Fungal Associates of the Moss Leucobryum candidum (Brid. ex P. Beauv.) Wilson in Southeast Queensland, Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Background: Subject Moss
2.2. Study Sites and Sampling Methods
2.3. Fungal Isolation and Preservation
2.4. Molecular-Level Characterisation of Fungal Isolates
2.4.1. DNA Extraction and PCR
2.4.2. Sequencing Amplicons
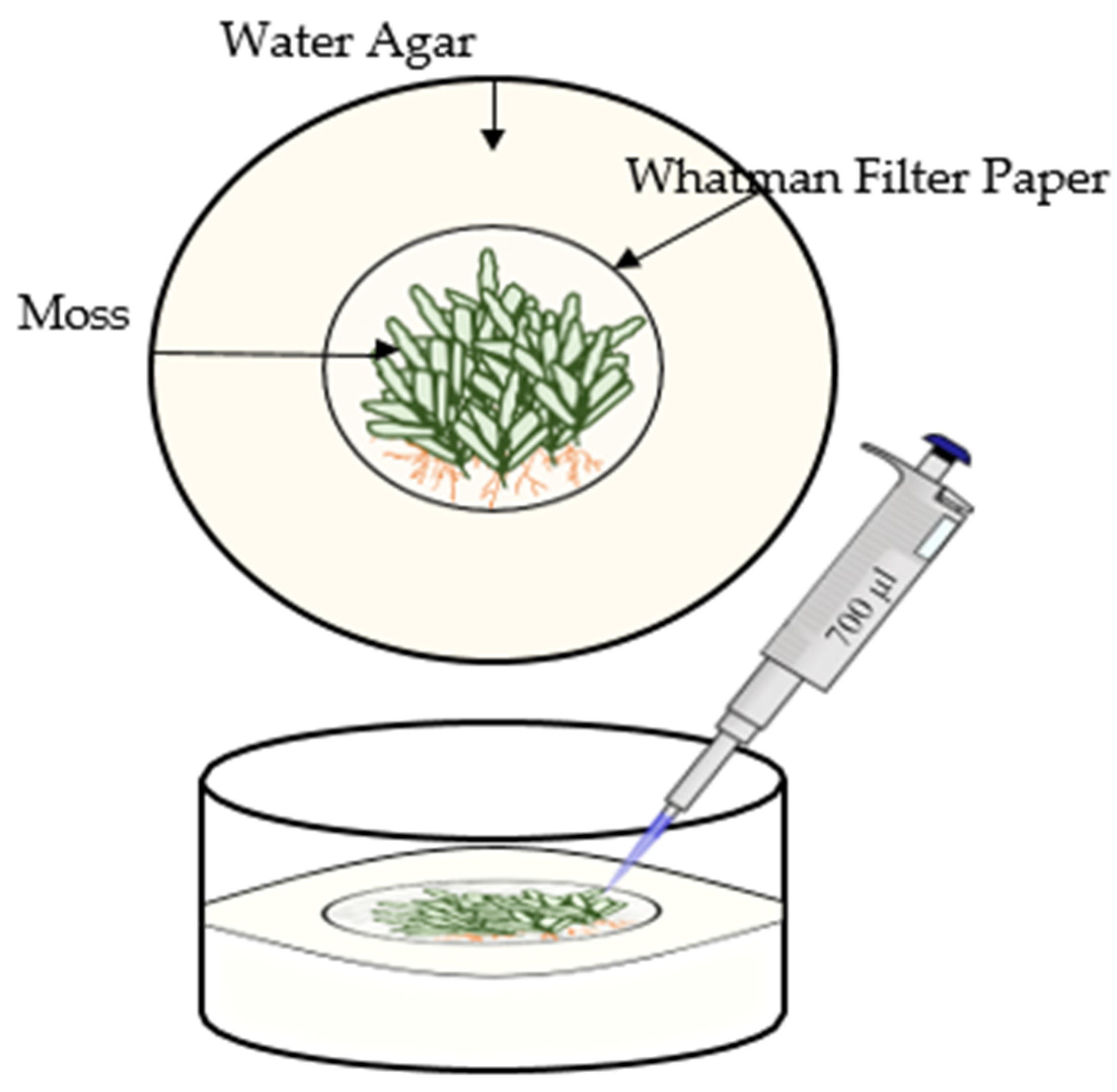
2.5. In Vitro Culture Assay
2.6. Bioassay
3. Results
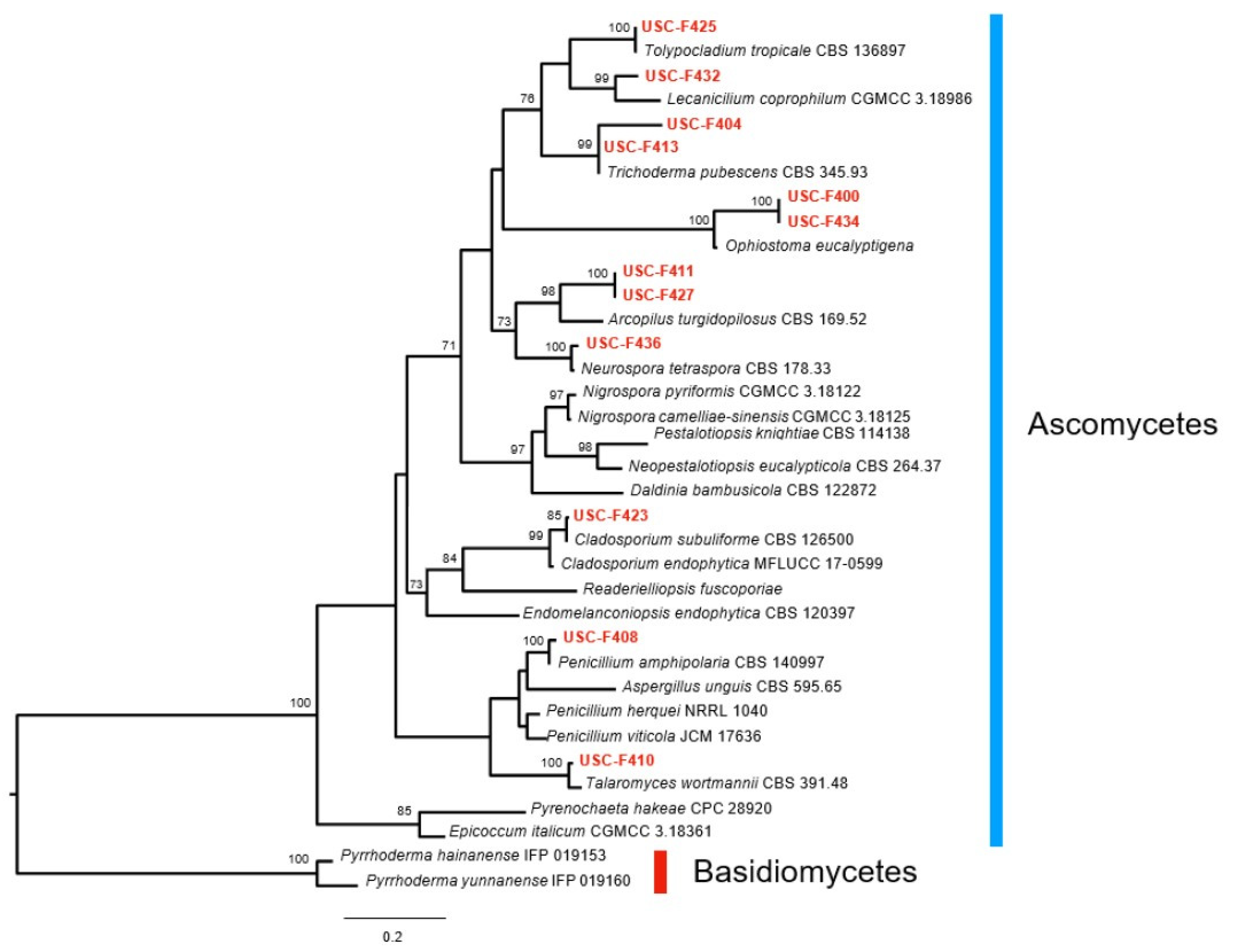
3.1. Fungal Isolates Cultured from Leucobryum candidum and Their Molecular Characterisation
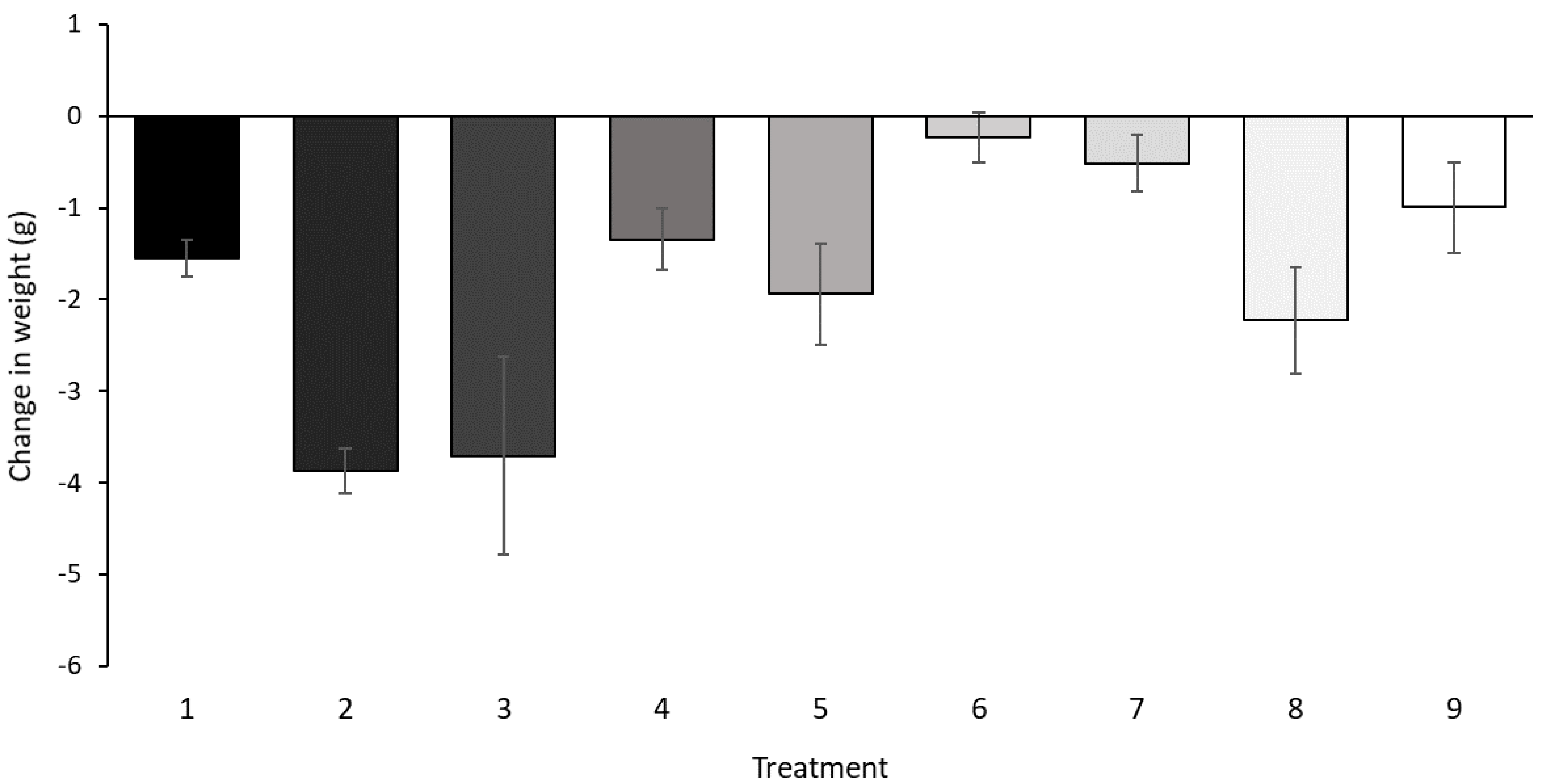
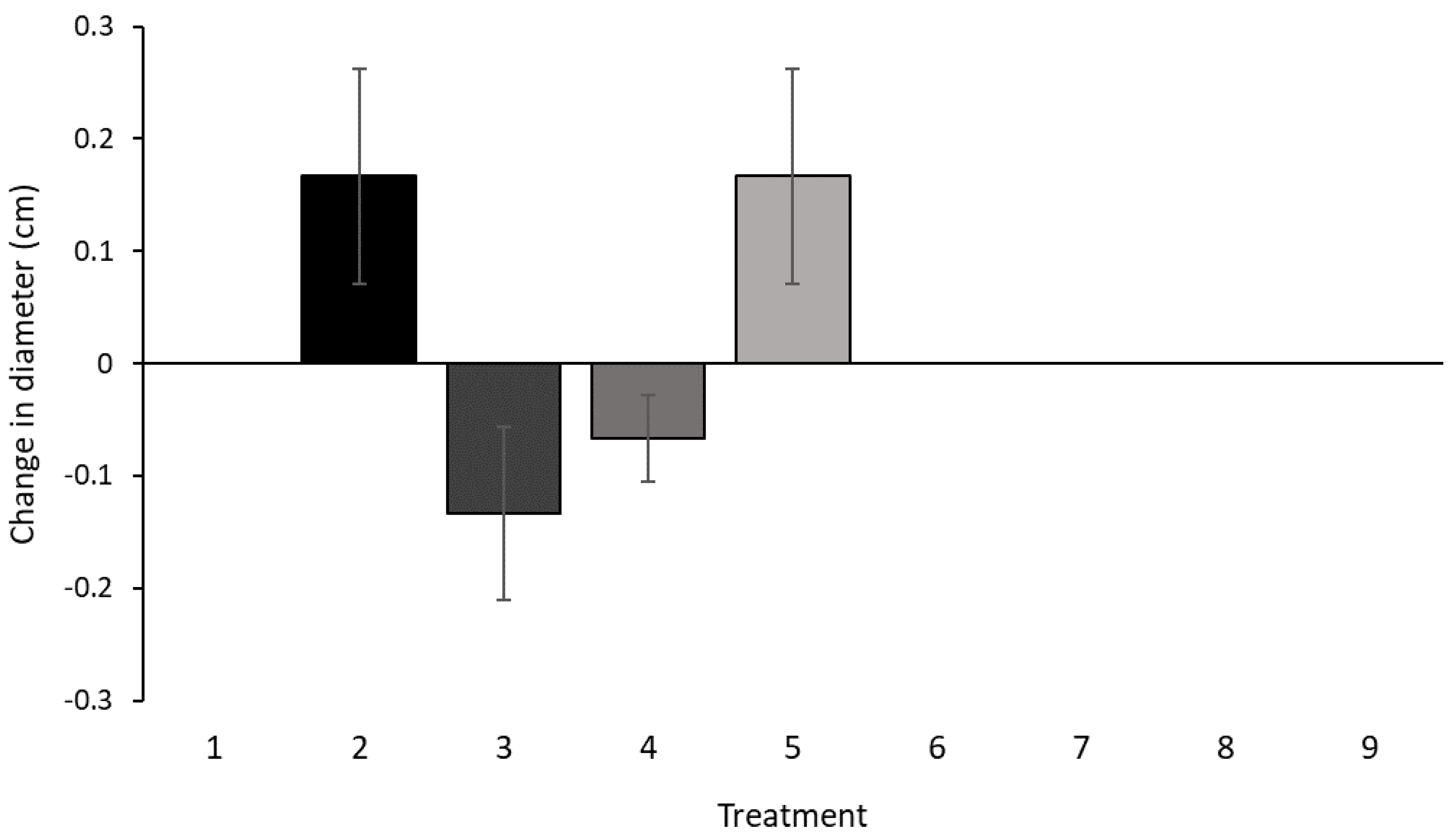
3.2. In Vitro Culture Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- During, H.J.; Tooren, B.F.V. Bryophyte interactions with other plants. Bot. J. Linn. Soc. 1990, 104, 79–98. [Google Scholar] [CrossRef]
- Schmid, E.; Oberwinkler, F.; Gomez, L. Light and electron microscopy of a host–fungus interaction in the roots of some epiphytic ferns from Costa Rica. Can. J. Bot. 1995, 73, 991–996. [Google Scholar] [CrossRef]
- Zuccaro, A.; Schulz, B.; Mitchell, J.I. Molecular detection of ascomycetes associated with Fucus serratus. Mycol. Res. 2003, 107, 1451–1466. [Google Scholar] [CrossRef]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Dearnaley, J. Further advances in orchid mycorrhizal research. Mycorrhiza 2007, 17, 475–486. [Google Scholar] [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef]
- Beike, A.K.; Horst, N.A.; Rensing, S.A. Axenic bryophyte in vitro cultivation. J. Endocytobiosis Cell Res. 2010, 1, 102–108. [Google Scholar]
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. Major transitions in the evolution of early land plants: A bryological perspective. Ann. Bot. 2012, 109, 851–871. [Google Scholar] [CrossRef]
- Bowen, W.R. The imperfect fungus Schizotrichella lunata on the moss Mnium cuspidatum. Bryologist 1968, 124–126. [Google Scholar] [CrossRef]
- Felix, H. Fungi on bryophytes: A review. Bot. Helv. 1988, 98, 239–269. [Google Scholar]
- Brown, D.H.; Bates, J.W. Bryophytes and nutrient cycling. Bot. J. Linn. Soc. 1990, 104, 129–147. [Google Scholar] [CrossRef]
- Read, D.J.; Duckett, J.G.; Francis, R.; Ligrone, R.; Russell, A. Symbiotic fungal associations in ‘lower’ land plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Döbbeler, P. Microniches occupied by bryophilous ascomycetes. Nova Hedwig. 2002, 75, 275–306. [Google Scholar] [CrossRef]
- Davey, M.L.; Currah, R.S. Interactions between mosses (Bryophyta) and fungi. Botany 2006, 84, 1509–1519. [Google Scholar] [CrossRef]
- Ptasznska, A.; Mułenko, W.; Żarnowiec, J. Bryophytes microniches inhabited by microfungi. Ann. UMCS Biol. 2009, 64, 35. [Google Scholar] [CrossRef]
- Stenroos, S.; Laukka, T.; Huhtinen, S.; Döbbeler, P.; Myllys, L.; Syrjänen, K.; Hyvönen, J. Multiple origins of symbioses between ascomycetes and bryophytes suggested by a five-gene phylogeny. Cladistics 2010, 26, 281–300. [Google Scholar] [CrossRef]
- Halbwachs, H.; Vohnik, M.; Rentmeister, J.; Heinrichs, J. Structure and seasonality of fungal communities associated with bryophytes in temperate forests. Fungal Ecol. 2013, 6, 17–27. [Google Scholar]
- Pressel, S.; Bidartondo, M.I.; Ligrone, R.; Duckett, J.G. Fungal symbioses in bryophytes: New insights in the twenty first century. Phytotaxa 2014, 9, 238–253. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Churchill, S.P.; Salazar-Allen, N.; Reiner-Drehwald, M.E. Guide to the Bryophytes of Tropical America; New York Botanical Garden Press: New York, NY, USA, 2001. [Google Scholar]
- Thieret, J.W. Bryophytes as economic plants. Econ. Bot. 1956, 10, 75. [Google Scholar] [CrossRef]
- Glime, J.M. Economic and ethnic uses of bryophytes. Flora N. Am. 2007, 27, 14–41. [Google Scholar]
- Furlan-Lopez, M.A.; Thomas, B.; Calatayud-Vernich, P.; Vidal, R.M. Natural and man-made disasters: Recovery in urban bryophyte communities. J. Bryol. 2022, 44, 45–59. [Google Scholar]
- Bais, H.; Sherrier, J. Advances in Botanical Research; Academic Press LTD-Elsevier Science: Cambridge, MA, USA, 2015. [Google Scholar]
- Osono, T. Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can. J. Microbiol. 2006, 52, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, B.; Wolski, G.J. Bryophilous species of the genus Galerina in peat bogs of Central Poland. Herzogia 2015, 28, 607–623. [Google Scholar] [CrossRef]
- Tosi, S.; Casado, B.; Gerdol, R.; Caretta, G. Fungi isolated from Antarctic mosses. Polar Biol. 2002, 25, 262–268. [Google Scholar] [CrossRef]
- Davey, M.L.; Heegaard, E.; Halvorsen, R.; Ohlson, M.; Kauserud, H. Seasonal trends in the biomass and structure of bryophyte-associated fungal communities explored by 454 pyrosequencing. New Phytol. 2012, 195, 844–856. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Phillips, A.J.L. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 140–144. [Google Scholar] [CrossRef]
- Downing, A.; Marner, S. The first moss to be collected in Australia? Leucobryum candidum—Collected by William Dampier in 1699. J. Bryol. 1998, 20, 237–240. [Google Scholar]
- Michel, P.; Overton, J.M.; Mason, N.; Hurst, J.; Lee, W. Species–environment relationships of mosses in New Zealand indigenous forest and shrubland ecosystems. Plant Ecol. 2011, 212, 353–367. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Hardy, G.E.S.J.; Sivasithamparam, K.; Hussein, A.M.; Kurtböke, D.I. The potential for the biological control of cavity-spot disease of carrots, caused by Pythium coloratum, by streptomycete and non-streptomycete actinomycetes. New Phytol. 1997, 137, 495–507. [Google Scholar] [CrossRef]
- Bakerspigel, A.; Miller, S.M. Fungi in soil and fungal diseases of plants. Am. J. Bot. 1953, 40, 201. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Kauserud, H.; Mathiesen, C.; Ohlson, M. High diversity of fungi associated with living parts of boreal forest bryophytes. Botany 2008, 86, 1326–1333. [Google Scholar] [CrossRef]
- Raja, H.A.; Shearer, C.A.; Miller, A.N.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Galtier, N.; Gouy, M.; Gautier, C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996, 12, 543–548. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.-H. Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. 2008, 4, 193–201. [Google Scholar] [CrossRef]
- Die Döbbeler, P.; Moosbewohnende Ascomyceten, I. Pyrenocarpen den Gametophyten besiedelnden Arten. Mitteilungen Bot. Staatssamml. München 1978, 14, 1–36. [Google Scholar]
- Bowman, E.A.; Arnold, A.E. Distributions of ectomycorrhizal and foliar endophytic fungal communities associated with Pinus ponderosa along a spatially constrained elevation gradient. Am. J. Bot. 2017, 105, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Rossman, A.Y. Where are all the undescribed fungi? Phytopathology 1997, 87, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Cannon, P.F.; Hawksworth, D.L. Fungal diversity in ecosystems. Mycol. Res. 1995, 99, 1057–1065. [Google Scholar]
- Zhang, Z.H.; Li, R.; Yang, D.; Wang, X.; Xiao, Y. Epiphytic bryophytes in Yunnan, China: Diversity, distribution, and conservation. J. Bryol. 2015, 37, 199–210. [Google Scholar]
- De Beer, Z.W.; Duong, T.A.; Dreyer, L.L.; Oberlander, K.C.; Roets, F. New species of Ophiostomatales from Scolytinae and Platypodinae beetles in the Cape Floristic Region, including the discovery of the sexual state of Raffaelea. Antonie Van Leeuwenhoek 2014, 108, 933–950. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J.; Crane, C.; Barrett, S.; Cano-Lira, J.F.; Le Roux, J.J.; Thangavel, R.; Guarro, J.; et al. Fungal Planet description sheets: 469–557. Persoonia Mol. Phylogeny Evol. Fungi 2015, 37, 218–403. [Google Scholar] [CrossRef]
- Davey, M.L.; Currah, R.S. A new species of Cladophialophora (hyphomycetes) from boreal and montane bryophytes. Mycol. Res. 2007, 111, 106–116. [Google Scholar] [CrossRef]
- Döbbeler, P. Polytrichadelphus magellanicus sensu lato (Musci) and its ascomycetes—Different fungi on different hosts. Sendtnera 1999, 7, 35–45. [Google Scholar]


| Sample | Location | Latitude | Longitude | Environment |
|---|---|---|---|---|
| Samples; 1A, 1B | D’ Aguilar National Park (NP) | −27.381297 | 152.778036 | Forested |
| Samples; 2A, 2B | Bunyaville Conservation Park (CP) | −27.368173 | 152.953509 | Urban |
| Samples; 3A, 3B | Mt. Mee | −27.097123 | 152.702889 | Forested |
| Samples 4A, 4B | Mt. Coolum NP | −26.563218 | 153.089238 | Urban |
| Samples; 5A, 5B | Noosa NP | −26.384495 | 153.102599 | Coastal |
| Samples; 6A, 6B | Maroochy River CP | −26.630635 | 153.094886 | Coastal |
| Broth | Inoculant |
|---|---|
| PDB | USC-F427 |
| PDB | USC-F426 |
| PDB | Combination; USC-F427, USC-F426 |
| MB | USC-F427 |
| MB | USC-F426 |
| MB | Combination; USC-F427, USC-F426 |
| Site | # Number of Fungal Isolates | Host | Substrate | Environment |
|---|---|---|---|---|
| D’Aguilar NP | 10 | L. candidum | Rotting Log | Forest |
| Bunyaville CP | 2 | L. candidum | Rotting Log | Urban |
| Mt. Mee | 3 | L. candidum | Rotting Log | Forest |
| Mt. Coolum NP | 4 | L. candidum | Rotting Log | Urban |
| Noosa NP | 9 | L. candidum | Rotting Log | Coastal |
| Maroochy River CP | 5 | L. candidum | Rotting Log | Coastal |
| Total | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misic, L.V.; Shapcott, A.; Franks, A.J.; Kurtböke, D.İ. Fungal Associates of the Moss Leucobryum candidum (Brid. ex P. Beauv.) Wilson in Southeast Queensland, Australia. Diversity 2025, 17, 370. https://doi.org/10.3390/d17060370
Misic LV, Shapcott A, Franks AJ, Kurtböke Dİ. Fungal Associates of the Moss Leucobryum candidum (Brid. ex P. Beauv.) Wilson in Southeast Queensland, Australia. Diversity. 2025; 17(6):370. https://doi.org/10.3390/d17060370
Chicago/Turabian StyleMisic, Lana Valeska, Alison Shapcott, Andrew J. Franks, and D. İpek Kurtböke. 2025. "Fungal Associates of the Moss Leucobryum candidum (Brid. ex P. Beauv.) Wilson in Southeast Queensland, Australia" Diversity 17, no. 6: 370. https://doi.org/10.3390/d17060370
APA StyleMisic, L. V., Shapcott, A., Franks, A. J., & Kurtböke, D. İ. (2025). Fungal Associates of the Moss Leucobryum candidum (Brid. ex P. Beauv.) Wilson in Southeast Queensland, Australia. Diversity, 17(6), 370. https://doi.org/10.3390/d17060370






