, R.T. Illustrated Genera of Ascomycetes; APS Press: St Paul, MN, USA, 1990. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 168, 688. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1964; pp. 1–117. [Google Scholar]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. Stat. Theory Appl. 1984, 11, 265–270. [Google Scholar]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.H. vegan: Community Ecology Package (R package version 2.5-5). 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 February 2025).
- Priyamvada, H.; Akila, M.; Singh, R.K.; Ravikrishna, R.; Verma, R.S.; Philip, L.; Marathe, R.R.; Sahu, L.K.; Sudheer, K.P.; Gunthe, S.S. Terrestrial Macrofungal Diversity from the Tropical Dry Evergreen Biome of Southern India and Its Potential Role in Aerobiology. PLoS ONE 2017, 12, e0169333. [Google Scholar] [CrossRef]
- Sharif, W.R.; Da Silva, P.N.B. Macrofungal Diversity in Selected Coastal Mangrove Ecosystems in Guyana, South America. Eur. Mod. Stud. J. 2023, 7, 38. [Google Scholar] [CrossRef]
- Huang, J.-M.; Xu, T.-M.; Zhao, W.; Mumin, R.; Zeng, L.; Sun, Y.-F.; Cui, B.-K. Species Diversity and Community Composition of Macrofungi in the Dongling Mountains, Western Beijing, China. J. Fungi 2025, 11, 155. [Google Scholar] [CrossRef]
- Schoch, C.L.; Sung, G.H.; López-Giráldez, F.; Townsend, J.P.; Miadlikowska, J.; Hofstetter, V.; Robbertse, B.; Matheny, P.B.; Kauff, F.; Wang, Z.; et al. The Ascomycota tree of life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 2009, 58, 224–239. [Google Scholar] [CrossRef]
- Oberwinkler, F. Evolutionary trends in Basidiomycota. Stapfia 2012, 96, 45–104. Available online: https://www.zobodat.at/pdf/STAPFIA_0096_0045-0104.pdf (accessed on 10 February 2025).
- Shen, C.; Wang, J.; He, J.Z.; Yu, F.H.; Ge, Y. Plant Diversity Enhances Soil Fungal Diversity and Microbial Resistance to Plant Invasion. Appl. Environ. Microbiol. 2021, 87, e00251-21. [Google Scholar] [CrossRef] [PubMed]
- Calabon, M.S.; Jones, E.B.G.; Promputtha, I.; Hyde, K.D. Fungal Biodiversity in Salt Marsh Ecosystems. J. Fungi 2021, 7, 648. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, E.; Wu, J.; Ma, D.; Zhang, C.; Zhang, B.; Liu, Y.; Zhang, Z.; Tian, F.; Zhao, H.; et al. Soil fungal community is more sensitive than bacterial community to modified materials application in saline-alkali land of Hetao Plain. Front. Microbiol. 2024, 15, 1255536. [Google Scholar] [CrossRef]
- Biológica, S.D. La Biodiversidad y la Agricultura: Salvaguardando la Biodiversidad Y Asegurando Alimentación Para El Mundo; Secretaría del Convenio Sobre Diversidad Biológica: Montreal, QC, Canada, 2008. [Google Scholar]




| Forest Type | Shannon (H’) | Simpson (D) |
|---|---|---|
| DF | 3.24 | 0.95 |
| SF | 2.97 | 0.94 |
| Df | SS | R2 | F | p-Value | |
|---|---|---|---|---|---|
| Season | 1 | 0.42565 | 0.18778 | 1.9520 | 0.93 |
| Forest type | 1 | 0.60564 | 0.26718 | 2.7775 | 0.012 * |
| Season: Forest Type | 1 | 0.14519 | 0.06405 | 0.6658 | 0.786 |
| Residual | 5 | 1.09027 | 0.48099 | ||
| Total | 8 | 2.26674 | 1.00000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masache, D.; López, F.; Benítez, Á.; Ochoa, T.; Cruz, D. Fungal Diversity in the Dry Forest and Salt Flat Ecosystems of Reserva Ecologica Arenillas, El Oro, Ecuador. Diversity 2025, 17, 422. https://doi.org/10.3390/d17060422
Masache D, López F, Benítez Á, Ochoa T, Cruz D. Fungal Diversity in the Dry Forest and Salt Flat Ecosystems of Reserva Ecologica Arenillas, El Oro, Ecuador. Diversity. 2025; 17(6):422. https://doi.org/10.3390/d17060422
Chicago/Turabian StyleMasache, Débora, Fausto López, Ángel Benítez, Teddy Ochoa, and Darío Cruz. 2025. "Fungal Diversity in the Dry Forest and Salt Flat Ecosystems of Reserva Ecologica Arenillas, El Oro, Ecuador" Diversity 17, no. 6: 422. https://doi.org/10.3390/d17060422
APA StyleMasache, D., López, F., Benítez, Á., Ochoa, T., & Cruz, D. (2025). Fungal Diversity in the Dry Forest and Salt Flat Ecosystems of Reserva Ecologica Arenillas, El Oro, Ecuador. Diversity, 17(6), 422. https://doi.org/10.3390/d17060422







