Distribution and Conservation Gaps of Nautilus pompilius: A Study Based on Species Distribution Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Data Collection and Preprocessing
2.2. Selection of Environmental Variables
2.3. Species Distribution Modeling and Evaluation
2.4. Conservation Gap Analysis
3. Results
3.1. Model Performance and Predictor Variable Analysis
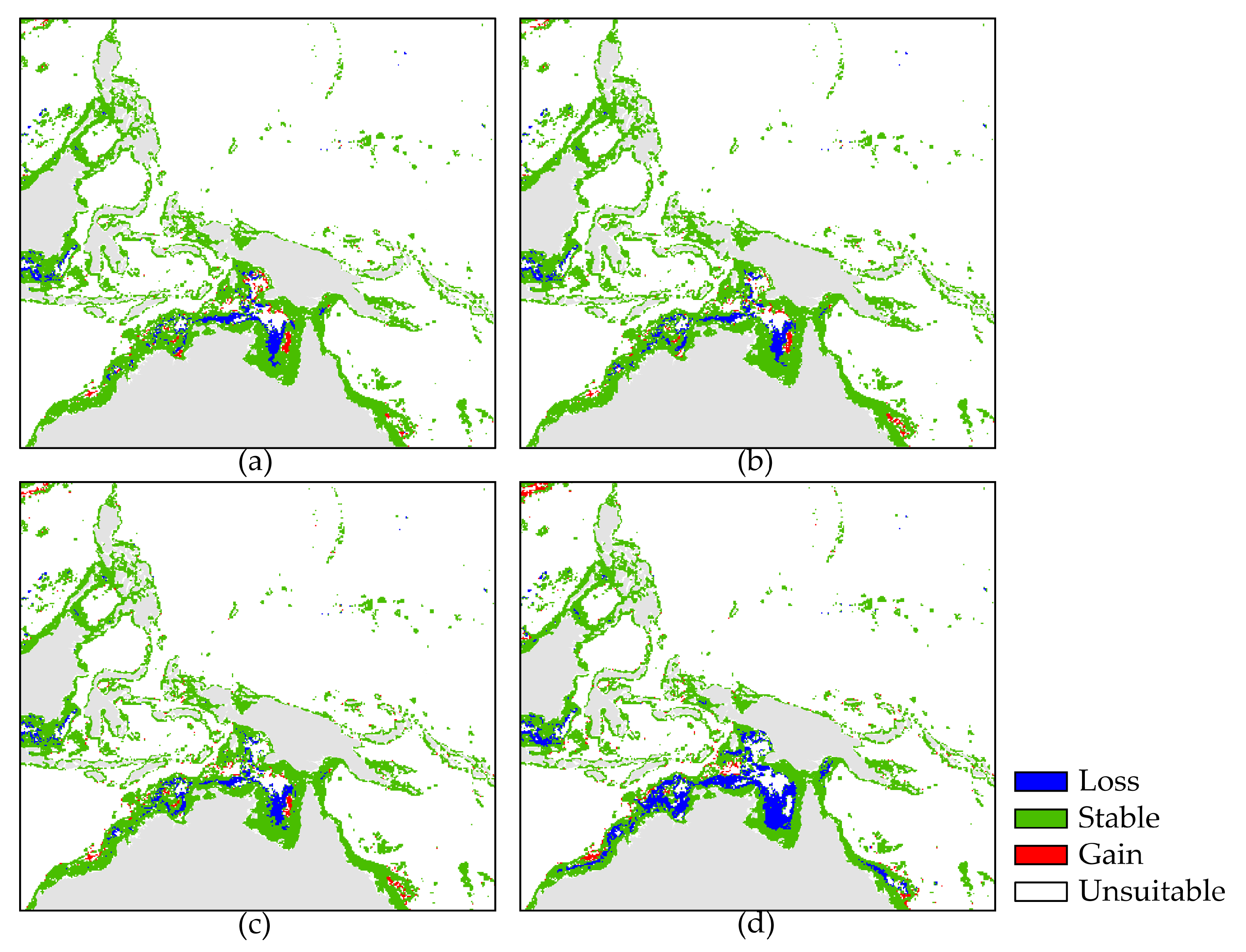
3.2. Current and Future Potential Distribution
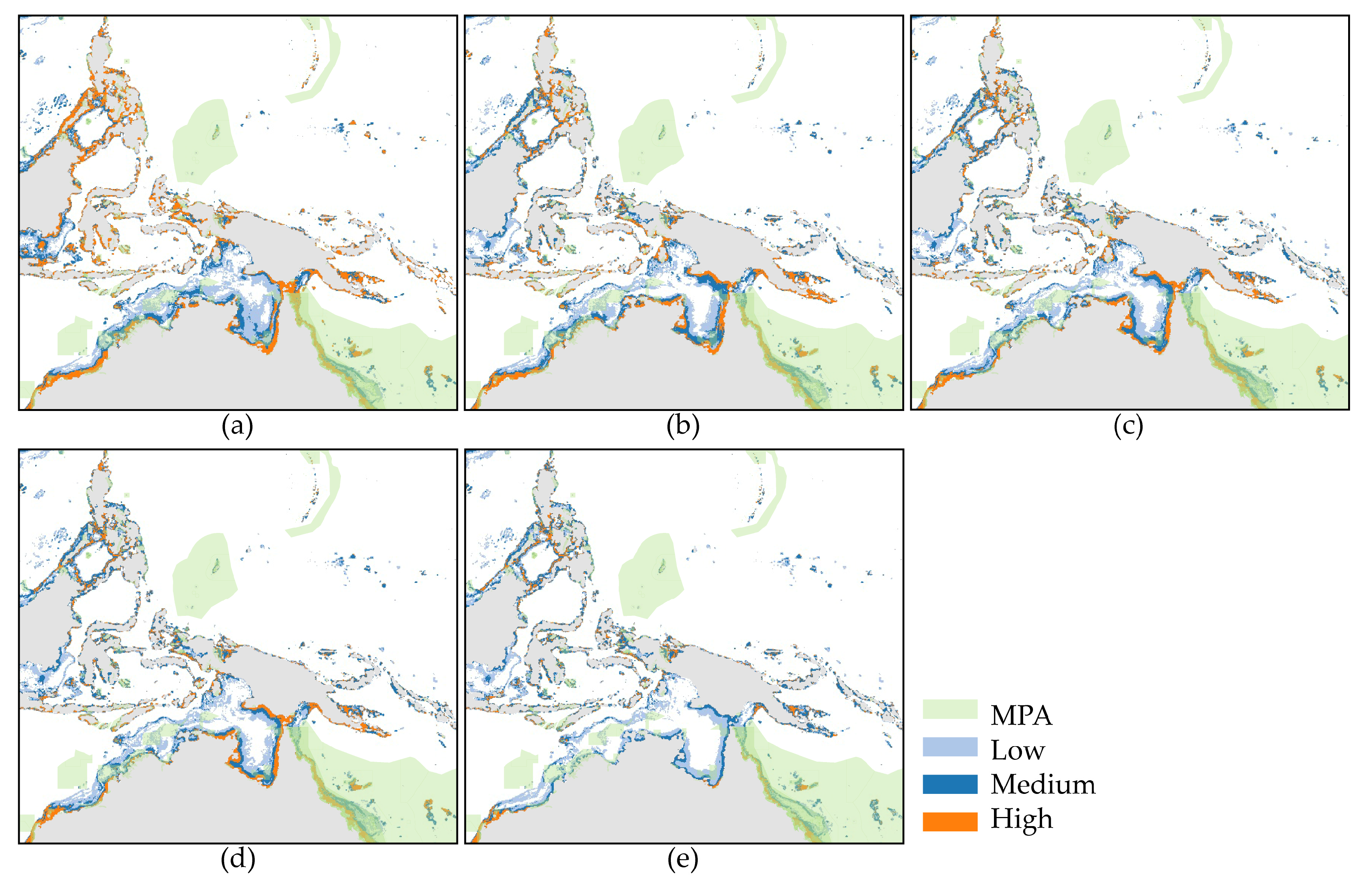
3.3. Analysis of the Conservation Status
4. Discussion
4.1. Vulnerability to Temperature
4.2. Changes in Suitable Habitat
4.3. Management and Conservation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| N. pompilius | Nautilus pompilius |
| MPAs | Marine protected areas |
| SDMs | Species distribution models |
| Gap analysis | A geographic approach to protect biological diversity |
References
- Mooney, H.; Larigauderie, A.; Cesario, M.; Elmquist, T.; Hoegh-Guldberg, O.; Lavorel, S.; Mace, G.M.; Palmer, M.; Scholes, R.; Yahara, T. Biodiversity, climate change, and ecosystem services. Curr. Opin. Environ. Sustain. 2009, 1, 46–54. [Google Scholar] [CrossRef]
- Priya, A.K.; Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Gaddam, M.K.R.; Velusamy, P.; Gomathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of climate change and anthropogenic activities on aquatic ecosystem—A review. Environ. Res. 2023, 238, 117233. [Google Scholar] [CrossRef]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.; et al. Scenarios for global biodiversity in the 21st century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Adla, K.; Dejan, K.; Neira, D.; Dragana, Š. Chapter 9—Degradation of ecosystems and loss of ecosystem services. In One Health; Prata, J.C., Ribeiro, A.I., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 281–327. [Google Scholar]
- Saunders, W.B.; Ward, P.D. Ecology, Distribution, and Population Characteristics of Nautilus. In Nautilus: Biology and Paleobiology of a Living Fossil; Saunders, W.B., Landman, N.H., Eds.; Plenum Press: New York, NY, USA, 2010; pp. 137–162. [Google Scholar]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A.; Pörtner, H.O.; Scanes, E.; Wright, J.M. Predicting the response of molluscs to the impact of ocean acidification. Biology 2013, 2, 651–692. [Google Scholar] [CrossRef]
- Williams, R.C.; Newman, S.J.; Sinclair, W. DNA barcoding in Nautilus pompilius (Mollusca: Cephalopoda): Evolutionary divergence of an ancient species in modern times. Invertebr. Syst. 2012, 26, 548–560. [Google Scholar] [CrossRef]
- Dunstan, A.J.; Ward, P.D.; Marshall, N.J. Nautilus pompilius life history and demographics at the Osprey Reef Seamount, Coral Sea, Australia. PLoS ONE 2011, 6, e16312. [Google Scholar] [CrossRef]
- Cochran, J.K.; Rye, D.M.; Landman, N.H. Growth-rate and habitat of Nautilus pompilius inferred from radioactive and stable isotope studies. Paleobiology 1981, 7, 469–480. [Google Scholar]
- Saunders, W.B. Nautilus growth and longevity: Evidence from marked and recaptured animals. Science 1984, 224, 990–992. [Google Scholar] [CrossRef]
- Boutilier, R.G.; West, T.G.; Pogson, G.H.; Mesa, K.A.; Wells, J.; Wells, M.J. Nautilus and the art of metabolic maintenance. Nature 1996, 382, 534–536. [Google Scholar] [CrossRef]
- Dunstan, A.; Alanis, O.; Marshall, J. Nautilus pompilius fishing and population decline in the Philippines: A comparison with an unexploited Australian Nautilus population. Fish. Res. 2010, 106, 239–247. [Google Scholar] [CrossRef]
- Forderer, E.M.; Rodder, D.; Langer, M.R. Global diversity patterns of larger benthic foraminifera under future climate change. Glob. Change Biol. 2023, 29, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 35. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Cong, B.; Yang, L.; Zhang, Z.; Zhao, L. Unveiling the suitable habitats and future conservation strategies of Tridacna maxima in the Indo-Pacific core area based on species distribution model. Ecol. Evol. 2024, 14, e70187. [Google Scholar] [CrossRef]
- Mohamed Nisin, K.M.N.; Sreenath, K.R.; Sreeram, M.P. Muscling mussels: Understanding the invasive potential of the South American bivalve Mytella strigata (Hanley, 1843) in the Northern Indian Ocean. Sci. Total Environ. 2024, 916, 170243. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, L.; Sui, J.; Li, X.; Wang, H.; Zhang, B. Potential effects of climate change on the habitat suitability of macrobenthos in the Yellow Sea and East China Sea. Mar. Pollut. Bull. 2022, 174, 113238. [Google Scholar] [CrossRef]
- França, S.; Cabral, H.N. Distribution models of estuarine fish species: The effect of sampling bias, species ecology and threshold selection on models’ accuracy. Ecol. Inform. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Kiser, A.H.; Cummings, K.S.; Tiemann, J.S.; Smith, C.H.; Johnson, N.A.; Lopez, R.R.; Randklev, C.R. Using a multi-model ensemble approach to determine biodiversity hotspots with limited occurrence data in understudied areas: An example using freshwater mussels in Mexico. Ecol. Evol. 2022, 12, 14. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 11. [Google Scholar] [CrossRef]
- Kriegl, M.; Ilosvay, X.E.E.; von Dorrien, C.; Oesterwind, D. Marine Protected Areas: At the Crossroads of Nature Conservation and Fisheries Management. Front. Mar. Sci. 2021, 8, 13. [Google Scholar] [CrossRef]
- Asaad, I.; Lundquist, C.J.; Erdmann, M.V.; Costello, M.J. Delineating priority areas for marine biodiversity conservation in the Coral Triangle. Biol. Conserv. 2018, 222, 198–211. [Google Scholar] [CrossRef]
- Fan, H.; Huang, M.; Chen, Y.; Zhou, W.; Hu, Y.; Wei, F. Conservation priorities for global marine biodiversity across multiple dimensions. Natl. Sci. Rev. 2023, 10, nwac241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zeng, C.; Cao, L. Mapping the biodiversity conservation gaps in the East China sea. J. Environ. Manag. 2023, 336, 117667. [Google Scholar] [CrossRef]
- Yang, L.; Zhuang, H.; Liu, S.; Cong, B.; Huang, W.; Li, T.; Liu, K.; Zhao, L. Estimating the Spatial Distribution and Future Conservation Requirements of the Spotted Seal in the North Pacific. Animals 2023, 13, 3260. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, L.; Liu, C.; Sun, B. Estimating the impact of climate change on the potential distribution of Indo-Pacific humpback dolphins with species distribution model. PeerJ 2021, 9, e12001. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Singer, A.; Millat, G.; Staneva, J.; Kröncke, I. Modelling benthic macrofauna and seagrass distribution patterns in a North Sea tidal basin in response to 2050 climatic and environmental scenarios. Estuar. Coast. Shelf Sci. 2017, 188, 99–108. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Yang, T.; Liu, X.; Han, Z. Predicting the Effects of Climate Change on the Suitable Habitat of Japanese Spanish Mackerel (Scomberomorus niphonius) Based on the Species Distribution Model. Front. Mar. Sci. 2022, 9, 927790. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2012, 36, 27–46. [Google Scholar] [CrossRef]
- Cecchetto, M.; Dettai, A.; Gallut, C.; Obst, M.; Kuklinski, P.; Balazy, P.; Chelchowski, M.; Małachowicz, M.; Poćwierz-Kotus, A.; Zbawicka, M.; et al. Seasonality of primary production explains the richness of pioneering benthic communities. Nat. Commun. 2024, 15, 8340. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Jackson, B.C.; Duvaux, L.; Dawson, D.A.; Burke, T.; Sinclair, W. The genetic structure of Nautilus pompiliu populations surrounding Australia and the Philippines. Mol. Ecol. 2015, 24, 3316–3328. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Basher, Z.; Bowden, D.A.; Costello, M.J. GMED: Global Marine Environment Datasets for environment visualisation and species distribution modelling. Earth Syst. Sci. Data Discuss. 2018, 2018, 1–62. [Google Scholar]
- Liu, K.; Tao, Y.; Huang, W.; Wang, B.; Liu, S.; Cong, B.; Zhou, M.; Zhao, L. Assessment of future habitat suitability and ecological vulnerability of Collichthys at population and species level. BMC Ecol. Evol. 2025, 25, 1. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.; Bede-Fazekas, Á.; Mammola, S.; Gu, J.; Zhou, J.; Qu, J.; Lin, Q. Cross-validation matters in species distribution models: A case study with goatfish species. Ecography 2024, 2025, e07354. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Fushiki, T. Estimation of prediction error by using K-fold cross-validation. Stat. Comput. 2011, 21, 137–146. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- He, B.H.; Zhao, Y.H.; Mao, W.; Griffin-Nolanb, R.J. Explainable artificial intelligence reveals environmental constraints in seagrass distribution. Ecol. Indic. 2022, 144, 10. [Google Scholar] [CrossRef]
- Petitpierre, B.; Broennimann, O.; Kueffer, C.; Daehler, C.; Guisan, A. Selecting predictors to maximize the transferability of species distribution models: Lessons from cross-continental plant invasions. Glob. Ecol. Biogeogr. 2017, 26, 275–287. [Google Scholar] [CrossRef]
- Austin, M.P. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Model. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Iannella, M.; Cerasoli, F.; Biondi, M. Unraveling climate influences on the distribution of the parapatric newts Lissotriton vulgaris meridionalis and L. italicus. Front. Zool. 2017, 14, 14. [Google Scholar] [CrossRef]
- Morato, T.; Gonzalez-Irusta, J.M.; Dominguez-Carrio, C.; Wei, C.L.; Davies, A.; Sweetman, A.K.; Taranto, G.H.; Beazley, L.; Garcia-Alegre, A.; Grehan, A.; et al. Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob. Change Biol. 2020, 26, 2181–2202. [Google Scholar] [CrossRef]
- Brown, S.K.; Buja, K.R.; Jury, S.H.; Monaco, M.E.; Banner, A. Habitat Suitability Index Models for Eight Fish and Invertebrate Species in Casco and Sheepscot Bays, Maine. N. Am. J. Fish. Manag. 2000, 20, 408–435. [Google Scholar] [CrossRef]
- She, Z.Y.; Tang, Y.M.; Chen, L.H.; Nong, X.Z.; Li, X.F. Determination of suitable ecological flow regimes for spawning of four major Chinese carps: A case study of the Hongshui River, China. Ecol. Inform. 2023, 76, 11. [Google Scholar] [CrossRef]
- Liu, C.R.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Xian, X.; Zhao, H.; Wang, R.; Huang, H.; Chen, B.; Zhang, G.; Liu, W.; Wan, F. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2023, 859, 160252. [Google Scholar] [CrossRef]
- Nisin, K.; Sreenath, K.R.; Sreeram, M.P. Change in habitat suitability of the invasive Snowflake coral (Carijoa riisei) during climate change: An ensemble modelling approach. Ecol. Inform. 2023, 76, 10. [Google Scholar] [CrossRef]
- Li, Y.P.; Gao, X.; An, Q.; Sun, Z.; Wang, H.B. Ecological niche modeling based on ensemble algorithms to predicting current and future potential distribution of African swine fever virus in China. Sci. Rep. 2022, 12, 11. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Barord, G.J. Comparative Population Assessments of Nautilus sp. in the Philippines, Australia, Fiji, and American Samoa Using Baited Remote Underwater Video Systems. PLoS ONE 2014, 9, 1. [Google Scholar] [CrossRef]
- Kanie, Y.; Fukuda, Y.; Nakayama, H.; Seki, K.; Hattori, M. Implosion of living Nautilus under increased pressure. Paleobiology 1980, 6, 44–47. [Google Scholar]
- Yasuhara, M.; Danovaro, R. Temperature impacts on deep-sea biodiversity. Biol. Rev. 2016, 91, 275–287. [Google Scholar] [CrossRef]
- Doi, H.; Yasuhara, M.; Ushio, M. Causal analysis of the temperature impact on deep-sea biodiversity. Biol. Lett. 2021, 17, 6. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Eikeset, A.M.; McCauley, D.J.; Payne, J.L.; Sunday, J.M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 2019, 569, 108–111. [Google Scholar] [CrossRef]
- Vermeij, G.J. The Natural History of Nautilus. Science 1988, 240, 1074–1075. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Morley, S.A.; Lai, C.H.; Clark, M.S.; Tan, K.S.; Bates, A.E.; Peck, L.S. Upper temperature limits of tropical marine ectotherms: Global warming implications. PLoS ONE 2011, 6, e29340. [Google Scholar] [CrossRef]
- Sweetman, A.K. Major impacts of climate change on deep-sea benthic ecosystems. Elem. Sci. Anth. 2017, 5, 4. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.J.W. Annual breeding cycles in marine invertebrates and environmental temperature: Probing the proximate and ultimate causes of reproductive synchrony. J. Therm. Biol. 1995, 20, 79–90. [Google Scholar] [CrossRef]
- Saunders, W.B.; Spinosa, C. Nautilus movement and distribution in palau, Western caroline islands. Science 1979, 204, 1199–1201. [Google Scholar] [CrossRef]
- Dunstan, A.J.; Ward, P.D.; Marshall, N.J. Vertical Distribution and Migration Patterns of Nautilus pompilius. PLoS ONE 2011, 6, 10. [Google Scholar] [CrossRef]
- Bennett, J.M.; Sunday, J.; Calosi, P.; Villalobos, F.; Martínez, B.; Molina-Venegas, R.; Araújo, M.B.; Algar, A.C.; Clusella-Trullas, S.; Hawkins, B.A.; et al. The evolution of critical thermal limits of life on Earth. Nat. Commun. 2021, 12, 9. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Selden, R.L.; Kitchel, Z.J. Climate-Driven Shifts in Marine Species Ranges: Scaling from Organisms to Communities. Ann Rev Mar Sci 2020, 12, 153–179. [Google Scholar] [CrossRef]
- Hoeksema, B.W. Delineation of the Indo-Malayan Centre of Maximum Marine Biodiversity: The Coral Triangle. In Biogeography, Time, and Place: Distributions, Barriers, and Islands; Renema, W., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 117–178. [Google Scholar]
- Assan, D.; Kuebutornye, F.K.A.; Mustapha, U.F.; Chen, H.; Li, G. Effects of Climate Change on Marine Organisms. Am. J. Clim. Change 2020, 09, 204–216. [Google Scholar] [CrossRef]
- Hastings, R.A.; Rutterford, L.A.; Freer, J.J.; Collins, R.A.; Simpson, S.D.; Genner, M.J. Climate Change Drives Poleward Increases and Equatorward Declines in Marine Species. Curr. Biol. 2020, 30, 1572–1577. [Google Scholar] [CrossRef]
- Donner, S.D.; Skirving, W.J.; Little, C.M.; Oppenheimer, M.; Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Chang Biol 2005, 11, 2251–2265. [Google Scholar] [CrossRef]
- Rose, G.A. Reconciling overfishing and climate change with stock dynamics of Atlantic cod (Gadus morhua) over 500 years. Can. J. Fish. Aquat. Sci. 2004, 61, 1553–1557. [Google Scholar]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.D.; Connell, S.D.; Mellin, C.; Brook, B.W.; Burnell, O.W.; Fordham, D.A. Predicting the distribution of commercially important invertebrate stocks under future climate. PLoS ONE 2012, 7, e46554. [Google Scholar] [CrossRef][Green Version]
- Joly, C.A. The Kunming-Montreal Global Biodiversity Framework. Biota Neotrop. 2022, 22, 2. [Google Scholar] [CrossRef]
- Villasante, S.; Ainsworth, G.B.; Pita, P.; Belgrano, A.; Bennett, N.; Sumaila, U.R. Chapter 2—The role of marine protected areas (MPAs) in providing ecosystem services to improve ocean and human health. In Oceans and Human Health, 2nd ed.; Fleming, L.E., Alcantara Creencia, L.B., Gerwick, W.H., Goh, H.C., Gribble, M.O., Maycock, B., Solo-Gabriele, H., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 23–37. [Google Scholar]
- Mora, C.; Andrèfouët, S.; Costello, M.J.; Kranenburg, C.; Rollo, A.; Veron, J.; Gaston, K.J.; Myers, R.A. Coral reefs and the global network of Marine Protected Areas. Science 2006, 312, 1750–1751. [Google Scholar] [CrossRef]



| Environment Variable | Unit | Source | Used (√) or Not (×) |
|---|---|---|---|
| Water temperature | °C | https://www.bio-oracle.org | √ |
| Dissolved molecular oxygen | mol·m−3 | https://www.bio-oracle.org | × |
| Primary productivity | g·m−3·day−1 | https://www.bio-oracle.org | √ |
| Light at bottom | - | https://www.bio-oracle.org | √ |
| Water depth | m | http://gmed.auckland.ac.nz | × |
| Slope | - | http://gmed.auckland.ac.nz | √ |
| Distance to land | km | http://gmed.auckland.ac.nz | √ |
| Future Climate Scenarios | Loss | Gain | Net Change |
|---|---|---|---|
| 2050s RCP 4.5 | 9.5 | 4.7 | −4.8 |
| 2050s RCP 8.5 | 9.7 | 4.4 | −5.3 |
| 2100s RCP 4.5 | 10.6 | 5.3 | −5.3 |
| 2100s RCP 8.5 | 20.5 | 5.1 | −15.4 |
| Value | Current | 2050s RCP 4.5 | 2050s RCP 8.5 | 2100s RCP 4.5 | 2100s RCP 8.5 |
|---|---|---|---|---|---|
| Low | 17.0 | 17.2 | 20.8 | 20.6 | 23.2 |
| Medium | 18.1 | 26.1 | 27.3 | 27.2 | 29.0 |
| High | 46.6 | 50.4 | 47.7 | 48.5 | 47.2 |
| Total | 28.3 | 29.85 | 30.07 | 30.16 | 29.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Zhao, L.; Huang, W.; Meilana, L.; Li, T.; Liu, K.; Wang, B.; Cong, B.; Liu, S. Distribution and Conservation Gaps of Nautilus pompilius: A Study Based on Species Distribution Models. Diversity 2025, 17, 243. https://doi.org/10.3390/d17040243
Lai X, Zhao L, Huang W, Meilana L, Li T, Liu K, Wang B, Cong B, Liu S. Distribution and Conservation Gaps of Nautilus pompilius: A Study Based on Species Distribution Models. Diversity. 2025; 17(4):243. https://doi.org/10.3390/d17040243
Chicago/Turabian StyleLai, Xianshui, Linlin Zhao, Wenhao Huang, Lusita Meilana, Tingting Li, Kaiyu Liu, Bei Wang, Bailin Cong, and Shenghao Liu. 2025. "Distribution and Conservation Gaps of Nautilus pompilius: A Study Based on Species Distribution Models" Diversity 17, no. 4: 243. https://doi.org/10.3390/d17040243
APA StyleLai, X., Zhao, L., Huang, W., Meilana, L., Li, T., Liu, K., Wang, B., Cong, B., & Liu, S. (2025). Distribution and Conservation Gaps of Nautilus pompilius: A Study Based on Species Distribution Models. Diversity, 17(4), 243. https://doi.org/10.3390/d17040243






