Advancing Regional Adaptation and Nitrogen Stress Resilience Through Integrative Phenotyping of Watkins Wheat Landraces via Source–Sink Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Screening and Selection
2.2. Controlled Growth Conditions and Hydroponic Protocols
2.3. Experimental Design and Nitrogen Treatments
2.4. Sampling and Measurements
2.5. Statistical Validation and Data Integration
2.6. Evaluating Stress Indices and Physiological Assay
3. Results
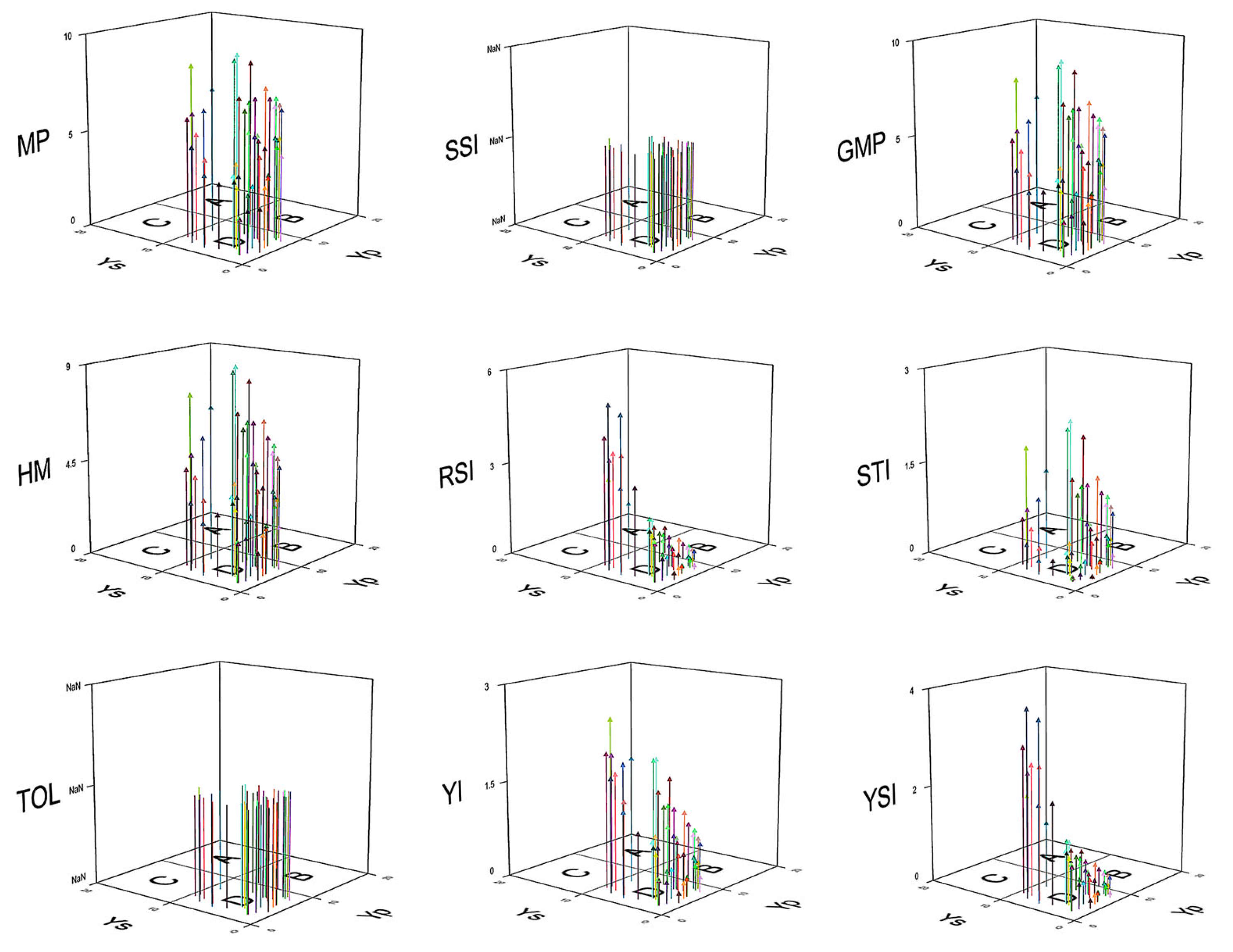
3.1. Three-Dimensional Scatter Plot Illustration
3.1.1. Yield Potential (Yp) and Stress Response (Ys)
3.1.2. Stress Adaptation and Stability Indices
3.1.3. Productivity and Tolerance Metrics
3.1.4. Tolerance and Yield Stability
3.1.5. Implications for Breeding and Selection
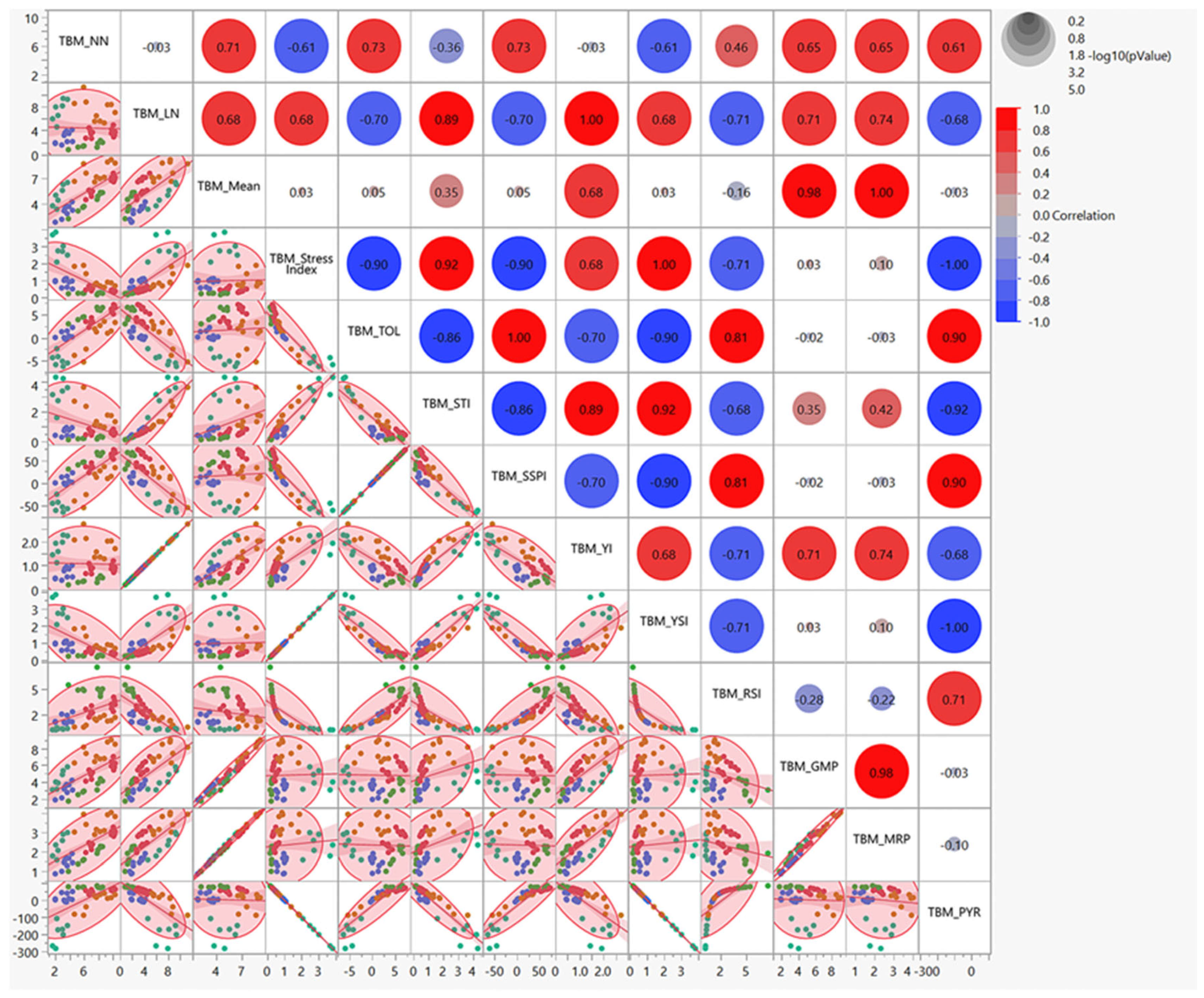
3.2. Multivariate Correlation Analysis of Nitrogen Stress Tolerance
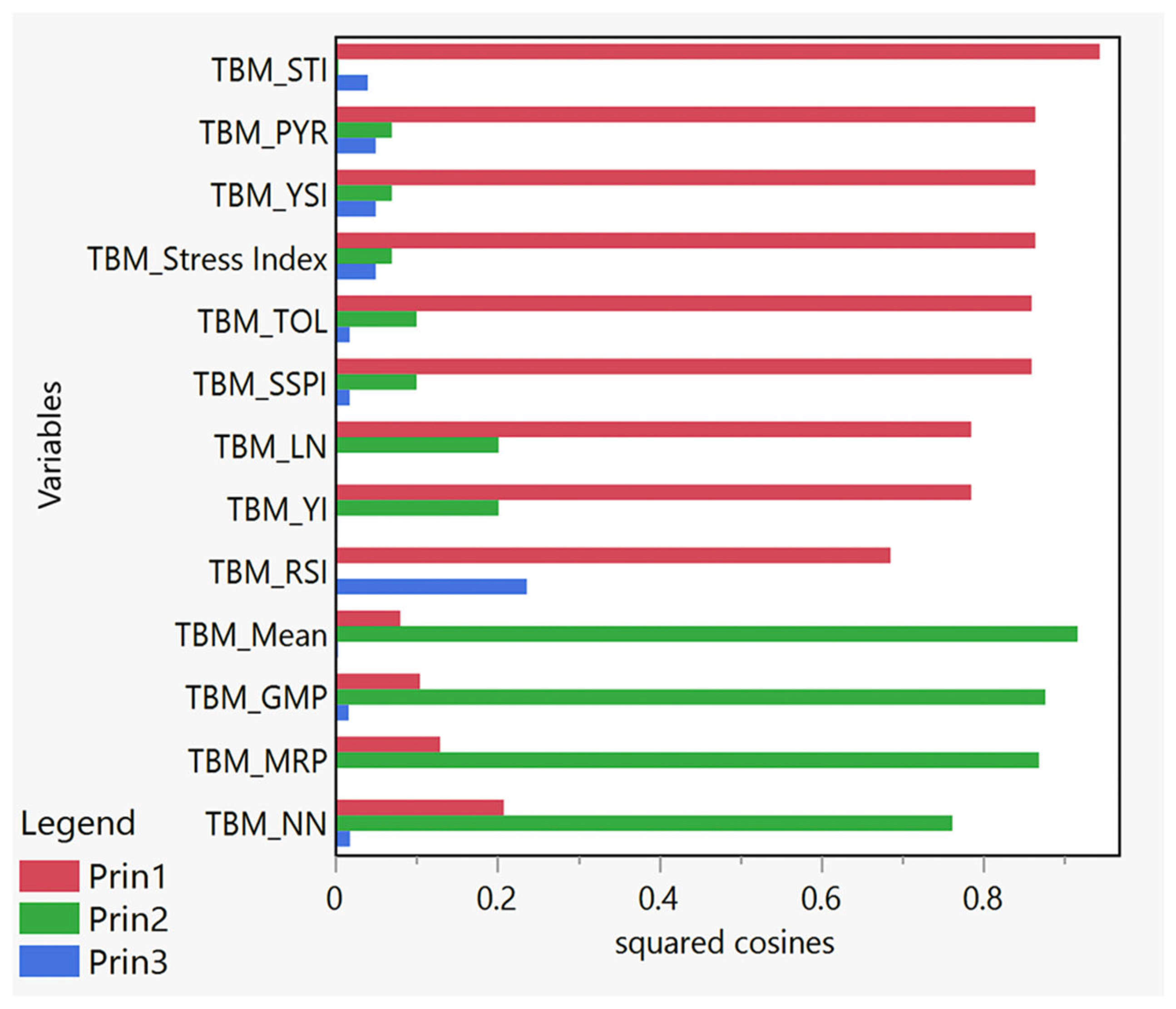
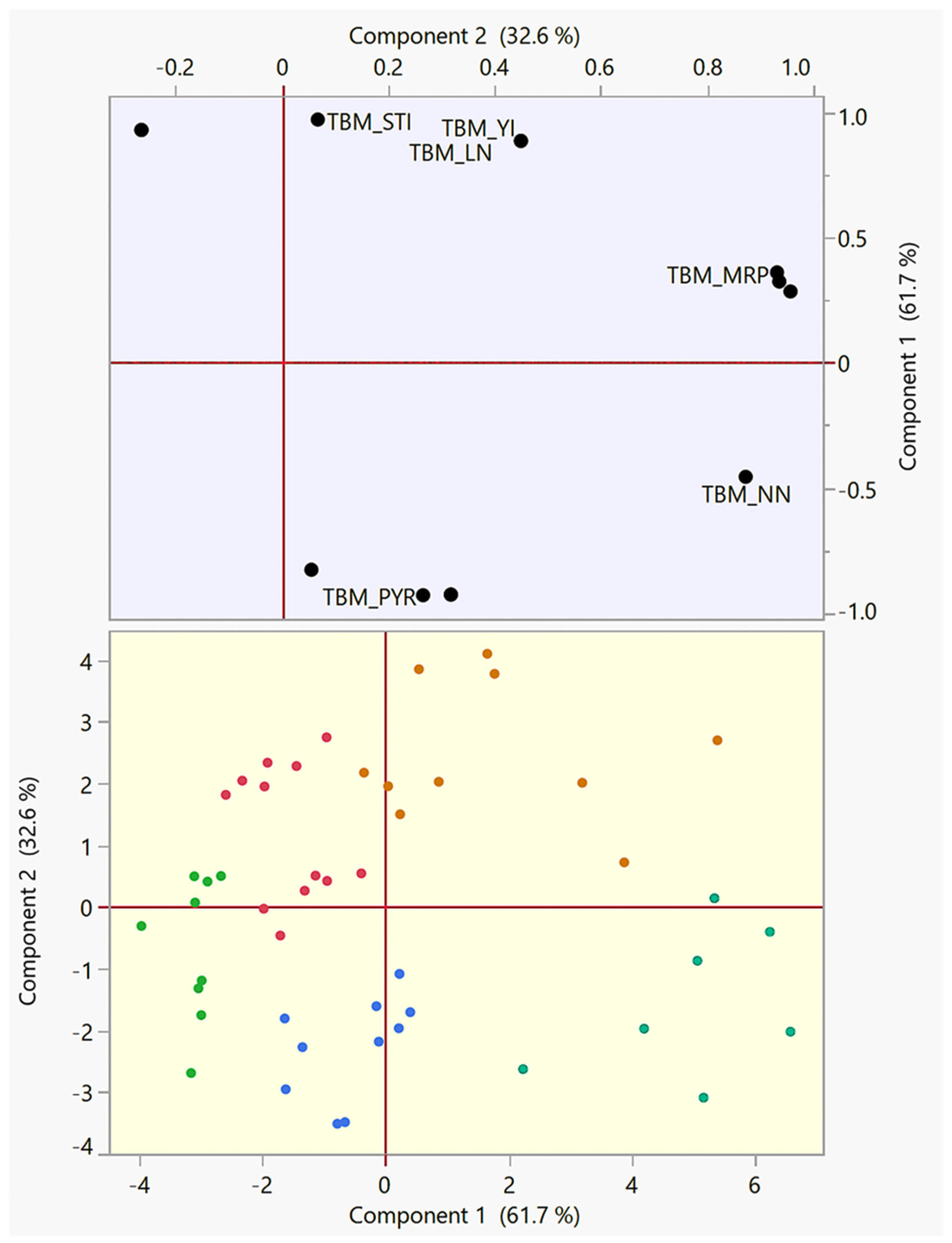
3.3. Squared Cosines Analysis
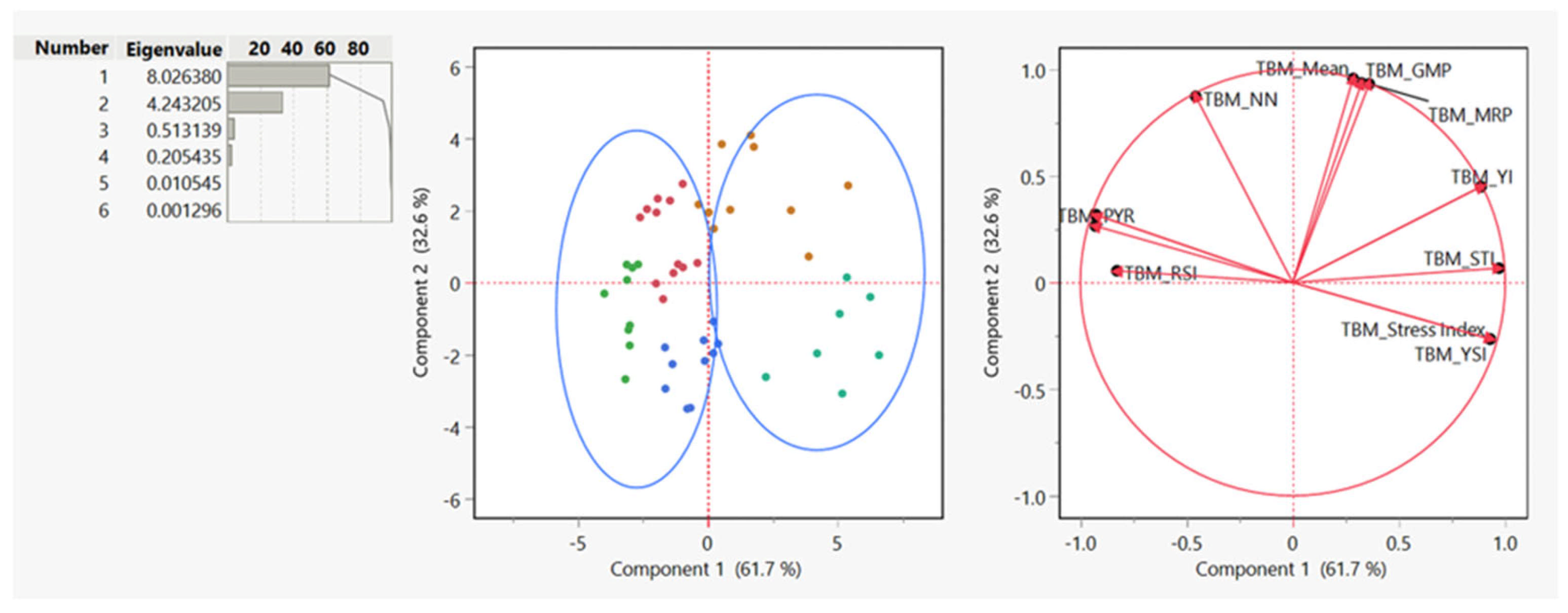
3.4. Principal Component Analysis (PCA)
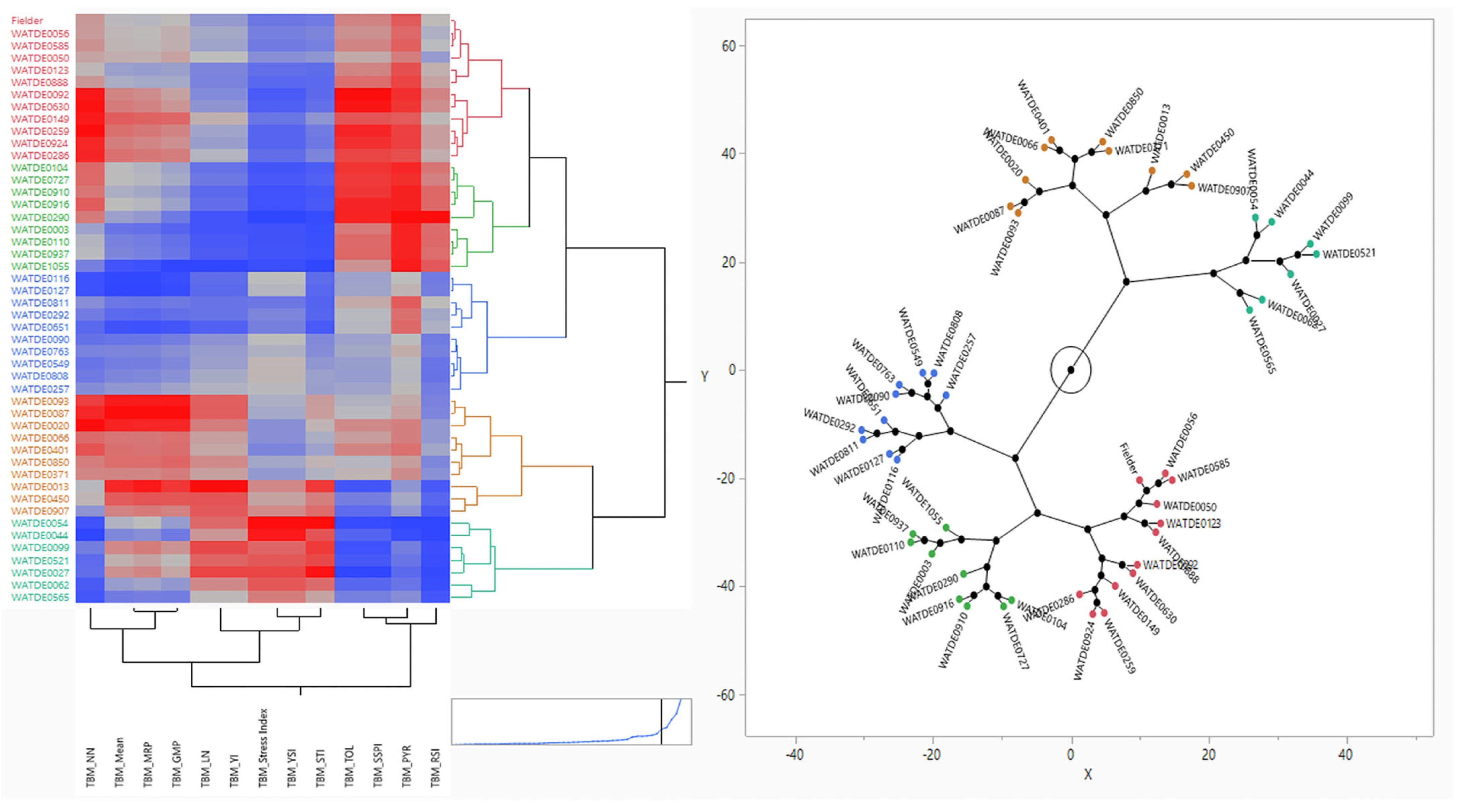
3.5. Hierarchical Clustering and Ranking Based on Stress Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, S.; Kashyap, P.; Sendhil, R.; Gupta, O. Wheat. In Trajectory of 75 Years of Indian Agriculture After Independence; Springer: Singapore, 2023; pp. 137–162. [Google Scholar]
- Amirhajloo, S.; Gheysari, M.; Shayannejad, M.; Shirvani, M. Selection of the best nitrogen fertilizer management scenario in wheat based on Palmer drought severity index with an environmental perspective. Eur. J. Agron. 2023, 151, 126980. [Google Scholar] [CrossRef]
- Fischer, R.; Edmeades, G.O. Breeding and cereal yield progress. Crop Sci. 2010, 50, S-85–S-98. [Google Scholar] [CrossRef]
- Ali, N.; Akmal, M. Wheat growth, yield, and quality under water deficit and reduced nitrogen supply. A review. Gesunde Pflanz. 2022, 74, 371–383. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The utilization and roles of nitrogen in plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Javed, T.; I, I.; Singhal, R.K.; Shabbir, R.; Shah, A.N.; Kumar, P.; Jinger, D.; Dharmappa, P.M.; Shad, M.A.; Saha, D. Recent advances in agronomic and physio-molecular approaches for improving nitrogen use efficiency in crop plants. Front. Plant Sci. 2022, 13, 877544. [Google Scholar] [CrossRef]
- Broccanello, C.; Bellin, D.; DalCorso, G.; Furini, A.; Taranto, F. Genetic approaches to exploit landraces for improvement of Triticum turgidum ssp. durum in the age of climate change. Front. Plant Sci. 2023, 14, 1101271. [Google Scholar] [CrossRef]
- Wingen, L.U.; Orford, S.; Goram, R.; Leverington-Waite, M.; Bilham, L.; Patsiou, T.S.; Ambrose, M.; Dicks, J.; Griffiths, S. Establishing the AE Watkins landrace cultivar collection as a resource for systematic gene discovery in bread wheat. Theor. Appl. Genet. 2014, 127, 1831–1842. [Google Scholar] [CrossRef]
- King, J.; Dreisigacker, S.; Reynolds, M.; Bandyopadhyay, A.; Braun, H.J.; Crespo-Herrera, L.; Crossa, J.; Govindan, V.; Huerta, J.; Ibba, M.I. Wheat genetic resources have avoided disease pandemics, improved food security, and reduced environmental footprints: A review of historical impacts and future opportunities. Glob. Change Biol. 2024, 30, e17440. [Google Scholar] [CrossRef]
- Mefleh, M.; Conte, P.; Fadda, C.; Giunta, F.; Piga, A.; Hassoun, G.; Motzo, R. From ancient to old and modern durum wheat varieties: Interaction among cultivar traits, management, and technological quality. J. Sci. Food Agric. 2019, 99, 2059–2067. [Google Scholar] [CrossRef]
- Gaju, O.; DeSilva, J.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M.J. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crops Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, M.D. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 2009, 149, 7–13. [Google Scholar] [CrossRef]
- Sabina, R.; Paul, J.; Sharma, S.; Hussain, N. Synthetic Nitrogen Fertilizer Pollution: Global Concerns and Sustainable Mitigating Approaches. In Agricultural Nutrient Pollution and Climate Change: Challenges and Opportunities; Springer: Cham, Switzerland, 2025; pp. 57–101. [Google Scholar]
- Zhang, X.; Sabo, R.; Rosa, L.; Niazi, H.; Kyle, P.; Byun, J.S.; Wang, Y.; Yan, X.; Gu, B.; Davidson, E.A. Nitrogen management during decarbonization. Nat. Rev. Earth Environ. 2024, 5, 717–731. [Google Scholar] [CrossRef]
- Tufail, M.A.; Ayyub, M.; Tariq, L.; Iltaf, J.; Asbat, A.; Bashir, I.; Umar, W. Nitrogen fertilizers and the future of sustainable agriculture: A deep dive into production, pollution, and mitigation measures. Soil Sci. Plant Nutr. 2024, 70, 457–477. [Google Scholar] [CrossRef]
- Ying, H.; Ye, Y.; Cui, Z.; Chen, X. Managing nitrogen for sustainable wheat production. J. Clean. Prod. 2017, 162, 1308–1316. [Google Scholar] [CrossRef]
- Link, J.; Batchelor, W.D.; Graeff, S.; Claupein, W. Evaluation of current and model-based site-specific nitrogen applications on wheat (Triticum aestivum L.) yield and environmental quality. Precis. Agric. 2008, 9, 251–267. [Google Scholar] [CrossRef]
- Argento, F.; Anken, T.; Abt, F.; Vogelsanger, E.; Walter, A.; Liebisch, F. Site-specific nitrogen management in winter wheat supported by low-altitude remote sensing and soil data. Precis. Agric. 2021, 22, 364–386. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.-L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V. Synchronization of developmental, molecular and metabolic aspects of source–sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Paul, M.J. Source/sink interactions underpin crop yield: The case for trehalose 6-phosphate/SnRK1 in improvement of wheat. Front. Plant Sci. 2014, 5, 418. [Google Scholar] [CrossRef] [PubMed]
- Ely, K.S.; Burnett, A.C.; Lieberman-Cribbin, W.; Serbin, S.P.; Rogers, A. Spectroscopy can predict key leaf traits associated with source–sink balance and carbon–nitrogen status. J. Exp. Bot. 2019, 70, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Miralles, D.J.; Slafer, G.A. Sink limitations to yield in wheat: How could it be reduced? J. Agric. Sci. 2007, 145, 139–149. [Google Scholar] [CrossRef]
- Asseng, S.; Cammarano, D.; Basso, B.; Chung, U.; Alderman, P.D.; Sonder, K.; Reynolds, M.; Lobell, D.B. Hot spots of wheat yield decline with rising temperatures. Glob. Change Biol. 2017, 23, 2464–2472. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Molero, G.; Trujillo-Negrellos, E.; Reynolds, M.; Foulkes, J. Estimating organ contribution to grain filling and potential for source upregulation in wheat cultivars with a contrasting source–sink balance. Agronomy 2020, 10, 1527. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Duan, J.; Shao, Y.; He, L.; Li, X.; Hou, G.; Li, S.; Feng, W.; Zhu, Y.; Wang, Y.; Xie, Y. Optimizing nitrogen management to achieve high yield, high nitrogen efficiency and low nitrogen emission in winter wheat. Sci. Total Environ. 2019, 697, 134088. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Durand, J.-L.; Gastal, F. Water deficit and nitrogen nutrition of crops. A review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F.B. Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef]
- Bihmidine, S.; Hunter III, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Rad, A.S.; Pazoki, A.; Nourmohammadi, G.; Zarghami, R. Wheat (Triticum aestivum L.) grain filling and dry matter partitioning responses to source: Sink modifications under postanthesis water and nitrogen deficiency. Acta Sci. Agron. 2010, 32, 145–151. [Google Scholar] [CrossRef]
- Mohammadi, R.; Abdulahi, A. Evaluation of durum wheat genotypes based on drought tolerance indices under different levels of drought stress. J. Agric. Sci. 2017, 62, 1–14. [Google Scholar] [CrossRef]
- Zhu, M.; Shabala, S.; Shabala, L.; Fan, Y.; Zhou, M. Evaluating predictive values of various physiological indices for salinity stress tolerance in wheat. J. Agron. Crop Sci. 2016, 202, 115–124. [Google Scholar] [CrossRef]
- Abd El-Mohsen, A.A.; Abd El-Shafi, M.; Gheith, E.; Suleiman, H. Using different statistical procedures for evaluating drought tolerance indices of bread wheat genotypes. Adv. Agric. Biol. 2015, 4, 19–30. [Google Scholar]
- Rosielle, A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Raman, A.; Verulkar, S.; Mandal, N.; Variar, M.; Shukla, V.; Dwivedi, J.; Singh, B.; Singh, O.; Swain, P.; Mall, A. Drought yield index to select high yielding rice lines under different drought stress severities. Rice 2012, 5, 31. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Food Crops to Temperature and Water Stress, Taiwan, China, 13–18 August 1992. [Google Scholar]
- Fischer, R.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campanile, R.; Ricciardi, G.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Poczai, P.; Siddique, K.H. STABILITYSOFT: A new online program to calculate parametric and non-parametric stability statistics for crop traits. Appl. Plant Sci. 2019, 7, e01211. [Google Scholar] [CrossRef]
- Ketata, H.; Yau, S.; Nachit, M. Relative consistency performance across environments. In Proceedings of the International Symposium on Physiology and Breeding of Winter Cereals for Stressed Mediterranean Environments, Montpellier, France, 3–6 July 1989; pp. 391–400. [Google Scholar]
- Bidinger, F.; Mahalakshmi, V.; Rao, G.D.P. Assessment of drought resistance in pearl millet (Pennisetum americanum (L.) Leeke). II. Estimation of genotype response to stress. Aust. J. Agric. Res. 1987, 38, 49–59. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W., Jr. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance 1. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source–sink ratios during grain filling in wheat and barley under contrasting environments. Field Crops Res. 2013, 150, 42–51. [Google Scholar] [CrossRef]
- Martínez-Peña, R.; Schlereth, A.; Höhne, M.; Encke, B.; Morcuende, R.; Nieto-Taladriz, M.T.; Araus, J.L.; Aparicio, N.; Vicente, R. Source-sink dynamics in field-grown durum wheat under contrasting nitrogen supplies: Key role of non-foliar organs during grain filling. Front. Plant Sci. 2022, 13, 869680. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Kamiji, Y.; Pang, J.; Milroy, S.P.; Palta, J.A. Shoot biomass in wheat is the driver for nitrogen uptake under low nitrogen supply, but not under high nitrogen supply. Field Crops Res. 2014, 165, 92–98. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Christina, M.; Laclau, J.-P.; Gonçalves, J.L.M.; Jourdan, C.; Nouvellon, Y.; Bouillet, J.-P. Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forests. Ecosphere 2011, 2, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.-Z.; Kang, M.-P.; Li, X.-Y. The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107836. [Google Scholar] [CrossRef]
- Yan, H.; Shi, H.; Hu, C.; Luo, M.; Xu, C.; Wang, S.; Li, N.; Tang, W.; Zhou, Y.; Wang, C. Transcriptome differences in response mechanisms to low-nitrogen stress in two wheat varieties. Int. J. Mol. Sci. 2021, 22, 12278. [Google Scholar] [CrossRef]
- Pathak, R.R.; Mandal, V.K.; Jangam, A.P.; Sharma, N.; Madan, B.; Jaiswal, D.K.; Raghuram, N. Heterotrimeric G-protein α subunit (RGA1) regulates tiller development, yield, cell wall, nitrogen response and biotic stress in rice. Sci. Rep. 2021, 11, 2323. [Google Scholar] [CrossRef]
- Zuluaga, D.L.; Sonnante, G. The use of nitrogen and its regulation in cereals: Structural genes, transcription factors, and the role of miRNAs. Plants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Dodig, D.; Božinović, S.; Nikolić, A.; Zorić, M.; Vančetović, J.; Ignjatović-Micić, D.; Delić, N.; Weigelt-Fischer, K.; Junker, A.; Altmann, T. Image-derived traits related to mid-season growth performance of maize under nitrogen and water stress. Front. Plant Sci. 2019, 10, 814. [Google Scholar] [CrossRef]
- Shi, W.; Muthurajan, R.; Rahman, H.; Selvam, J.; Peng, S.; Zou, Y.; Jagadish, K.S. Source–sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013, 197, 825–837. [Google Scholar] [CrossRef]
- Andrianasolo, F.N.; Champolivier, L.; Debaeke, P.; Maury, P. Source and sink indicators for determining nitrogen, plant density and genotype effects on oil and protein contents in sunflower achenes. Field Crops Res. 2016, 192, 33–41. [Google Scholar] [CrossRef]
- Mangi, N.; Nazir, M.F.; Wang, X.; Iqbal, M.S.; Sarfraz, Z.; Jatoi, G.H.; Mahmood, T.; Ma, Q.; Shuli, F. Dissecting source-sink relationship of subtending leaf for yield and fiber quality attributes in upland cotton (Gossypium hirsutum L.). Plants 2021, 10, 1147. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Jiang, Z.; Wang, W.; Xu, R.; Wang, Q.; Zhang, Z.; Li, A.; Liang, Y.; Ou, S. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 2021, 590, 600–605. [Google Scholar] [CrossRef]
- Patil, B.S.; Bhat, J.S. Genomic Approaches for Achieving Higher Nutrient Use Efficiency. In Breeding Climate Resilient and Future Ready Oilseed Crops; Springer: Singapore, 2025; p. 163. [Google Scholar]
- Schillaci, C.; Perego, A.; Acutis, M.; Botta, M.; Tadiello, T.; Gabbrielli, M.; Barsali, T.; Tozzi, F.; Chiaramonti, D.; Jones, A. Assessing marginality of Camelina (C. sativa L. Crantz) in rotation with barley production in Southern Europe: A modelling approach. Agric. Ecosyst. Environ. 2023, 357, 108677. [Google Scholar] [CrossRef]
- Balmaceda, M.A.; Bainotti, C.; Gomez, D.; González, F.G.; Helguera, M.; Ruiz, M.B. Selection of wheat genotypes for water deficit tolerance using different drought tolerance indices. Cereal Res. Commun. 2024, 52, 1175–1185. [Google Scholar] [CrossRef]
- Zafar, M.M.; Iqbal, M.S.; Kamal, H.; Khan, A.I.; Sarfraz, Z.; Saeed, A.; Razzaq, A.; Shakeel, A. Advancing heat tolerance in cotton through integration of multiple stress tolerance indices and multivariate analyses. Int. J. Agric. Biosci. 2024, 13, 65–75. [Google Scholar]
- Kumar, Y.; Devi, S.; Phougat, D.; Chaurasia, H.; Choudhary, S. Assessment of Barley genotypes for salinity tolerance based on various indices under field condition. Assess. Curr. Agric. Res. J. 2024, 12, 586–598. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, C.; Wang, Y.; Sha, Y.; Hao, Z.; Chen, F.; Yuan, L.; Mi, G. Nitrogen allocation and remobilization contributing to low-nitrogen tolerance in stay-green maize. Field Crops Res. 2021, 263, 108078. [Google Scholar] [CrossRef]
- Yan, Z.; Bin, C.; Zhengwen, S.; Zhengwen, L.; Yanru, C.; Ke, H.; Zhicheng, W.; Liqiang, W.; Guiyin, Z.; Wang, G.; et al. A large-scale genomic association analysis identifies a fragment in Dt11 chromosome conferring cotton Verticillium wilt resistance. Plant Biotechnol. J. 2021, 19, 2126–2138. [Google Scholar] [CrossRef]
- Cai, Z.; Xie, T.; Xu, J. Source–sink manipulations differentially affect carbon and nitrogen dynamics, fruit metabolites and yield of Sacha Inchi plants. BMC Plant Biol. 2021, 21, 160. [Google Scholar] [CrossRef]
- Burgess, A.J.; Masclaux-Daubresse, C.; Strittmatter, G.; Weber, A.P.; Taylor, S.H.; Harbinson, J.; Yin, X.; Long, S.; Paul, M.J.; Westhoff, P. Improving crop yield potential: Underlying biological processes and future prospects. Food Energy Secur. 2023, 12, e435. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Shah, M.M.; Iqbal, M.S.; Nazir, M.F.; Al-Ashkar, I.; Rehmani, M.I.A.; Shahid Iqbal, M.; Ullah, N.; El Sabagh, A. Rendering multivariate statistical models for genetic diversity assessment in A-genome diploid wheat population. Agronomy 2021, 11, 2339. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Stoddard, F.L.; Ortiz, R. Genomic-based root plasticity to enhance abiotic stress adaptation and edible yield in grain crops. Plant Sci. 2020, 295, 110365. [Google Scholar] [CrossRef]
- Biradar, S.S.; Patil, M.K.; Desai, S.; Singh, S.K.; Naik, V.R.; Lamani, K.; Joshi, A.K. Nitrogen use efficiency in bread wheat: Genetic variation and prospects for improvement. PLoS ONE 2024, 19, e0294755. [Google Scholar] [CrossRef]
- Mubushar, M.; El-Hendawy, S.; Tahir, M.U.; Alotaibi, M.; Mohammed, N.; Refay, Y.; Tola, E. Assessing the suitability of multivariate analysis for stress tolerance indices, biomass, and grain yield for detecting salt tolerance in advanced spring wheat lines irrigated with saline water under field conditions. Agronomy 2022, 12, 3084. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, M.; Hossain, M.A.; Rohman, M.M.; Uddin, M.N.; Haque, M.S.; Ahmed, J.U.; Hossain, A.; Hassan, M.M.; Mostofa, M.G. Multivariate analysis of morpho-physiological traits reveals differential drought tolerance potential of bread wheat genotypes at the seedling stage. Plants 2021, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.M.; Jia, X.; Shakeel, A.; Sarfraz, Z.; Manan, A.; Imran, A.; Mo, H.; Ali, A.; Youlu, Y.; Razzaq, A. Unraveling heat tolerance in upland cotton (Gossypium hirsutum L.) using univariate and multivariate analysis. Front. Plant Sci. 2022, 12, 727835. [Google Scholar] [CrossRef]
- Amara, V.; Samura, A.E.; Norman, P.E.; Kanu, S.A.; Karim, K.Y.; Tiendrebeogo, F.; Eni, A.O.; Pita, J.S. Characterization of the Cassava (Manihot esculenta) germplasm in Sierra Leone Based on agro-morphological and culinary traits. Horticulturae 2024, 10, 640. [Google Scholar] [CrossRef]
- Peng, Z.; Rehman, A.; Li, X.; Jiang, X.; Tian, C.; Wang, X.; Li, H.; Wang, Z.; He, S.; Du, X. Comprehensive evaluation and transcriptome analysis reveal the salt tolerance mechanism in semi-wild cotton (Gossypium purpurascens). Int. J. Mol. Sci. 2023, 24, 12853. [Google Scholar] [CrossRef]
- Konishi, M.; McLaren, D.G.; Engen, H.; Smallwood, J. Shaped by the past: The default mode network supports cognition that is independent of immediate perceptual input. PLoS ONE 2015, 10, e0132209. [Google Scholar] [CrossRef]
- Sandhu, N.; Sethi, M.; Kaur, H.; Dhillon, A.; Kumar, A.; Kaur, A.; Kaur, S.; Bentley, A.R.; Chhuneja, P. Mining natural genetic variations for nitrogen use efficiency utilizing nested synthetic hexaploid wheat introgression libraries. Environ. Exp. Bot. 2023, 212, 105394. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From zero to hero: The past, present and future of grain amaranth breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef]
- Vieira, R.A.; Nogueira, A.P.O.; Fritsche-Neto, R. Optimizing the selection of quantitative traits in plant breeding using simulation. Front. Plant Sci. 2025, 16, 1495662. [Google Scholar] [CrossRef]

| Stress Index | Abbreviation | Formula | Reference |
|---|---|---|---|
| Tolerance Index | TOL | Yp − Ys | [38] |
| Mean Productivity | MP | [38] | |
| Geometric Mean | GMP | [40] | |
| Harmonic Mean | HM | [45] | |
| Stress Susceptibility Index | SSI | [41] | |
| Stress Tolerance Index | STI | [40] | |
| Yield Index | YI | [42] | |
| Yield Stability Index | YSI | [46] | |
| Relative Stress Index | RSI | [41] |
| Trait | NN | LN | Mean | Stress Index | TOL | STI | SSPI | YI | YSI | RSI | GMP | MRP | PYR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NN | 1 | −0.031 | 0.714 | −0.611 | 0.735 | −0.365 | 0.735 | −0.031 | −0.611 | 0.462 | 0.653 | 0.655 | 0.611 |
| LN | −0.031 | 1 | 0.677 | 0.678 | −0.701 | 0.886 | −0.701 | 1 | 0.678 | −0.707 | 0.712 | 0.735 | −0.678 |
| Mean | 0.714 | 0.677 | 1 | 0.025 | 0.050 | 0.352 | 0.050 | 0.677 | 0.025 | −0.155 | 0.979 | 0.997 | −0.025 |
| Stress Index | −0.611 | 0.678 | 0.025 | 1 | −0.896 | 0.918 | −0.896 | 0.678 | 1 | −0.708 | 0.027 | 0.098 | −1 |
| TOL | 0.735 | −0.701 | 0.050 | −0.896 | 1 | −0.861 | 1 | −0.701 | −0.896 | 0.809 | −0.018 | −0.032 | 0.896 |
| STI | −0.365 | 0.886 | 0.352 | 0.918 | −0.861 | 1 | −0.861 | 0.886 | 0.918 | −0.683 | 0.347 | 0.423 | −0.918 |
| SSPI | 0.735 | −0.701 | 0.050 | −0.896 | 1 | −0.861 | 1 | −0.701 | −0.896 | 0.809 | −0.018 | −0.032 | 0.896 |
| YI | −0.031 | 1 | 0.677 | 0.678 | −0.701 | 0.886 | −0.701 | 1 | 0.678 | −0.707 | 0.712 | 0.735 | −0.678 |
| YSI | −0.611 | 0.678 | 0.025 | 1 | −0.896 | 0.918 | −0.896 | 0.678 | 1 | −0.708 | 0.027 | 0.098 | −1 |
| RSI | 0.462 | −0.707 | −0.155 | −0.708 | 0.809 | −0.683 | 0.809 | −0.707 | −0.708 | 1 | −0.285 | −0.221 | 0.708 |
| GMP | 0.653 | 0.712 | 0.979 | 0.027 | −0.018 | 0.347 | −0.018 | 0.712 | 0.027 | −0.285 | 1 | 0.981 | −0.027 |
| MRP | 0.655 | 0.735 | 0.997 | 0.098 | −0.032 | 0.423 | −0.032 | 0.735 | 0.098 | −0.221 | 0.981 | 1 | −0.098 |
| PYR | 0.611 | −0.678 | −0.025 | −1 | 0.896 | −0.918 | 0.896 | −0.678 | −1 | 0.708 | −0.027 | −0.098 | 1 |
| Number | Eigenvalue | Percent | Cum Percent | ChiSquare | DF | Prob > ChiSq |
|---|---|---|---|---|---|---|
| 1 | 8.02638 | 61.741 | 61.741 | 425.017 | 76.727 | <0.0001 * |
| 2 | 4.243205 | 32.64 | 94.381 | 60.56 | 77.98 | 0.9279 |
| 3 | 0.513139 | 3.947 | 98.329 | 0 | 72.584 | 1 |
| 4 | 0.205435 | 1.58 | 99.909 | 0 | 61.125 | 1 |
| 5 | 0.010545 | 0.081 | 99.99 | 0 | 50.321 | 1 |
| 6 | 0.001296 | 0.01 | 100 | 0 | 40.462 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waheed, A.; Iqbal, M.S.; Sarfraz, Z.; Wei, Y.; Hou, J.; Li, S.; Song, B.; Cheng, S. Advancing Regional Adaptation and Nitrogen Stress Resilience Through Integrative Phenotyping of Watkins Wheat Landraces via Source–Sink Dynamics. Diversity 2025, 17, 359. https://doi.org/10.3390/d17050359
Waheed A, Iqbal MS, Sarfraz Z, Wei Y, Hou J, Li S, Song B, Cheng S. Advancing Regional Adaptation and Nitrogen Stress Resilience Through Integrative Phenotyping of Watkins Wheat Landraces via Source–Sink Dynamics. Diversity. 2025; 17(5):359. https://doi.org/10.3390/d17050359
Chicago/Turabian StyleWaheed, Abdul, Muhammad Shahid Iqbal, Zareen Sarfraz, Yanping Wei, Junliang Hou, Sixing Li, Bo Song, and Shifeng Cheng. 2025. "Advancing Regional Adaptation and Nitrogen Stress Resilience Through Integrative Phenotyping of Watkins Wheat Landraces via Source–Sink Dynamics" Diversity 17, no. 5: 359. https://doi.org/10.3390/d17050359
APA StyleWaheed, A., Iqbal, M. S., Sarfraz, Z., Wei, Y., Hou, J., Li, S., Song, B., & Cheng, S. (2025). Advancing Regional Adaptation and Nitrogen Stress Resilience Through Integrative Phenotyping of Watkins Wheat Landraces via Source–Sink Dynamics. Diversity, 17(5), 359. https://doi.org/10.3390/d17050359







