Abstract
To learn about the yeast biodiversity of Hungarian honeys and to isolate osmotolerant yeasts, fifteen different honey varieties, beeswax, and bee bread were purchased, and samples of another, but highly osmotic material, tree sap (cherry, sour cherry, and plum), were collected from the northeastern region of the country. In total, 60 yeast strains were isolated and their taxonomic positions were determined by barcode sequences using ITS1-NL4 primers. The honey products contained mostly Zygosaccharomyces and Starmerella species. In addition, Hanseniaspora uvarum, Rhodotorula mucilaginosa and diobovata, Sporobolomyces roseus, Filobasidium magnum, Naganishia sp., and Aureobasidium pullulans were also present in smaller numbers. In contrast, tree saps contained Metschnikowia and Pichia fermentas cells. Further results suggest that some of the yeasts in honey can only “survive”, while others can propagate at high sugar levels, generally between 600 and 700 mg/g, with a predominance of fructose. Properties important for pathogenicity, such as invasive hyphae production, gelatin melting ability, and growth at 37 °C, were also examined. Hanseniaspora uvarum and Pichia fermentans representatives seemed to be negative for gelatin hydrolysis, while the other strains were able to melt gelatin. Although some of the strains could produce hyphae-like structures at 25 °C, none of them could grow at 37 °C.
1. Introduction
Honey is a natural sweetener that has been widely consumed and used by mankind since ancient times due to its high nutritional value and health-related properties. Honey is made by several species of bees. The vast majority of commercialized honey is produced by the best-known species, the western honeybee (Apis mellifera), but interest has increased for honeys made by stingless bees as well [1,2,3]. According to the Codex Alimentarius, honey is produced from nectar or honeydew, which honeybees collect, transform, and combine with specific substances of their own, store, and leave in their honeycombs to ripen and mature [4].
Honey is an aromatic, sweet food with considerable nutritional value. Surveys of honey composition have long revealed that the three major components of honey are fructose, glucose, and water [5] and that the sugar composition as well as the fructose/glucose ratio may show significant differences between different honey types [6]. In most types of honey, fructose is the predominant carbohydrate [7]. The sugar composition of honeys depends mainly on its botanical and geographical origin and is affected by climate, processing, and storage [7,8]. In addition to the high carbohydrate content that makes honey an excellent source of energy, honeys contain proteins, amino acids, organic acids, vitamins, minerals, phenolic compounds, and volatile compounds. Several of these nutritional components have been proven to possess various beneficial properties [9,10]. Honey has long been appreciated for its positive health effects. Traditionally, it was mainly used to treat wounds and various gastrointestinal diseases. More recently, antioxidant, anti-inflammatory, antimicrobial, and anticancer effects have been linked to honey consumption, increasing interest in this food. Studies attribute these beneficial effects of honey to its content of phenolic compounds [11,12].
Honeys may contain microorganisms, which influence the quality and safety of the product. Microbial contamination may originate from primary sources such as pollen, the digestive tracts of honeybees, and the environment, or from the post-harvest processing of honey [13,14]. Studies using traditional microbiological assays and modern DNA-based analysis agree that the most common microorganisms in honey are spore-forming bacteria and yeasts [13,15]. Due to its low water activity, honey provides a special environment for microbial growth. Some yeast species, termed sugar-tolerant or osmophilic, can survive and grow in this environment of high sugar content and high osmotic pressure [16,17,18]. Sugar-tolerant/osmotolerant yeasts are a problem in the honey industry, as they can cause fermentation of honey and thereby a significant economic loss to the honey producers. Species of the Zygosaccharomyces genus are often responsible for spoilage in honey [19,20]. On the other hand, high osmotolerance can be an advantage in special biotechnological processes; thus, yeasts isolated from honey can be well-suited for use in the biotechnological industry. Yeast strains with high sugar tolerance are therefore searched for and tested, e.g., for high-concentration ethanol fermentation or for the production of special compounds [21,22,23].
Honey production is a significant economic activity in Hungary; with 25,000 tons, Hungary was the fifth largest producer of honey in the European Union in 2022 [24]. A wide variety of different monofloral, multifloral, and honeydew honeys are available in Hungary; some of the most commonly sold are acacia (Robinia pseudoacacia), linden (Tilia spp.), rape (Brassica napus), and sunflower (Helianthus annuus). In recent years, interest has increased in the investigation of Hungarian honeys. Most studies have focused on the examination of the floral origin and nutritional properties of different Hungarian honey types or their potential medical applications. Several investigations have combined pollen spectrum analysis with antioxidant capacity, physicochemical, and element analysis to demonstrate the quality and botanical origin of honeys [25,26,27,28,29]. Other studies have been related to detecting the effect of different treatments on honeys or revealing honey adulteration [30,31,32]. Analysis of the mineral content of different honey types has been carried out to ensure food quality and safety or to demonstrate that mineral content can serve as an indicator of environmental changes [33,34,35,36]. Studies on the potential medical application of honeys have mostly investigated their antibacterial and antibiofilm effects. The antibacterial activity of different monofloral honeys has been tested against respiratory tract pathogens [37,38], wound-associated bacteria [39], and food-borne pathogens [40].
Studies on the microbiological analysis of Hungarian honeys are scarce. Except for the study of Čadež and co-workers [18], who isolated the osmophilic species Zygosaccharomyces favi from Hungarian honey and bee bread, we have no knowledge of any studies on the detailed microbial analysis of Hungarian honeys. Therefore, we decided to analyze mono- and multifloral honey samples from the northeastern region of Hungary. In this study, we focused on the isolation and identification of yeast strains, as they can reveal the yeast flora of honeys and can be used for further biotechnological applications in subsequent studies. In addition, we examined the propagation ability of the yeast isolates under certain conditions and their potential pathogenicity. Moreover, the sugar content of certain honey varieties was also determined.
2. Materials and Methods
2.1. Source of Yeast Strains
We acquired different types of honey (rape, acacia, sunflower, linden, multifloral, buckwheat, apple, forest, pine, wild raspberry, goldenrod (Solidago gigantea), bastard indigo (Amorpha fruticose), dandelion (Taraxacum officinale), honey flavored with blackberries, sea buckthorn, propolis black walnut, bee bread, and beeswax from honey producers or markets. We collected the sap of fruit trees (cherry, sour cherry, and plum), as well. A total of 52 samples were collected, all from the northeastern region of Hungary (Figure 1). The geographical coordinates of the sampling sites in larger cities were obtained from https://www.maps.ie/coordinates.html (accessed on 13 February 2025).
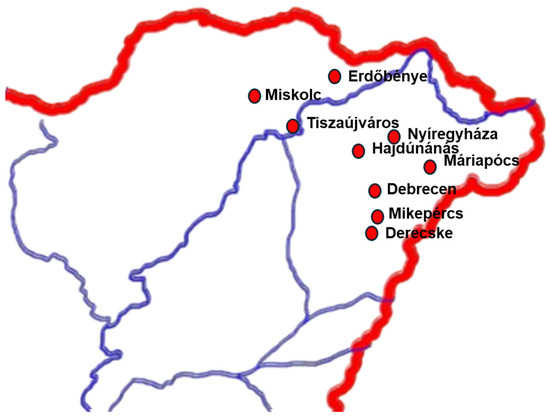
Figure 1.
The geographical locations of the sampling sites. Coordinates of larger cities: Debrecen: Latitude—47.531399, Longitude—21.6259782; Nyíregyháza: Latitude—47.9557802, Longitude—21.7167982; Tiszaújváros: Latitude—47.9280341, Longitude—21.0467056; Miskolc: Latitude—48.1030643, Longitude—20.7900429.
2.2. Isolation of Yeast Strains
Honey samples were streaked on the surface of a complex medium (YPA) (1% yeast extract, 2% peptone, 2% glucose, and 2% agar) (VWR International Ltd., Debrecen, Hungary), or a modified YPA medium with elevated glucose (5%), or placed in the same liquid medium (YPL) prepared without agar (5 mL). The cultures were incubated at room temperature for two weeks. Yeast colonies with different colors and/or morphologies were randomly isolated and spread on a fresh medium to obtain pure cultures from single cells. This step was repeated three times. The samples were aseptically treated, and the final isolates were cryopreserved at −80 °C. Their origin is shown in Table S1.
2.3. Amplification of DNA Barcoding Sequences
A colony PCR technique was used to amplify the barcoding sequences [41]. When this method was unsuccessful, genomic DNA (extracted from overnight cultures grown in YPL) was used as a template for PCR reactions [42]. The barcoding sequences (chromosomal segments corresponding to rDNA genes) were amplified using Dream Taq DNA Polymerase (Thermo Fisher Scientific) and ITS1 (5′-TCCTCCGCTTATTGATATGC-3′) [43] and NL-4 (5′-GGTCCGTGTTTCAAGACGGR-3′) primers [44]. These primers amplify a region containing ITS1-5.8S rDNA-ITS2 and a partial region from 28S rDNA. The PCR conditions were as follows: after an initial denaturation step (94 °C for 30 s), the DNA was amplified in 30 cycles (95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min), which was followed by a final elongation step (72 °C for 10 min). The PCR products were checked on a 1% agarose gel (1 × TBE, 120 V, 45 min), purified with a PCR purification KIT (VWR International Ltd., Debrecen, Hungary), and sequenced with either the ITS1 or the NL4 primer (Microsynth AG, Vienna, Austria).
2.4. Sequence Analyses
Sequences were analyzed with the NCBI BLASTn program (https://blast.ncbi.nlm.nih.gov, accessed on 21 February 2025) (megablast, core nucleotide database, sequences from type material [45]). In certain cases, the sequences were also aligned to sequences of non-type material. These data are marked with (*) in Table S2. In general, we accepted the results with the highest Max Scores, Query covers of 99–100%, and E values of 0. The results were cross-validated in the 28S rDNA and ITS RefSeq databases [46] and in the Unite database [47,48]. Conflict between the results of NCBI and Unite searches was resolved with phylogenetic analyses. For species-level identification, 99–100% sequence identity was accepted. In cases of two or more identical search results, lower sequence identity, or lower coverage, only the genus was determined, except for Metschnikowia species. Due to the high intragenomic diversity of Metschnikowia barcoding sequences (two nucleotides alternate in certain positions) [49] and unclear species boundaries [50], Metschnikowia sequences were accepted with 98% sequence identity and designated Metschnikowia aff. pulcherrima. Synonyms of species names were collected from https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi (accessed on 21 February 2025).
2.5. Phylogenetic Analyses
28S rDNA and ITS sequences were searched in the RefSeq database, and the 10 most similar sequences of type strains from the concerned genera were downloaded. A phylogenetic workflow was assembled using the Phylogeny.fr platform [51] for phylogenetic tree constructions. The collected and sequenced DNA sequences were aligned with MUSCLE v3.8.31 (full mode, maximum iteration: 16) [52], and the ambiguous regions were removed with GBLOCKS v0.91b [53]. The PhyML v3.0 [54] algorithm, either with the GTR or with the HKY85 substitution model, was used for the phylogenies. The number of substitution rate categories was adjusted to 4. Gamma distribution parameters, proportions of invariable sites, and transition/transversion ratios were all estimated. Branch support was estimated with the approximate likelihood ratio test [55]. The trees were displayed with FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 21 February 2025) or with TreeDyn v198.3 at the website of Phylogeny.fr [51].
2.6. Survival Ability of Yeasts at High Sugar Levels
Drop tests were used to reveal the cell propagation abilities of selected yeast strains at high sugar levels.
Certain yeast strains isolated from a particular acacia honey were tested on YEA (1% yeast extract, 2% glucose, and 2% agar) (VWR International Ltd., Debrecen, Hungary) and modified YEA, which had similar sugar contents to the acacia honey from which they were derived (Bánk) (379 g/L fructose and 239 g/L glucose, pH 4).
Cell suspensions were prepared in sterile MQ water from yeast cells (cultured for one day on YEA at 25 °C). A total of 20 µL of cell suspension (OD595: 0.2) and its serial dilutions (10×, 100×, and 1000×) were dropped onto the surface of agar plates, which were incubated at 25 °C, and the growth was monitored for two weeks. The experiment was repeated three times.
2.7. Pathogenicity Tests of Yeast Strains
To examine the potential pathogenicity of the isolated yeast strains, three virulence factors were investigated: the propagation ability at human physiological temperature (~37 °C), invasive hyphal growth, and substrate hydrolyzing ability [56,57].
One representative of each species (Zygosaccharomyces mellis: 11-2311, Metschnikowia aff. pulcherrima: 11-2294, Rhodotorula mucilaginosa: 11-2330, Naganishia sp.: 11-2328, Sporobolomyces rosea: 11-2331, Hanseniaspora uvarum: 11-2309, Starmerella magnoliae: 11-2374, Filobasidium magnum: 11-2326, Aureobasidium pullulans: 11-2322, and Pichia fermentans: 11-2296) was selected for testing. Yeast strains cultured on YEA for three days were used to prepare cell suspensions in sterile MQ water. A total of 15 µL of cell suspension (OD595: 0.2) and its serial dilutions (10×, 100×, and 1000×) were dropped onto the surface of YEA and modified YEA (solidified with 8% gelatin (WVR) instead of agar). To determine whether yeast cells are able to grow at 37 °C or not, the agar plates (YEA) were incubated at 37 °C and 25 °C as controls. For testing substrate hydrolyzing we used gelatin plates incubated at 25 °C. Growth at 37 °C and melting of gelatin around the yeast cells were monitored for two weeks. In addition, hyphae production of the yeasts was also monitored. The experiments were repeated two times.
2.8. Sample Preparation and Determination of Fructose and Glucose Contents of Honeys
For each honey sample, 200 ± 1 mg was weighed and dissolved in 10 mL of deionized water. The samples were filtered using 0.22 µm PTFE syringe filters (Lab-Ex Ltd., Budapest, Hungary) and diluted tenfold with deionized water. Two independent weightings and sugar content determinations were performed from each sample. A Shimadzu LC-20 Prominence HPLC system equipped with an evaporative light scattering detector (ELSD) (Shimadzu, Kyoto, Japan) was used for the separation and determination of fructose and glucose contents of honey samples. The compounds were separated on a Supelco apHera NH2 Polymer column (150 mm × 4.6 mm i. d. 5 μm) thermostated at 30 °C (±1 °C). Acetonitrile (A) and water (B) (both from VWR International Ltd., Debrecen, Hungary) were used as eluents. The flow rate was maintained at 700 μL/min. The gradient elution was performed as follows: 0–1 min: 80% of mobile phase A; 1–13 min: → 63% A; 13–14 min: → 80% A; 14–18 min: 80% A. Retention time of fructose—6.2 min; retention time of glucose—7.4 min. The ELSD detector was thermostated at 35 °C (±1 °C), and the pressure of nitrogen gas (Linde 5.0) was set to 350 kPa. Standard solutions with the following concentrations were used to determine the calibration curves: 250 µg/mL, 500 µg/mL, 1000 µg/mL, and 1500 µg/mL. The injection volume was 10 µL for both standards and samples.
3. Results and Discussion
To learn about the yeast biodiversity of Hungarian honeys and isolate osmotolerant yeasts, 52 samples of 15 different honey varieties were purchased (see materials and methods). All honey came from the northeastern region of the country (Figure 1). In addition, beeswax, bee bread, and a completely different substance, the sap of fruit trees, were also examined.
To obtain information on the yeast biodiversity of our samples, we streaked them on a complex medium that allowed living cells to divide. There were 22 samples which contained viable yeast cells, so we were able to isolate 49 yeast strains from rape, acacia, bastard indigo, sunflower, linden, multifloral honey, beeswax, and bee bread and 11 strains from tree saps (Table S1). In contrast, although several attempts have been made to isolate yeast cells from certain samples, no yeasts have been isolated from buckwheat, apple, forest, pine, wild raspberry, goldenrod (Solidago gigantea), dandelion (Taraxacum officinale) honey, and honey flavored with blackberries, sea buckthorn, propolis, or black walnut. As the latter honey varieties originated from different locations and apiaries, we assume that this negative result is not necessarily due to the honey-making process but also to the antifungal activity of these honeys. This may be supported by results indicating strong antimicrobial properties of propolis and some honeys [38,40,58,59,60,61,62,63].
Later, the taxonomic position of the isolated strains was determined by barcode sequencing using the ITS1-NL4 primers [43,44]. The amplified rDNA regions included ITS1-5.8S rDNA-ITS2 and partial 28S rDNA (D1/D2 region). The PCR products were sequenced (Table S2), and the sequences were analyzed using BLASTn in different databases [45,46,47,48]. Various yeasts were identified (Tables S3 and S4). However, many times we observed conflict between the NCBI Type Material and UNITE search results (Tables S3 and S4). The reason for this phenomenon could be the different approaches and data availability in the used databases. Furthermore, certain species could be delineated rather by their D1/D2 region, while others by their ITS regions. To resolve the conflict between the different results, we used phylogenetic-based taxonomic approaches in certain cases (Figure 2 and Figures S1–S6) [64]. For example, a BLASTn search using the Type Material database at NCBI resulted in Starmerella vacinii as the best hit, while the UNITE search showed S. magnoliae as the most probable hit in the case of strain 11-2391. However, the phylogenetic analysis of the Starmerella-like strains indicated that all those strains most probably belong to the species S. magnoliae (Figure 2A).
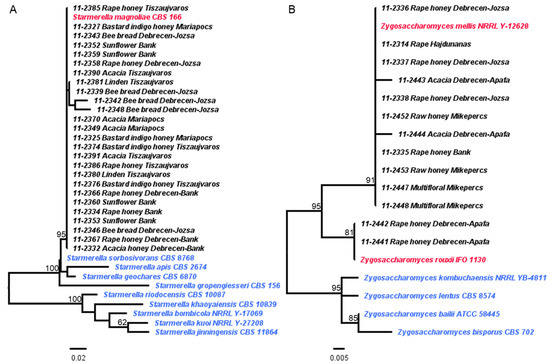
Figure 2.
Phylogenetic trees of selected species of the genera Starmerella (A) and Zygosaccharomyces (B). The strains isolated in this study are listed in black letters, while the selected type strains of the genera are indicated with blue and red letters. (A) The isolated strains most probably belong to the species S. magnoliae. However, to evaluate the exact taxonomic position of some bee bread-originating species (11-2342 and 11-2348), further studies are needed. (B) Most of the isolated strains belong to the species Z. mellis (indicated with red letters), while a small portion of the isolated strains belong to the species Z. rouxii (also indicated with red letters). Two strains (11-2443 and 11-2444) of the isolated strains showed some sequence divergence compared to the Z. mellis-like strains, but to evaluate whether the exhibited difference is species- or strain-level diversity needs further studies. Four sequences from the isolated Zygosaccharomyces-like strains were not included in the phylogenetic analysis because of insufficient sequence length. The trees were created with PhyML 3.0 [54] at the website of Phylogeny.fr [51] and based on 28S rDNA sequences (297 and 134 selected nucleotide sites, respectively) of the type strains. Branch support was estimated with aLRT [55]. aLRT values lower than 50% are not shown.
The honey products contained mostly Starmerella magnoliae species (21 strains) (Figure 2A, Table S5) and Zygosaccharomyces (17 strains: Z. mellis, Z. rouxii, and other Zygosaccharomyces spp.) (Figure 2B, Table S5). They were found in different honey varieties and sugar concentrations, indicating that they may be well-adapted species. In addition, Hanseniaspora uvarum, Rhodotorula mucilaginosa and diobovata, Sporobolomyces roseus, Filobasidium magnum, Naganishia sp., and Aureobasidium pullulans were also present in smaller numbers (Figures S1–S6, Table S5). These data correlate well with the results of Sinacori [65] or Echeverrigaray [3], where Zygosaccharomyces species were among the most frequently isolated microbes, although these honeys were made by different bees: honeybees [65] or stingless bees [3]. As Zygosaccharomyces species have been isolated from honey from different countries, such as Canada [66], Portugal [67], or China [21], they tend to be widespread yeasts in honey. However, their presence may not be beneficial, as these species are often referred to as food and beverage spoilage yeasts [21,68,69,70,71,72]. Starmerella magnoliae (synonyms: Torulopsis magnoliae and Candida magnoliae), which has also been isolated from Polish [23], Italian [73], and Portuguese honey [67], may also be widespread.
Interestingly, the yeasts identified from tree sap were very different from the honey samples. Tree saps contained mainly Metschnikowia isolates and two Pichia fermentas, which were not detected in honey (Table S5). The sequences of Metschnikowia were most similar to M. pulcherrima (Tables S3 and S4). However, their species-level identification is somewhat uncertain due to their high intragenomic diversity and unclear species boundaries (Figure S7) [49,50].
Since honey samples are a special habitat for microorganisms due to their high osmolarity [74], the question was also raised whether living yeast cells can “just” tolerate the high osmolarity of honey or whether they can divide under such hard conditions. To answer this question, we determined the exact sugar content of the honey samples from which the yeast cells were isolated using HPLC analyses. As shown in Table 1, the fructose + glucose concentrations were generally between 600 and 700 mg/g, which is slightly lower than those of honey collected in, for example, Tanzania or China [58,75]. In general, fructose dominated, as in many other honeys [29,58,75,76,77]. Most acacia honey had a fructose/glucose ratio of 1.6, similar to acacia honey collected in other regions of the country (F/G: 1.55) [29]. The lowest F/G ratio was in rape honey (0.8), similar to, for example, the results of Estonian or Romanian samples [78,79], while the highest (2.4) was in sunflower honey.

Table 1.
Sugar content of the selected honeys.
Thereafter, we performed a drop test with four strains isolated from a certain acacia honey (11-2330, 11-2331, 11-2332, 11-2333). As shown in Figure 3, the cell propagation abilities of the selected strains were different on the high sugar-containing medium (379 g/L fructose and 239 g/L glucose). The Starmerella magnoliae strain 11-2332 could divide both on the control medium (YEA prepared with 2% glucose, pH 4) (Figure 3A) and under high osmolarity (Figure 3B). In contrast, cells of Rhodotorula diobovata (11-2333) and Sporobolomyces roseus (11-2331) did not propagate under high sugar levels (Figure 3D), only on the control medium (Figure 3C). Thus, it can be assumed that some of the yeasts in honey only “survive”, while others are also able to propagate at high sugar levels.
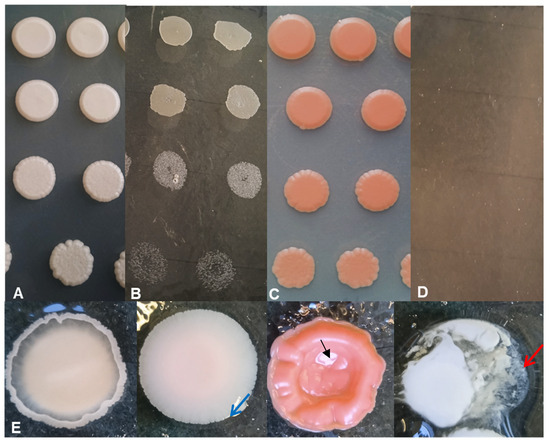
Figure 3.
Drop tests for growth at high sugar content and gelatin melting capacity. The Starmerella magnoliae strain (11-2332) could propagate both on the control medium (YEA) (A) and a high sugar-containing medium (B). In contrast, Rhodotorula mucilaginosa (11-2330) cells could only propagate on the control (C) and not on high sugar content (D). (Sporobolomyces roseus (11-2331) and Rhodotorula diobovata (11-2333) yielded similar results.) Top to bottom: OD595:0.2 and its 10×, 100×, and 1000× dilutions. Growth on gelatin-containing media and gelatin hydrolysis (E). Some strains did not, while others melted gelatin to varying degrees. (From left to right: Hanseniaspora uvarum (11-2309) did not melt the gelatin but produced hyphae (Pichia fermentans 11-2296 was similar)). Filobasidium magnum (11-2326) slightly melted gelatin (marked with a blue arrow) and submerged in the medium. Rhodotorula mucilaginosa (11-2330) produced red droplets with a cave in the middle (marked with a black arrow), indicating that gelatin in the middle region of the droplet had melted. Starmerella magnolie (11-2374) completely melted the gelatin (marked with a red arrow).
Considering the fact that honey is generally consumed without heat treatment, it is important to know whether the yeast strains inhabiting the honey exhibit potential pathogenicity. To test that eventuality, we examined the strains’ ability to undergo cell division at 37 °C, hydrolyze gelatin, and produce invasive hyphae. These features are important in the assessment of GRAS status (generally recognized as safe). Therefore, representatives of species were cultured on gelatin-solidified medium and agar plates (YEA). According to our data, Hanseniaspora uvarum (11-2309) and Pichia fermentans (11-2296) seemed to be negative for gelatin hydrolysis; however, they formed hyphae (Figure 3E and Figure S8). While the other strains were able to melt gelatin around the droplets to different extents, Filobasidium magnum (11-2326) and Rhodotorula mucilaginosa (11-2330) slightly melted the gelatin and were submerged in the culture medium (Figure S9A). In contrast, Starmerella magnoliae (11-2374) completely melted the gelatin-containing medium around itself (Figure 3E and Figure S9B). Although some strains could produce hyphae, while others melted gelatin, none of them grew on YEA at 37 °C. Thus, it might be easily concluded that the yeast flora of the tested honey products is safe for human consumption.
Taken together, this study provides an insight into the yeast biodiversity of Hungarian honeys from the northeastern region. In addition, the capacity for cell propagation at high sugar content and some GRAS conditions were also investigated. The isolated strains can be used for biotechnological applications after further testing.
4. Conclusions
Certain yeast species, such as Starmerella magnoliae, Zygosaccharomyces spp., Hanseniaspora uvarum, Rhodotorula mucilaginosa and diobovata, Sporobolomyces roseus, Filobasidium magnum, Naganishia sp., and Aureobasidium pullulans are able to maintain their viability in honey despite high osmolarity. Starmerella magnoliae and Zygosaccharomyces may be well adapted species, as they have been isolated in larger numbers from different honey varieties and different sugar concentrations. Some yeasts only “survive”, while others can divide at a sugar content similar to honey. The presence of these yeasts seems to be safe for human consumption as the yeast strains tested failed to grow at 37 °C.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17050325/s1, Figure S1: Phylogenetic trees of selected species of the genus Filobasidium; Figure S2: Phylogenetic trees of selected species of the genus Hanseniaspora; Figure S3: Phylogenetic trees of selected species of the genus Naganishia; Figure S4–S5: Phylogenetic trees of selected species of the genus Rhodotorula; Figure S6: Phylogenetic trees of selected species of the genus Sporobolomyces; Figure S7: High intragenomic diversity of Metschnikowia barcoding sequences; Figure S8: Hyphae production of Hanseniaspora uvarum (11-2309); Figure S9: Gelatin melting ability of the yeast strains. Table S1: Isolated yeast strains and their origin; Table S2: Barcoding sequences; Table S3: Results of the BLASTn analyses at the website of NCBI; Table S4: Results of the UNITE analyses; Table S5: Taxonomic identification of the isolates.
Author Contributions
Conceptualization, M.M., L.Á.-S. and I.M.; methodology, M.M., L.Á.-S., L.A.P., Z.C. and M.M.; validation, L.Á.-S.; formal analysis, I.M. and L.Á.-S.; investigation, M.M., Z.C. and I.M.; resources, I.M. and M.M.; data curation, L.A.P.; writing—original draft preparation, M.M. and I.M.; writing—review and editing, L.Á.-S. and L.A.P.; visualization, I.M. and L.Á.-S.; funding acquisition, I.M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. TKP2021-EGA-18 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. The project was also supported by the Scientific Council of the University of Nyíregyháza, under the project no. IHK/206-19/2024. The APC of this publication was supported by the University of Debrecen Program for Scientific Publication.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Files). The sequences generated during the current study are available in the GenBank repository with the following accession numbers: PV231358, PV231412-PV231415, PV241705-PV241731, and PV243997-PV244024.
Acknowledgments
The authors thank Ilona Lakatos for skillful technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crane, E. Honey from Honeybees and Other Insects. Ethol. Ecol. Evol. 1991, 3, 100–105. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical Profiles of Stingless Bee (Apidae: Meliponini) Honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Scariot, F.J.; Foresti, L.; Schwarz, L.V.; Rocha, R.K.M.; Da Silva, G.P.; Moreira, J.P.; Delamare, A.P.L. Yeast Biodiversity in Honey Produced by Stingless Bees Raised in the Highlands of Southern Brazil. Int. J. Food Microbiol. 2021, 347, 109200. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Revised Codex Standard for Honey Codex Stan 12–1981; Codex Alimentarius Commission: Rome, Italy, 2001. [Google Scholar]
- Doner, L.W. The Sugars of Honey—A Review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef]
- Mateo, R.; Bosch-Reig, F. Sugar Profiles of Spanish Unifloral Honeys. Food Chem. 1997, 60, 33–41. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Tedesco, R.; Scalabrin, E.; Malagnini, V.; Strojnik, L.; Ogrinc, N.; Capodaglio, G. Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs). Foods 2022, 11, 2441. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Lin, X.; Bai, W.; Xiao, G.; Liu, G. Composition, Functional Properties and Safety of Honey: A Review. J. Sci. Food Agric. 2023, 103, 6767–6779. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Palma-Morales, M.; Huertas, J.; Rodríguez-Pérez, C. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, J.A.; Cliver, D.O. Microorganisms in Honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Klein, G. Microbiology and Food-Borne Pathogens in Honey. Crit. Rev. Food Sci. Nutr. 2017, 57, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.R.; Sogin, J.H.; Worobo, R.W. Microbiome Analysis of Raw Honey Reveals Important Factors Influencing the Bacterial and Fungal Communities. Front. Microbiol. 2023, 13, 1099522. [Google Scholar] [CrossRef]
- Munitis, M.T.; Cabrera, E.; Rodriguez-Navarro, A. An Obligate Osmophilic Yeast from Honey. Appl. Environ. Microbiol. 1976, 32, 320–323. [Google Scholar] [CrossRef]
- Park, Y.K.; Koo, M.H.; Oliveira, I.M.D.A. Biochemical Characteristics of Osmophilic Yeasts Isolated from Pollens and Honey. Biosci. Biotechnol. Biochem. 1996, 60, 1872–1873. [Google Scholar] [CrossRef]
- Čadež, N.; Fülöp, L.; Dlauchy, D.; Péter, G. Zygosaccharomyces favi Sp. Nov., an Obligate Osmophilic Yeast Species from Bee Bread and Honey. Antonie Van Leeuwenhoek 2015, 107, 645–654. [Google Scholar] [CrossRef]
- Hulin, M.; Wheals, A. Rapid Identification of Zygosaccharomyces with Genus-Specific Primers. Int. J. Food Microbiol. 2014, 173, 9–13. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Cheng, N.; Zhao, H.; Zhang, Y.; Liu, C.; He, L.; Ma, T.; Li, Y.; Cao, W. Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey. Foods 2023, 12, 2730. [Google Scholar] [CrossRef]
- Liu, G.; Tao, C.; Zhu, B.; Bai, W.; Zhang, L.; Wang, Z.; Liang, X. Identification of Zygosaccharomyces mellis Strains in Stored Honey and Their Stress Tolerance. Food Sci. Biotechnol. 2016, 25, 1645–1650. [Google Scholar] [CrossRef]
- Iwata, K.; Maeda, M.; Kashiwagi, Y.; Maehashi, K.; Yoshikawa, J. Isolation of Zygosaccharomyces siamensis Kiy1 as a Novel Arabitol-Producing Yeast and Its Arabitol Production. AMB Expr. 2023, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Ziuzia, P.; Janiec, Z.; Wróbel-Kwiatkowska, M.; Lazar, Z.; Rakicka-Pustułka, M. Honey’s Yeast—New Source of Valuable Species for Industrial Applications. Int. J. Mol. Sci. 2023, 24, 7889. [Google Scholar] [CrossRef] [PubMed]
- Honey Market Overview (Autumn 2024); 2024. Available online: https://agriculture.ec.europa.eu/farming/animal-products/honey_en (accessed on 25 January 2025).
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, Antioxidant Activity and Multielement Analysis of Two Types of Early Spring Honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Bodor, Z.; Kovacs, Z.; Benedek, C.; Hitka, G.; Behling, H. Origin Identification of Hungarian Honey Using Melissopalynology, Physicochemical Analysis, and Near Infrared Spectroscopy. Molecules 2021, 26, 7274. [Google Scholar] [CrossRef]
- Kocsis, M.; Bodó, A.; Kőszegi, T.; Csepregi, R.; Filep, R.; Hoffmann, G.; Farkas, Á. Quality Assessment of Goldenrod, Milkweed and Multifloral Honeys Based on Botanical Origin, Antioxidant Capacity and Mineral Content. Int. J. Mol. Sci. 2022, 23, 769. [Google Scholar] [CrossRef]
- Dominkó, E.; Németh, Z.I.; Rétfalvi, T. Classification of Acacia, Rape and Multifloral Hungarian Honey Types. Heliyon 2024, 10, e30498. [Google Scholar] [CrossRef]
- Czipa, N.; Phillips, C.J.C.; Kovács, B. Composition of Acacia Honeys Following Processing, Storage and Adulteration. J. Food Sci. Technol. 2019, 56, 1245–1255. [Google Scholar] [CrossRef]
- Bodor, Z.; Kovacs, Z.; Rashed, M.S.; Kókai, Z.; Dalmadi, I.; Benedek, C. Sensory and Physicochemical Evaluation of Acacia and Linden Honey Adulterated with Sugar Syrup. Sensors 2020, 20, 4845. [Google Scholar] [CrossRef]
- Bodor, Z.; Benedek, C.; Aouadi, B.; Zsom-Muha, V.; Kovacs, Z. Revealing the Effect of Heat Treatment on the Spectral Pattern of Unifloral Honeys Using Aquaphotomics. Molecules 2022, 27, 780. [Google Scholar] [CrossRef]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of Essential and Toxic Elements in Hungarian Honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Poór, P.; Ördög, A.; Tari, I.; Bátori, Z. Mineral Content Analysis of Unifloral Honeys from the Hungarian Great Plain. J. Elem. 2016, 22, 271–281. [Google Scholar] [CrossRef]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental Analysis of Hungarian Honey Samples and Bee Products by MP-AES Method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Varga, T.; Sajtos, Z.; Gajdos, Z.; Jull, A.J.T.; Molnár, M.; Baranyai, E. Honey as an Indicator of Long-Term Environmental Changes: MP-AES Analysis Coupled with 14C-Based Age Determination of Hungarian Honey Samples. Sci. Total Environ. 2020, 736, 139686. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, Á. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods 2021, 10, 1632. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Radványi, L.; Balázs, V.L.; Kocsis, B.; Csikós, E.; Ángyán, V.D.; Szabó, P.; Biró, V.; Kocsis, M.; Farkas, Á. Antibacterial Activity of Hungarian Varietal Honeys against Respiratory Pathogens as a Function of Storage Time. Sci. Rep. 2024, 14, 10200. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy-Radványi, L.; Bencsik-Kerekes, E.; Koloh, R.; Szabó, D.; Kocsis, B.; Kocsis, M.; Farkas, Á. Antibacterial and Antibiofilm Effect of Unifloral Honeys against Bacteria Isolated from Chronic Wound Infections. Microorganisms 2023, 11, 509. [Google Scholar] [CrossRef]
- Farkas, Á.; Balázs, V.L.; Kõszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked With Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef]
- Acs-Szabo, L.; Papp, L.A.; Takacs, S.; Miklos, I. Disruption of the Schizosaccharomyces Japonicus Lig4 Disturbs Several Cellular Processes and Leads to a Pleiotropic Phenotype. JoF 2023, 9, 550. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; ISBN 978-1-936113-42-2. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- O’Donnel, K. Fusarium and Its near Relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Federhen, S. Type Material in the NCBI Taxonomy Database. Nucleic Acids Res. 2015, 43, D1086–D1098. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Tedersoo, L.; Nilsson, R.H.; Vellak, K.; Saar, I.; Veldre, V.; Parmasto, E.; Prous, M.; Aan, A.; Ots, M.; et al. PlutoF—A Web Based Workbench for Ecological and Taxonomic Research, with an Online Implementation for Fungal ITS Sequences. Evol. Bioinform. Online 2010, 6, EBO.S6271. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE Database for Molecular Identification and Taxonomic Communication of Fungi and Other Eukaryotes: Sequences, Taxa and Classifications Reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M.; Czentye, K.; Kállai, Z. High Intragenomic, Intergenomic, and Phenotypic Diversity in Pulcherrimin-Producing Metschnikowia Yeasts Indicates a Special Mode of Genome Evolution. Sci. Rep. 2024, 14, 10521. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and Related Pulcherrimin-Producing Yeasts: Fuzzy Species Boundaries and Complex Antimicrobial Antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.Fr: Robust Phylogenetic Analysis for the Non-Specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Anisimova, M.; Gascuel, O. Approximate Likelihood-Ratio Test for Branches: A Fast, Accurate, and Powerful Alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Muñoz, C.J.; Combina, M.; Toro, M.E.; De Figueroa, L.I.C.; Vazquez, F. Biocontrol of Botrytis cinerea in Table Grapes by Non-Pathogenic Indigenous Saccharomyces cerevisiae Yeasts Isolated from Viticultural Environments in Argentina. Postharvest Biol. Technol. 2012, 64, 40–48. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.F.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile Organic Compounds from Starmerella bacillaris to Control Gray Mold on Apples and Modulate Cider Aroma Profile. Food Microbiol. 2020, 89, 103446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, H.; Wang, Q.; Wu, F.; Cao, W. A Novel Chinese Honey from Amorpha fruticosa L.: Nutritional Composition and Antioxidant Capacity In Vitro. Molecules 2020, 25, 5211. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Sariguzel, F.; Berk, E.; Koc, A.N.; Sav, H.; Demir, G. Antifungal Activity of Propolis Against Yeasts Isolated From Blood Culture: In Vitro Evaluation. Clin. Lab. Anal. 2016, 30, 513–516. [Google Scholar] [CrossRef]
- Sadowska, B.; Budzyńska, A.; Stochmal, A.; Żuchowski, J.; Różalska, B. Novel Properties of Hippophae rhamnoides L. Twig and Leaf Extracts—Anti-Virulence Action and Synergy with Antifungals Studied in Vitro on Candida spp. Model. Microb. Pathog. 2017, 107, 372–379. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Fernandes, L.; Ribeiro, H.; Oliveira, A.; Sanches Silva, A.; Freitas, A.; Henriques, M.; Rodrigues, M.E. Portuguese Honeys as Antimicrobial Agents against Candida Species. J. Tradit. Complement. Med. 2021, 11, 130–136. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An Update on Its Chemistry and Pharmacological Applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Sinacori, M.; Francesca, N.; Alfonzo, A.; Cruciata, M.; Sannino, C.; Settanni, L.; Moschetti, G. Cultivable Microorganisms Associated with Honeys of Different Geographical and Botanical Origin. Food Microbiol. 2014, 38, 284–294. [Google Scholar] [CrossRef]
- Shiwa, Y.; Kanesaki, Y.; Ishige, T.; Mura, K.; Hori, T.; Tamura, T. Draft Genome Sequence of Zygosaccharomyces mellis CA-7, Isolated from Honey. Microbiol. Resour. Announc. 2019, 8, e00449-19. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Meirinho, S.; Estevinho, M.L.F.; Choupina, A. Yeast Species Associated with Honey: Different Identification Methods. ARCH ZOOTEC 2010, 59, 103–113. [Google Scholar] [CrossRef]
- Dakal, T.C.; Solieri, L.; Giudici, P. Adaptive Response and Tolerance to Sugar and Salt Stress in the Food Yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014, 185, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Colautti, A.; Comi, G. Zygosaccharomyces rouxii Is the Predominant Species Responsible for the Spoilage of the Mix Base for Ice Cream and Ethanol Is the Best Inhibitor Tested. Food Microbiol. 2022, 102, 103929. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Long, F.; Guo, C.; Yuan, Y.; Yue, T. Early Detection of Zygosaccharomyces rouxii—Spawned Spoilage in Apple Juice by Electronic Nose Combined with Chemometrics. Int. J. Food Microbiol. 2016, 217, 68–78. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Q.; Geng, J.; Liu, Y.; Jiang, J.; Cai, X.; Cao, H.; Wu, Y.; Ren, Y.; Liu, K.; et al. Detection of Viable Zygosaccharomyces rouxii in Honey and Honey Products via PMAXX-qPCR. J. Food Qual. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Csoma, H.; Kállai, Z.; Antunovics, Z.; Czentye, K.; Sipiczki, M. Vinification without Saccharomyces: Interacting Osmotolerant and “Spoilage” Yeast Communities in Fermenting and Ageing Botrytised High-Sugar Wines (Tokaj Essence). Microorganisms 2020, 9, 19. [Google Scholar] [CrossRef]
- Craparo, V.; Viola, E.; Vella, A.; Prestianni, R.; Pirrone, A.; Naselli, V.; Amato, F.; Oliva, D.; Notarbartolo, G.; Guzzon, R.; et al. Oenological Capabilities of Yeasts Isolated from High-Sugar Matrices (Manna and Honey) as Potential Starters and Co-Starters for Winemaking. Beverages 2024, 10, 48. [Google Scholar] [CrossRef]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.D.; Sancho, M.T.; Pascual-Maté, A. Composition and Properties of Apis Mellifera Honey: A Review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ruhembe, C.C. Sugar Profile and Sensory Properties of Honey from Different Geographical Zones and Botanical Origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef]
- Cavia, M.M.; Fernández-Muiño, M.A.; Gömez-Alonso, E.; Montes-Pérez, M.J.; Huidobro, J.F.; Sancho, M.T. Evolution of Fructose and Glucose in Honey over One Year: Influence of Induced Granulation. Food Chem. 2002, 78, 157–161. [Google Scholar] [CrossRef]
- Dobre, I.; Georgescu, L.A.; Alexe, P.; Escuredo, O.; Seijo, M.C. Rheological Behavior of Different Honey Types from Romania. Food Res. Int. 2012, 49, 126–132. [Google Scholar] [CrossRef]
- Kirs, E.; Pall, R.; Martverk, K.; Laos, K. Physicochemical and Melissopalynological Characterization of Estonian Summer Honeys. Procedia Food Sci. 2011, 1, 616–624. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Ropciuc, S.; Oroian, M. Advanced Characterization of Monofloral Honeys from Romania. Agriculture 2022, 12, 526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).