Integrative Description of Temnothorax siculus sp. n.: A New Ant Species from Sicily, Italy (Hymenoptera, Formicidae) †
Abstract
1. Introduction
2. Materials and Methods
2.1. Examined Materials
2.2. Morphometrics
2.2.1. Measured Specimens
2.2.2. Protocol for Morphometric Character Recording
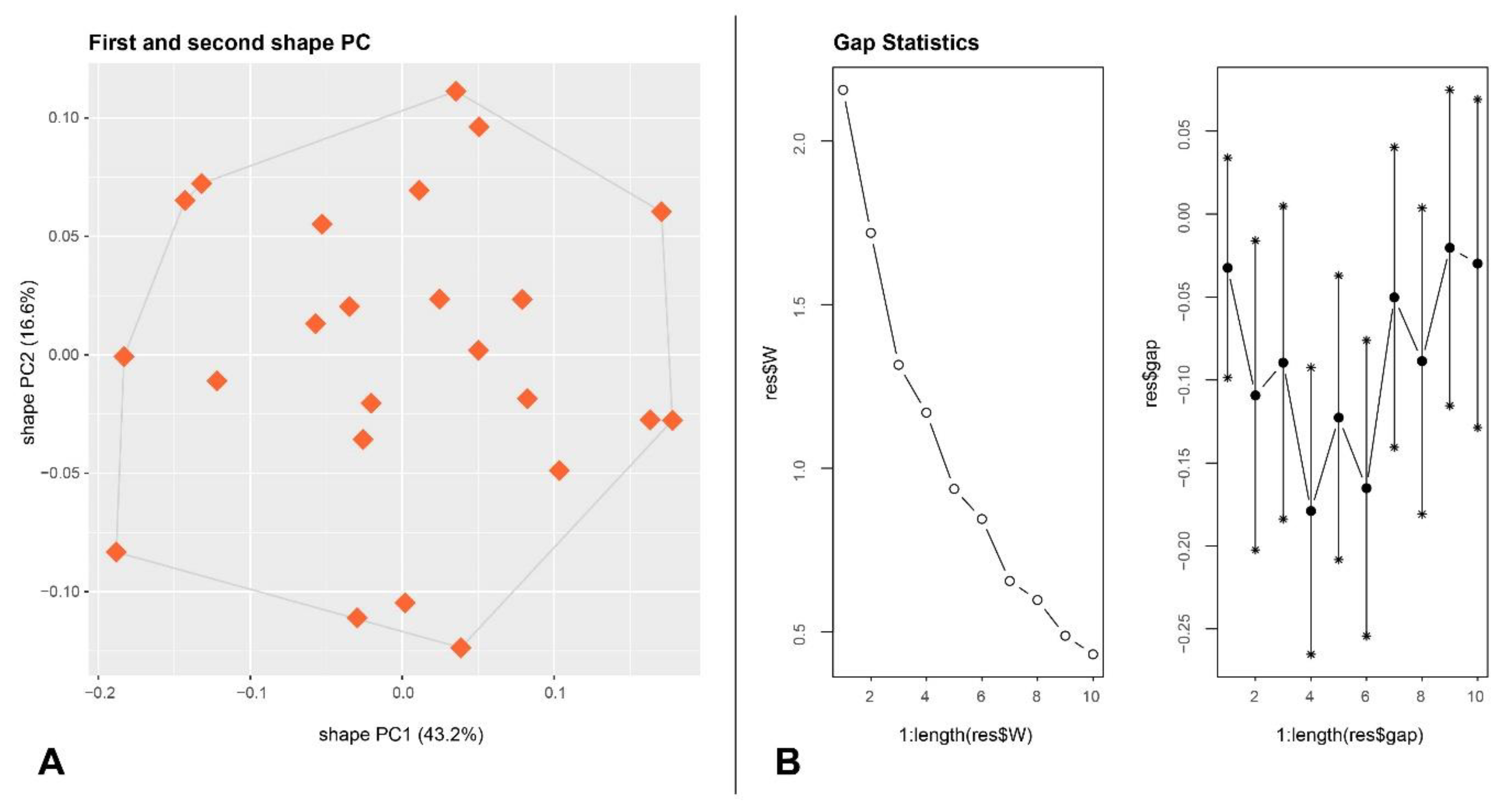
2.2.3. Exploratory Analyses Through sPCA in Combination with Gap Statistics
2.2.4. Hypothesis Testing via Confirmatory Analyses
2.3. Phylogenomics
2.3.1. Molecular Data Generation
2.3.2. Dataset Construction
2.3.3. Phylogenetic Inference
3. Results
3.1. Morphometrics
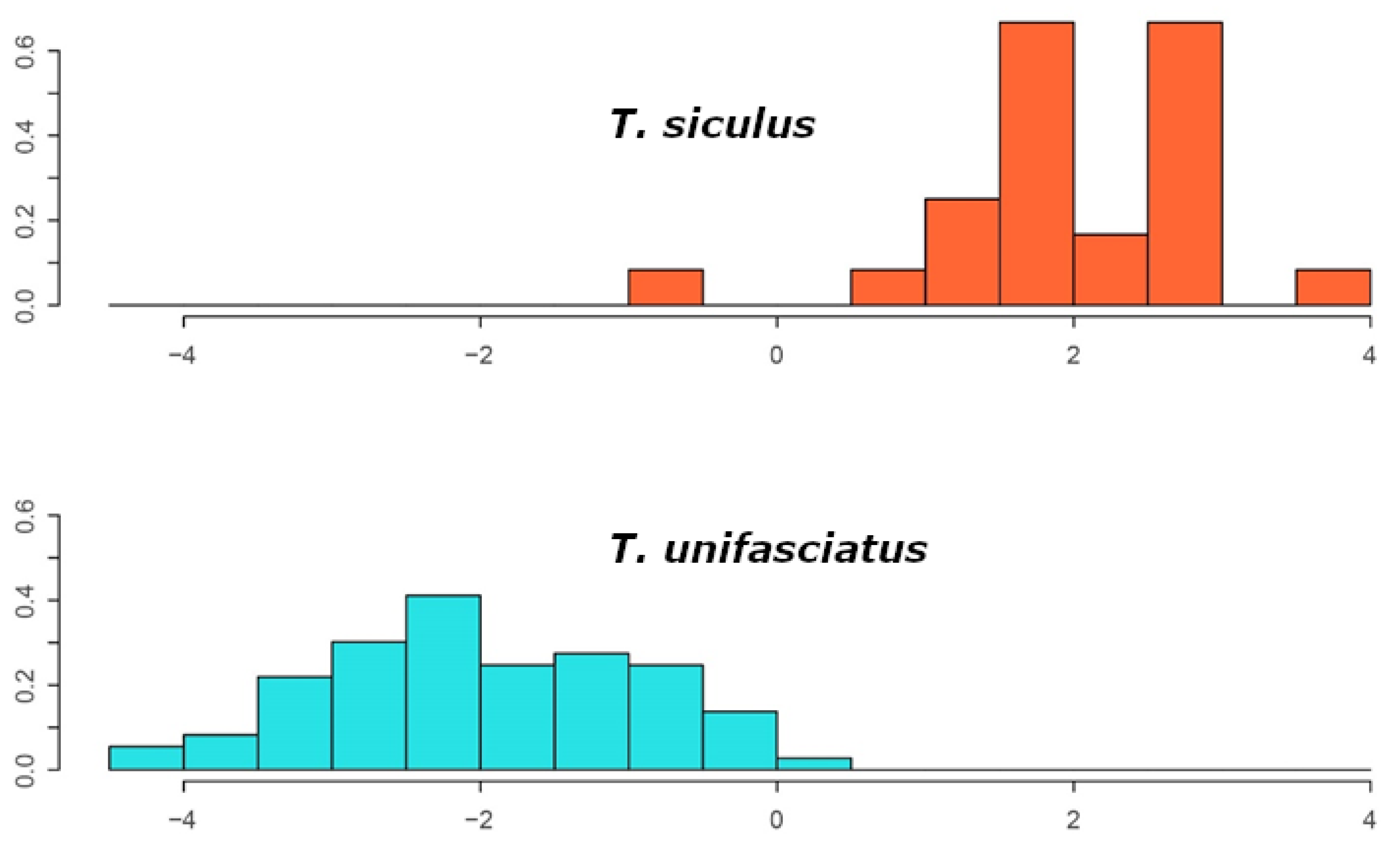
Morphometric Separation from Similar Sympatric Species
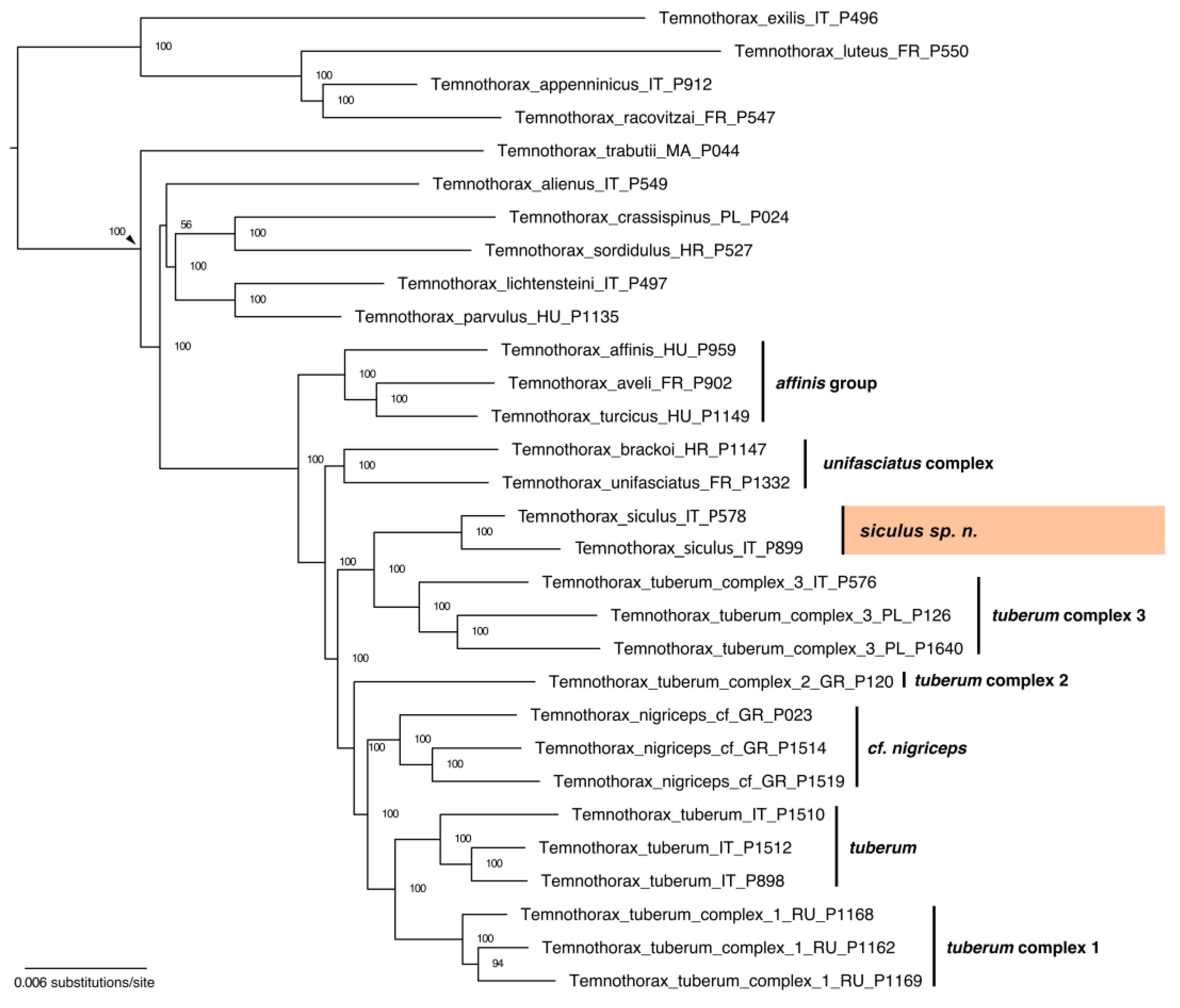
3.2. Phylogenomics
3.2.1. Sequence Capture and Processing
3.2.2. Phylogenetic Inference
3.3. Description of Temnothorax siculus sp. n.
3.3.1. Etymology
3.3.2. Formal Description of the Worker
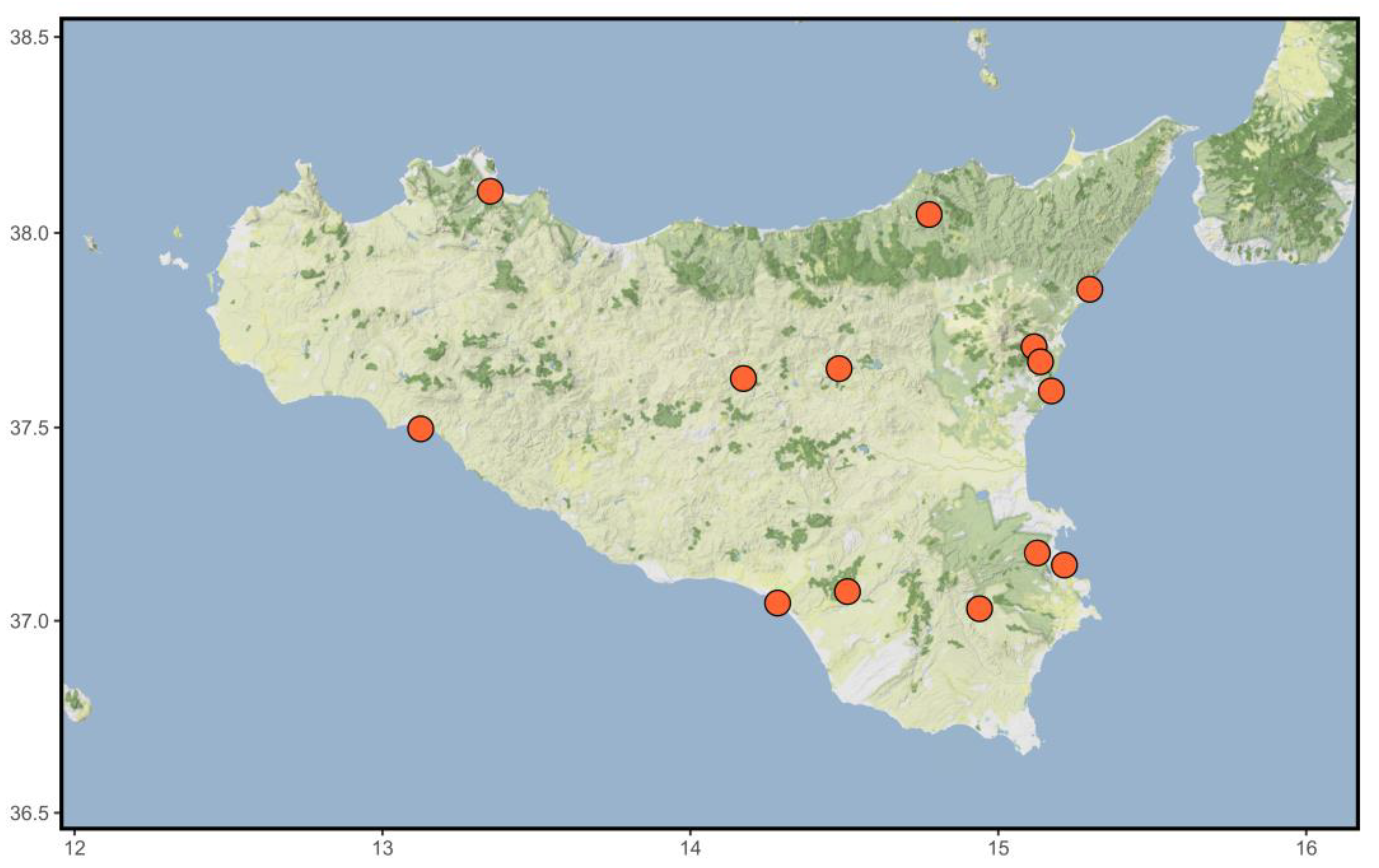
3.3.3. Distribution and Ecology
3.3.4. Qualitative Diagnostic Traits and Worker-Based Key to the Sicilian Temnothorax Fauna
| 1. | Frontal carinae extending to the level of the posterior eye margin, ventral postpetiole with an acute denticle………………………………………………………T. muellerianus [6] |
| - Frontal carinae short, ventral postpetiole without denticles……………………………2 | |
| 2. |
Petiole in lateral profile view with a very broad lobiform process on the ventral side… …………………………………………………………………………………T. algerianus [17] |
| - Petiole in lateral profile view only with very small, non-lobiform processes…………3 | |
| 3. | Antennae normally with 11 segments……………………………………T. flavicornis [7,13] |
| - Antennae with 12 segments…………………………………………………………………4 | |
| 4. | Very long scapi, metanotal impression deeply marked with a very strong discontinuity in the lateral profile of the mesosoma between the propodeum and the rest, body covered with several long setae, sculpture extremely reduced……………T. recedens [6] |
| - Shorter scapi, lateral profile of the mesosoma less discontinuous, shorter and thicker setae on the body………………………………………………………………………………5 | |
| 5. | Whole head and body dark-blackish, only the mesosoma may be occasionally reddish ……………………………………………………………………………………………………6 |
| - Head and body yellowish to ferruginous, only the gaster may be partly or entirely blackish, and sometimes the frons……………………………………………………………8 | |
| 6. | Head and mesosoma with a very strong sculpture, petiole in profile view subrectangular…………T. rottenbergii or its cryptic sister species under description [10] |
| - Head sculpture much reduced, petiole in profile view subtriangular…………………7 | |
| 7. | Long propodeal spines, clypeus with a middle carina, petiole lateral profile triangular, without a clear anterior peduncle. Arboreal species, typically wood-nesting…………… ………………………………………………………………………………T. mediterraneus [48] |
| - Propodeal spines short, clypeus without a middle carina, petiole typically pedunculate. Ground nesting………T. laestrygon (pending a revision of the exilis group) | |
| 8. | Antennal club normally darker than the rest of the antenna, mesosoma lateral profile not consistently characterized by a metanotal impression…………………………………9 |
| - Antennae concolor light, a metanotal impression may or may not be present………11 | |
| 9. | Long scapi [SL/CW: 0.87 (0.82–0.91)]. Mesosoma dorsal profile in lateral view rounded, antennal club only very slightly infuscate, gaster with a weak dark band, body otherwise yellowish, high elevation species (1400–2000 m asl).………T. apenninicus [14] |
| - Shorter scapi. Either a lowland species or the dorsal profile of the mesosoma is largely straight. Antennal clubs normally clearly darkened or blackish, and gaster more extensively dark-colored………………………………………………………………………10 | |
| 10. | Dorsal profile of the mesosoma straight, surface sculpturing coarser. Mid-high elevations (1180–1975 m asl). Can be told apart using the discriminant function illustrated in Section Morphometric Separation from Similar Sympatric Species: D4 (+0.073 * MW + 0.092 * FR − 0.090 * SL − 0.078 * PEH + 13.032) = −1.993 [−4.051, +0.016] …………………………………………………………………………………T. unifasciatus [9] |
| - Dorsal profile of the mesosoma rounded, sculpture weaker. Low elevations (8–620 m asl). D4 = +1.993 [−0.654, 3.851]…………………………………………………T. siculus sp. n | |
| 11. | Bulky, high and short petiole in lateral view, with a slightly convex frontal face forming a rounded-rectangular transition with the dorsal crest, and straight mesosoma dorsum. Rare, relatively large-sized arboreal species………………………………T. clypeatus [6,28] |
| - Character combination different……………………………………………………………12 | |
| 12. | In lateral view, petiole narrow and high, without or with a very minimally developed subpetiolar process, the dorsal profile of the mesosoma is interrupted by a marked metanotal impression………………………………………………………nylanderi group, 13 |
| - Character combination different……………………………………………………………14 | |
| 13. | Long propodeal spines deviating by <28.5° from the mesosoma axis. Lower altitudes …………………………………………………………………………………T. lichtensteini [13] |
| - Spies shorter (SPST/CS), deviating by 32–42° from the mesosoma axis. High altitudes …………………………………………………………………………………T. nylanderi [13,28] | |
| 14. | Normally larger in size (CL: 636–771, ML: 671–905), color ferrugineous, stronger sculpture with several striae except for a notable smooth shiny area in the frons, spines long……………………………………………………………………………………T. poldii [7] |
| - Smaller size, color yellow, sculpture much weaker but more homogeneous (no smooth area in the frons), spines variable……………………………………………………………15 | |
| 15. | Spines long, subpetiolar process forming a small denticle……………………T. lagrecai [7] |
| - Spines short, subpetiolar process reduced…………………………………………………16 | |
| 16. | Dorsal profile of the petiole blunt, including a horizontal plane. South-western Sicily… …………………………………………………………………………………………T. marae [7] |
| - Profile of the petiole forming a sharp triangle, with no horizontal dorsum. North- western Sicily………………………………………………………………………T. vivianoi [7] |
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, P.S.; Brady, S.G.; Fisher, B.L.; Schultz, T.R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 2015, 40, 61–81. [Google Scholar] [CrossRef]
- Bolton, B. An Online Catalog of the Ants of the World. 2024. Available online: https://antcat.org (accessed on 20 February 2024).
- Prebus, M. Palearctic elements in the old world tropics: A taxonomic revision of the ant genus Temnothorax Mayr (Hymenoptera, Formicidae) for the Afrotropical biogeographical region. ZooKeys 2015, 483, 23. [Google Scholar] [CrossRef] [PubMed]
- Prebus, M. Insights into the evolution, biogeography and natural history of the acorn ants, genus Temnothorax Mayr (Hymenoptera: Formicidae). BMC Evol. Biol. 2017, 17, 250. [Google Scholar] [CrossRef] [PubMed]
- Guénard, B.; Weiser, M.D.; Gomez, K.; Narula, N.; Economo, E.P. The Global Ant Biodiversity Informatics (GABI) database: Synthesizing data on ant species geographic distribution. Myrmecol. News 2017, 24, 83–89. [Google Scholar] [CrossRef]
- Seifert, B. Ants of Northern and Central Europe; Lutra Verlags-und Vertriebsgesellschaft: Tauer, Germany, 2018. [Google Scholar]
- Schifani, E.; Prebus, M.M.; Alicata, A. Integrating morphology with phylogenomics to describe four island endemic species of Temnothorax from Sicily and Malta (Hymenoptera, Formicidae). Eur. J. Taxon. 2022, 833, 143–179. [Google Scholar] [CrossRef]
- Seifert, B.; Csősz, S.; Schulz, A. NC-Clustering demonstrates heterospecificity of the cryptic ant species Temnothorax luteus (Forel, 1874) and T. racovitzai (Bondroit, 1918) (Hymenoptera: Formicidae). Beitr. Entomol. 2014, 64, 47–57. [Google Scholar] [CrossRef]
- Csősz, S.; Alicata, A.; Báthori, F.; Galkowski, C.; Schifani, E.; Yusupov, Z.; Herczeg, G.; Prebus, M.M. Integrative taxonomy reveals inflated biodiversity in the European Temnothorax unifasciatus complex (Hymenoptera: Formicidae). Zool. Scr. 2025, 54, 33–49. [Google Scholar] [CrossRef]
- Csősz, S.; Taheri, A.; Schifani, E.; Reyes-López, J.-L.; Alicata, A.; Báthori, F.; Prebus, M. Taxonomic revision of the Mediterranean Temnothorax rottenbergii species group via integrated morphological and molecular approaches. Insect Syst. Divers. 2025; in press. [Google Scholar]
- Schlick-Steiner, B.C.; Steiner, F.M.; Seifert, B.; Stauffer, C.; Christian, E.; Crozier, R.H. Integrative taxonomy: A multisource approach to exploring biodiversity. Annu. Rev. Entomol. 2010, 55, 421–438. [Google Scholar] [CrossRef]
- Oberski, J.T.; Griebenow, Z.; Adams, R.M.M.; Andersen, A.; Andrade-Silva, J.; Barden, P.; Borowiec, M.; Brady, S.; Csősz, S.; Dias, A.M.; et al. Ant Systematics: Past, Present, and Future. Insect Syst. Divers. 2024; in press. [Google Scholar]
- Csősz, S.; Heinze, J.; Mikó, I. Taxonomic synopsis of the Ponto-Mediterranean ants of Temnothorax nylanderi species-group. PLoS ONE 2015, 10, e0140000. [Google Scholar] [CrossRef]
- Csősz, S.; Schifani, E.; Seifert, B.; Alicata, A.; Prebus, M.M. A new species of yellow acorn ant discovered in Italy via integrative taxonomy (Temnothorax luteus-complex, Formicidae). Evol. Syst. 2024, 8, 183. [Google Scholar] [CrossRef]
- Prebus, M.M. Phylogenomic species delimitation in the ants of the Temnothorax salvini group (Hymenoptera: Formicidae): An integrative approach. Syst. Entomol. 2021, 46, 307–326. [Google Scholar] [CrossRef]
- Prebus, M.M. Taxonomic revision of the Temnothorax salvini clade (Hymenoptera: Formicidae), with a key to the clades of New World Temnothorax. PeerJ 2021, 9, e11514. [Google Scholar] [CrossRef] [PubMed]
- Báthori, F.; Seifert, B.; Heinze, J.; Kiran, K.; Karaman, C.; Csősz, S. Taxonomy of the Palearctic socially parasitic Temnothorax (Myrmoxenus) ants (Hymenoptera: Formicidae). PLoS ONE 2024, 19, e0308712. [Google Scholar] [CrossRef]
- Kass, J.M.; Guénard, B.; Dudley, K.L.; Jenkins, C.N.; Azuma, F.; Fisher, B.L.; Parr, C.L.; Gibb, H.; Longino, J.T.; Ward, P.S.; et al. The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 2022, 8, eabp9908. [Google Scholar] [CrossRef]
- Borowiec, L. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus 2014, 25, 1–340. [Google Scholar]
- Wang, R.; Kass, J.M.; Galkowski, C.; Garcia, F.; Hamer, M.T.; Radchenko, A.; Salata, S.D.; Schifani, E.; Yusupov, Z.M.; Economo, E.P.; et al. Geographic and climatic constraints on bioregionalization of European ants. J. Biogeogr. 2023, 50, 503–514. [Google Scholar] [CrossRef]
- Salata, S.; Borowiec, L.; Trichas, A. Taxonomic revision of the Cretan fauna of the genus Temnothorax Mayr, 1861 (Hymenoptera: Formicidae), with notes on the endemism of ant fauna of Crete. Ann. Zool. 2018, 68, 769–808. [Google Scholar] [CrossRef]
- Salata, S.; Borowiec, L.; Trichas, A. Review of ants (Hymenoptera: Formicidae) of Crete, with keys to species determination and zoogeographical remarks. Dep. Nat. Hist. Up. Silesian Mus. 2020. [Google Scholar] [CrossRef]
- Lapeva-Gjonova, A.; Csősz, S.; Mifsud, D. Further records of social parasitic ants in Europe and review of the Bulgarian species. Biodivers. Data J. 2024, 12, e123575. [Google Scholar] [CrossRef]
- Alicata, A.; Schifani, E. Three endemic Aphaenogaster from the Siculo-Maltese archipelago and the Italian Peninsula: Part of a hitherto unrecognized species group from the Maghreb? (Hymenoptera: Formicidae: Myrmicinae). Acta Entomol. Mus. Natl. Pragae 2019, 59, 1–16. [Google Scholar] [CrossRef]
- Schifani, E.; Alicata, A. Exploring the myrmecofauna of Sicily: Thirty-two new ant species recorded, including six new to Italy and many new aliens (Hymenoptera, Formicidae). Pol. J. Entomol. 2018, 87, 323–348. [Google Scholar] [CrossRef]
- Schär, S.; Menchetti, M.; Schifani, E.; Hinojosa, J.C.; Platania, L.; Dapporto, L.; Vila, R. Integrative biodiversity inventory of ants from a Sicilian archipelago reveals high diversity on young volcanic islands (Hymenoptera: Formicidae). Org. Divers. Evol. 2020, 20, 405–416. [Google Scholar] [CrossRef]
- Schifani, E.; Scupola, A.; Alicata, A. Morphology, ecology and biogeography of Myrmecina sicula André, 1882, rediscovered after 140 years (Hymenoptera, Formicidae). Biogeographia 2020, 35, 105–116. [Google Scholar] [CrossRef]
- Schifani, E.; Csősz, S.; Viviano, R.; Alicata, A. Ant diversity on the largest Mediterranean islands: On the presence or absence of 28 species in Sicily (Hymenoptera, Formicidae). Nat. Hist. Sci. 2021, 8, 55–70. [Google Scholar] [CrossRef]
- Menchetti, M.; Schifani, E.; Alicata, A.; Vila, R. Quantitative morphology and mtDNA reveal that Lasius maltaeus is not endemic to the Maltese Islands (Hymenoptera, Formicidae). J. Hymenopt. Res. 2023, 95, 129–142. [Google Scholar] [CrossRef]
- Menchetti, M.; Schifani, E.; Alicata, A.; Cardador, L.; Sbrega, E.; Toro-Delgado, E.; Vila, R. The invasive ant Solenopsis invicta is established in Europe. Curr. Biol. 2023, 33, R896–R897. [Google Scholar] [CrossRef]
- Baur, H.; Leuenberger, C. Analysis of ratios in multivariate morphometry. Syst. Biol. 2011, 60, 813–825. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Nilsen, G.; Borgan, Ø.; Liestøl, K.; Lingjærde, O.C. Identifying clusters in genomics data by recursive partitioning. Stat. Appl. Genet. Mol. Biol. 2013, 12, 637–652. [Google Scholar] [CrossRef]
- Faircloth, B.C.; Branstetter, M.G.; White, N.D.; Brady, S.G. Target enrichment of ultraconserved elements from arthropods provides a genomic perspective on relationships among Hymenoptera. Mol. Ecol. Resour. 2015, 15, 489–501. [Google Scholar] [CrossRef]
- Branstetter, M.G.; Longino, J.T.; Ward, P.S.; Faircloth, B.C. Enriching the ant tree of life: Enhanced UCE bait set for genome-scale phylogenetics of ants and other Hymenoptera. Methods Ecol. Evol. 2017, 8, 768–776. [Google Scholar] [CrossRef]
- Glenn, T.C.; Nilsen, R.A.; Kieran, T.J.; Sanders, J.G.; Bayona-Vásquez, N.J.; Finger, J.W.; Faircloth, B.C.; Pierson, T.W.; Bentley, K.E.; Hoffberg, S.L.; et al. Adapterama I: Universal stubs and primers for 384 unique dual-indexed or 147,456 combinatorially-indexed Illumina libraries (iTru & iNext). PeerJ 2019, 7, e7755. [Google Scholar] [CrossRef] [PubMed]
- Rohland, N.; Reich, D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012, 22, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Faircloth, B.C. PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 2016, 32, 786–788. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Buckley, T.R.; Simon, C.; Chambers, G.K. Exploring among-site rate variation models in a maximum likelihood framework using empirical data: Effects of model assumptions on estimates of topology, branch lengths, and bootstrap support. Syst. Biol. 2001, 50, 67–86. [Google Scholar] [CrossRef]
- Tagliacollo, V.A.; Lanfear, R. Estimating improved partitioning schemes for ultraconserved elements. Mol. Biol. Evol. 2018, 35, 1798–1811. [Google Scholar] [CrossRef]
- Freitas, F.V.; Branstetter, M.G.; Franceschini-Santos, V.H.; Dorchin, A.; Wright, K.W.; López-Uribe, M.M.; Griswold, T.; Silveira, F.A.; Almeida, E.A.B. UCE phylogenomics, biogeography, and classification of long-horned bees (Hymenoptera: Apidae: Eucerini), with insights on using specimens with extremely degraded DNA. Insect Syst. Divers. 2023, 7, 3. [Google Scholar] [CrossRef]
- Sullivan, J.; Swofford, D.L. Should we use model-based methods for phylogenetic inference when we know that assumptions about among-site rate variation and nucleotide substitution pattern are violated? Syst. Biol. 2001, 50, 723–729. [Google Scholar] [CrossRef]
- Yang, Z. Computational Molecular Evolution; OUP Oxford: Oxford, UK, 2006. [Google Scholar]
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 1. [Google Scholar] [CrossRef]
- Galkowski, C.; Cagniant, H. Contribution à la connaissance des fourmis du groupe angustulus dans le genre Temnothorax (Hymenoptera, Formicidae). Rev. L’association Roussillonnaise d’Entomologie 2017, 26, 180–191. [Google Scholar]
- Radchenko, A.; Elmes, G.W.; Alicata, A. Taxonomic revision of the schencki-group of the ant genus Myrmica Latreille (Hymenoptera: Formicidae) from the Palaearctic region. Ann. Zool. 2006, 56, 499–538. [Google Scholar]
- Schifani, E. First record of the vulnerable social parasite ant Plagiolepis grassei in Italy (Hymenoptera: Formicidae). Fragm. Entomol. 2017, 49, 61–64. [Google Scholar] [CrossRef]
- Schifani, E.; Giannetti, D.; Csősz, S.; Castellucci, F.; Luchetti, A.; Castracani, C.; Spotti, F.A.; Mori, A.; Grasso, D.A. Is mimicry a diversification-driver in ants? Biogeography, ecology, ethology, genetics and morphology define a second West-Palaearctic Colobopsis species (Hymenoptera: Formicidae). Zool. J. Linn. Soc. 2022, 194, 1424–1450. [Google Scholar] [CrossRef]
- Schifani, E.; Nalini, E.; Gentile, V.; Alamanni, F.; Ancona, C.; Caria, M.; Cillo, D.; Bazzato, E. Ants of Sardinia: An updated checklist based on new faunistic, morphological and biogeographical notes. Redia 2021, 104, 21–35. [Google Scholar] [CrossRef]
- Báthori, F.; Heinze, J.; Trindl, A.; Seifert, B.; Herczeg, G.; Csősz, S. Host-switching events are not always the driver of speciation in social parasites: A case study in Temnothorax (Myrmoxenus) ants (Hymenoptera, Formicidae). J. Zool. 2024, 322, 221–231. [Google Scholar] [CrossRef]


| Abbr. | Definition of the Trait |
|---|---|
| CL: | Maximum cephalic length in median line; the head must be carefully tilted to the position with the true maximum. Excavations of hind vertex and/or clypeus, if any, reduce cl. |
| CS: | Cephalic size; the arithmetic mean of CL and cwb. |
| CW: | Maximum width of head, including eyes; measured across the most distant contour lines of the two compound eyes when the head is in full face view. |
| CWb: | Maximum width of head capsule, measured posterior to the eyes. |
| EL: | Maximum diameter of the compound eye. |
| FRS: | Minimum distance between the frontal carinae. |
| ML: | Mesosoma length from caudal-most point of propodeal lobe to the transition point between the anterior pronotal slope and anterior propodeal shield (preferentially measured in lateral view; if the transition point is not well defined, use the dorsal view and take the center of the dark-shaded borderline between the pronotal slope and pronotal shield as the anterior reference point). |
| MW: | Maximum mesosoma width; pronotal width in workers |
| NOH: | Maximum height of the petiolar node, measured in lateral view from the uppermost point of the petiolar node perpendicular to a reference line set from the petiolar spiracle to the imaginary midpoint of the transition between the dorso-caudal slope and dorsal profile of the caudal cylinder of the petiole. |
| NOL: | Length of the petiolar node, measured in lateral view from petiolar spiracle to dorso-caudal corner of caudal cylinder. Do not erroneously take as reference point the dorso-caudal corner of the helcium, which is sometimes visible. |
| PEH: | Maximum petiole height. The chord of ventral petiolar profile at node level is the reference line perpendicular to which the maximum height of the petiole is measured. |
| PEL | Diagonal petiolar length measured from the base of the anteroventral subpetiolar process to the dorsocaudal corner of the caudal cylinder. |
| PEW: | Maximum width of petiole |
| POC: | Postocular distance. Use a cross-scaled ocular micrometer and adjust the head to the measuring position of cl. Caudal measuring point: median occipital margin; frontal measuring point: median head at the level of the posterior eye margin. |
| PLST | Distance between the most dorsocaudal point of the propodeal lobe and the center of the propodeal stigma. |
| PPW: | Maximum width of postpetiole. |
| SL: | Maximum straight line scape length excluding the articular condyle. |
| SPBA: | The smallest distance between the lateral margins of the spines at their base. This should be measured in dorsofrontal view, since the wider parts of the ventral propodeum do not interfere with the measurement in this position. If the lateral margins of spines diverge continuously from the tip to the base, the smallest distance at the base is not defined. In this case, SPBA is measured at the level of the bottom of the interspinal meniscus. |
| SPST: | Distance between the center of the propodeal stigma and spine tip. The stigma center refers to the midpoint defined by the outer cuticular ring but not to the center of the real stigma opening that may be positioned eccentrically. |
| SPWI: | Maximum distance between outer margins of spines; measured in the same position as SPBA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schifani, E.; Alicata, A.; Prebus, M.M.; Csősz, S. Integrative Description of Temnothorax siculus sp. n.: A New Ant Species from Sicily, Italy (Hymenoptera, Formicidae). Diversity 2025, 17, 294. https://doi.org/10.3390/d17040294
Schifani E, Alicata A, Prebus MM, Csősz S. Integrative Description of Temnothorax siculus sp. n.: A New Ant Species from Sicily, Italy (Hymenoptera, Formicidae). Diversity. 2025; 17(4):294. https://doi.org/10.3390/d17040294
Chicago/Turabian StyleSchifani, Enrico, Antonio Alicata, Matthew M. Prebus, and Sándor Csősz. 2025. "Integrative Description of Temnothorax siculus sp. n.: A New Ant Species from Sicily, Italy (Hymenoptera, Formicidae)" Diversity 17, no. 4: 294. https://doi.org/10.3390/d17040294
APA StyleSchifani, E., Alicata, A., Prebus, M. M., & Csősz, S. (2025). Integrative Description of Temnothorax siculus sp. n.: A New Ant Species from Sicily, Italy (Hymenoptera, Formicidae). Diversity, 17(4), 294. https://doi.org/10.3390/d17040294








