Abstract
In the north and center of Chile, there has been a significant environmental contamination by copper due to natural factors, mining activities and use of agricultural fertilizers and pesticides. Copper (Cu) soil contamination is of important concern in agriculture, food safety, and human health. Soil copper concentrations higher than 100–150 mg/kg can be toxic for plants and other organisms. Therefore, identification of copper-tolerant crops is of great interest for sustainable cultivation purposes. Quinoa is a promising candidate as a copper-tolerant crop, owing to its wide genetic diversity, high adaptability to different environmental conditions, and tolerance to abiotic stresses. In this work, we evaluated the effect of copper on 21 accessions of quinoa (including 19 accessions from different geographical locations of Chile) in order to identify tolerant and sensitive accessions. Our results show that (1) Germination parameters of quinoa are negatively affected in the presence of increasing Cu concentrations, with differential inhibition values among accessions. (2) Early seedling growth of accessions was differentially affected in the presence of Cu. (3) Plant biomass production (relative fresh and dry weights) was also affected by Cu, with significant differences between accessions. A Principal Component Analysis (PCA) of these data identified accessions based on Cu tolerance. (4) A clear regional pattern was observed when comparing accessions from northern, central, and southern Chile, suggesting local adaptation to Cu-rich soils. Thus, significant differences in copper tolerance between accessions were observed, revealing genetic diversity in copper tolerance among quinoa accessions. Tolerant accessions of quinoa can have important applications in sustainable agriculture.
1. Introduction
Copper (Cu) is an essential micronutrient for plants, as it is a redox transition metal required as cofactor in several enzymes involved in electron transport chain, respiration, photosynthesis, oxidative stress response, hormone signal transduction, and other physiological and biochemical processes underlying growth and development [1,2,3,4].
In excess, Cu may have toxic effects in plants due to alterations of enzymatic activities (by binding to sulfhydral groups), water uptake and nutrient imbalance, impairment of thylakoid structure and of PSII function by Cu accumulation, and impairment of respiration, photosynthesis, and electron transport. Copper can induce generation of reactive oxygen species (ROS) through Fenton and Haber–Weiss reactions, which can produce oxidation and damage of DNA, proteins, lipids, and other biomolecules, disrupting many metabolic pathways [5,6,7,8]. As consequence, excessive levels of Cu can disrupt seed germination, inhibit plant growth and development, and reduce yield, posing a significant challenge to agricultural systems [4,9].
Plants have developed several mechanisms to cope with Cu stress. Plants can prevent the entry of Cu by secreting chelating organic acids and other compounds from roots to the rizosphere [10]. Plants can also regulate Cu transport and accumulation in apoplast, vacuoles, and different tissues by modulating the expression and activity of several Cu and ion transporters and chaperones (as COPT: copper transporters, HMA: Heavy Metal ATPases, YSL: Yellow Stripe-Like, ZIP, and ABC transporters, among others), and chelators binding to Cu (as phytochelatins and metallothioneins), in order to minimize the toxic effects of Cu [9]. In addition, plants can induce expression and activity of antioxidant enzymes (catalase, superoxide dismutase (SOD), ascorbate peroxidase (APX), and others) and of non-enzymatic antioxidants (as glutathione, melatonin, and carotenoids), in order to neutralize ROS and regulate Cu homeostasis [9,11]. Different transcription factors (as members of SPL7 and WRKY families) and different Cu-activated miRNAs are known to be involved in Cu homeostasis [12].
Cu contamination in soil originates from both natural and anthropogenic sources. Naturally, Cu derives primarily from source rocks and exists in various oxidation states, with the divalent form (Cu2+) being the most prevalent and bioavailable in acidic conditions [13,14]. Its solubility and bioavailability are influenced by soil properties such as organic matter, clay content, and pH [7,15].
Anthropogenic activities, including mining, smelting, excessive use of Cu-based agrochemicals (e.g., fungicides and fertilizers), and industrial waste disposal, have significantly increased Cu accumulation in soils [4,16]. Copper is not susceptible to degradation, leading to Cu accumulation in the environment, with the corresponding concerns in agriculture production, food chain security, and human and livestock health [4,9].
Regulatory limits vary by country, with maximum permissible concentrations of up to 150 mg/kg in Canada and 200 mg/kg in Germany [17,18]. However, in contaminated sites, Cu concentrations can exceed 1700 mg/kg, posing severe risks to ecosystems and agriculture [19].
In Chile, a world’s leading Cu producer, significant environmental contamination has been produced due to mining activities, particularly in the northern and central regions [20,21]. Additionally, the prolonged use of Cu-based pesticides in fruit cultivation has exacerbated Cu accumulation in agricultural soils of central–south of Chile, with concentrations reaching up to 400 mg/kg in some areas [21,22]. This contamination threatens agriculture, food safety, and human health, highlighting the need to identify Cu-tolerant plant species for sustainable cultivation [6,23].
Quinoa (Chenopodium quinoa Willd.), a native Andean pseudo-cereal, has gained attention for its exceptional adaptability to abiotic stresses, including salinity and drought [24,25]. Quinoa exhibits a wide genetic diversity and can be classified into five agro-ecological zones: Highlands (Altiplano type); Inter-Andean Valleys (with semi dry cold climate, rainfall of 450–600 mm); Yungas (grown under tropical conditions); Salares (high altitude salt lakes and growing under limited volume of annual rainfall (150–300 mm)); and Coastal/Lowlands (with annual rainfall from 500 to 1500 mm) [26,27]. Among these, the Coastal/Lowlands ecotype is of particular importance due its wide longitudinal distribution, which enhances photoperiod adaptation and facilitates quinoa cultivation across diverse climatic regions [28,29,30]. Notably, coastal Chilean landraces have been used as elite accessions in European quinoa-breeding programs [28,29].
Quinoa’s high nutritional value and tolerance to stress make it a promising crop for cultivation in marginal soils [31,32]. Previous studies have demonstrated its tolerance to heavy metals, such as Cd, Pb, and Zn [33,34]. However, research on screening quinoa’s response to Cu stress across different accessions at the germination and seedling stages remains limited. The genetic diversity of quinoa, particularly among the Chilean accessions, provides a valuable resource for identifying Cu-tolerant genotypes suitable for agriculture applications [35]. Despite increasing interest in quinoa’s stress tolerance, comprehensive studies on its response to Cu toxicity—especially in early developmental stages—are lacking. Moreover, systematic comparisons of quinoa germplasms from different geographical origins for tolerance under Cu stress have not been performed up to date. This study aims to evaluate the effects of Cu stress on germination and early seedling growth in twenty-one quinoa accessions, identify Cu-tolerant and sensitive accessions based on germination traits and seedling performance, and assess the potential of quinoa for sustainable agriculture in Cu-contaminated soils.
2. Materials and Methods
2.1. Plant Material
Seeds of 21 accessions of Chenopodium quinoa (Willd.) were used in this study, including 19 accessions from Chile (spanning the Altiplano, central, and southern zones), one accession from the Peruvian Altiplano, and one from the Bolivian Altiplano. All of them are natural quinoa accessions adapted to the soil and natural environmental conditions of their growing zones. The seeds were obtained from the National Seed Bank of Chile (managed by the Genetic Resources section of the National Institute of Agricultural Research, INIA-Intihuasi, Vicuña, Chile), the Department of Botany at the Universidad de Concepción, and the U.S. Department of Agriculture (USDA). Table S1 provides details on each accession, including codes, geographical origins, and sources.
To ensure sterility, seeds were treated with 5% (v/v) commercial bleach for 5 min, followed by rinsing with distilled water (one wash for 1 min, then four washes for 5 min each, and a final wash for 15 min). Seeds were then placed on filter paper in Petri dishes moistened with 4 mL of distilled water. Dishes were sealed with Parafilm™ (Heathrow ScientificTM ParafilmTM Laboratory Film, Thermo Fisher Scienficic, Waltham, MA, USA) to prevent desiccation and incubated at 23 °C in the dark for 72 h. Germinated seeds were transferred to 11 L pots (2 plantlets per pot) filled with a 1:4 (v/v) mixture of peat (H2–H4 Sphagnum brown peat) and sand (lampa sand). Pots were placed in a shaded area and irrigated with 200–300 mL of water twice weekly. After 2–3 months, seeds were harvested and stored at 4 °C under dry conditions.
2.2. Germination Assays
To evaluate the effects of copper (Cu) on germination, seeds of each accession were sterilized as described above and placed on filter paper in Petri dishes (30 seeds per dish). Fresh CuSO4·5H2O (MilliporeSigma, Burlington, MA, USA) solutions were prepared in distilled water at concentrations of 400, 600 and 800 mM, with a resulting final pH of 3.0 (due to acidification by CuSO4·5H2O), measured with a multiparameter pHmeter (AD8000, Adwa Instruments, Ethiopia). Seeds in Petri dishes were moistened with 4 mL of fresh Cu solution. Control plates were moistened with 4 mL of distilled water (adjusted to pH 3.0 with HCl). Another series of control plates moistened with distilled water adjusted to pH 6.0 were also prepared for testing putative effects of pH on germination. Plates were incubated at 23 °C in the dark, in a plant growth chamber with controlled temperature, and germination was recorded at 24, 48, and 72 h. The pH was checked during the experiment. A seed was considered germinated when the radicle protruded through the seed coat. Each treatment was replicated in three Petri dishes, and at least two independent assays were performed.
The following germination parameters were assessed:
where n = number of seeds germinated on each day, d = number of days from the beginning of the test, and N = total number of seeds germinated at the end of the experiment [36,37].
where n1, n2, and n3 designate the number of germinated seeds after the first, second, and third day of germination for each treatment [38].
ref. [38,39].
Germination Percentage [GP = (number of germinated seeds in the presence of copper/number of germinated seeds in control plates, that is, in the absence of copper) × 100],
Mean Germination Time [MGT = ∑(n × d)/N],
Germination Index [GI = (3 × n1) + (2 × n2) + (1 × n3)],
Inhibition of Germination [IG = 100 − (GI of treatment/GI of control) × 100]
2.3. Early Growth Assays in Petri Dishes
To assess and characterize the lower Cu concentrations affecting early seedling growth, sterilized seeds were germinated in Petri dishes (15 seeds per dish) containing Hoagland nutrient solution [40] (diluted 1:10 in distilled water) supplemented with CuSO4·5H2O from a water solution freshly prepared at final concentrations of 5 µM or 12.5 µM of CuSO4·5H2O; the resulting pH was 5.8. Control plates contained Hoagland (1:10) nutrient solution adjusted to pH 5.8 with NaOH/HCl. The plates were incubated in a growth chamber with controlled temperature at 23 °C in the dark, and seedlings were collected after 70 h. Root length was measured for both control and Cu-treated seedlings. The relative root length (RRL) was calculated as follows:
RRL = (Root length in the presence of Cu/Root length in the absence of Cu) × 100
2.4. Growth Assays in Hydroponic Pouches
To evaluate the effects of Cu on biomass production, seedlings with a root length of 1 ± 0.2 cm were transferred to hydroponic pouches (CYG Seed Germination Pouches, Mega International, Roseville, MN, USA) containing 40 mL of Hoagland nutrient solution supplemented with 350 µM of CuSO4·5H2O (added from a water stock freshly prepared, with resulting pH 5.6) for 10 days in a plant growth chamber under controlled conditions (23 °C, 200 µmol photons m−2s−1, 12/12 h photoperiod, 50% relative humidity). Control seedlings were grown in a nutrient solution at pH 5.6 (adjusted with NaOH/HCl). Fresh and dry weights of roots and shoots were measured, and relative growth parameters were calculated.
2.5. Statistical Analysis
Data were analyzed using INFOSTAT v. 2020 [41] and RStudio version 4.3.2 [42] software. Normality and homogeneity of variance were tested, and a non-parametric Kruskal–Wallis test was used to detect significant differences (p < 0.05). A Mann–Whitney test with Bonferroni correction for post hoc analysis was performed when comparisons were significant.
For data of effects of Cu on plant growth in the hydroponic system, a Principal Component Analysis (PCA) was performed to explore the correlation between relative parameters of all accessions. Then, to test differences among accessions, a permutational multivariate ANOVA (PERMANOVA) test was performed (using adonis2 function in vegan R package) with 999 permutations, and a post hoc pairwise comparison test (RVAideMemoire R package) was conducted. Then, the Kruskal–Wallis test was conducted to all relative parameters followed by the Mann–Whitney test with Bonferroni correction for post hoc analysis when comparisons were significant.
To test if accessions had a correlation with its geographical origin (Altiplano, Central Chile and Southern Chile), another PCA was conducted only with Chilean accessions. A PERMANOVA test was performed with the geographical origin as a fixed factor, and a post hoc pairwise comparison test (RVAideMemoire R package v. 0.9-83-7) was performed.
3. Results
3.1. Effect of Cu on Seed Germination
Copper (Cu) significantly affected seed germination across all quinoa accessions, with pronounced differences in tolerance levels. No significant differences were observed when comparing controls at different pHs. As Cu concentrations increased, the Germination Percentage (GP) decreased. Significant effects were observed in all accessions in the presence of 600 mM and 800 mM of Cu. Most affected accessions in the presence of the highest Cu concentration (800 mM) were Kello Juira, Regalona, BO-78, BO-25, Villarrica, and Chadmo, showing complete inhibition of germination (Table S2).
Mean Germination Time (MGT) also increased, indicating delayed germination. In the absence of Cu, the highest MGT values were observed for accessions Ancovinto (1.71 ± 0.1 days), BO-25 (1.40 ± 0.08), and BO-78 (1.76 ± 0.04) (Table S3). The other accessions have MGT values from 1.0 to 1.26 in the absence of Cu. In the presence of Cu, lower increases in MGT were observed in Lisio Putre, Socaire, Paredones, Roja Paredones, and Dorado Paredones. Higher increases in MGT values were observed in Kello Juira, UdeC9, Faro, Regalona, BO-25, BO-78, Villarrica, and Chadmo (Table S3).
The Germination Index (GI) was negatively impacted by increasing Cu concentrations in all tested accessions. In the absence of Cu, the highest GI values were observed in accessions Roja Paredones and Villarrica (105 ± 0.0 germinated seeds), and lower values were observed in Ancovinto (29.83 ± 3.17), BO-25 (76.83 ± 7.05), and BO-78 (66.67 ± 5.77). In the presence of Cu, lower decreases in GI were observed in Chucapaca, Socaire, Peñablanca, Paredones, Roja Paredones, and Dorado Paredones. Higher decreases in GI values were observed in Kello Juira, Ancovinto, UdeC9, Regalona, BO-25, BO-78, Villarrica, and Chadmo (Table S3).
Inhibition of Germination (IG) increased with the Cu concentration (Table S3). Significant differences in IG values among accessions are shown in Figure 1A (at 400 mM Cu), Figure 1B (at 600 mM Cu), and Figure 1C (at 800 mM Cu).
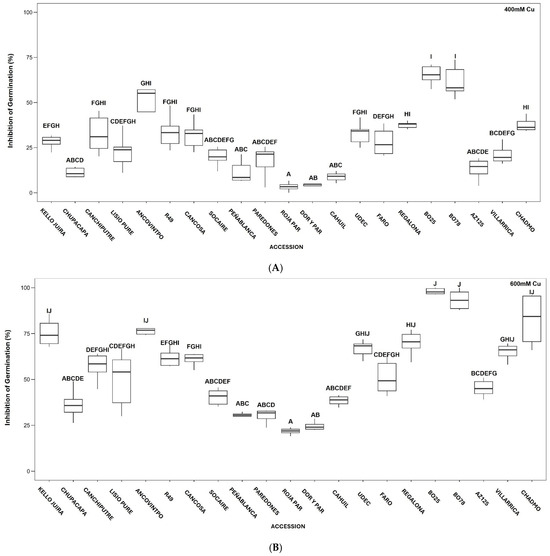
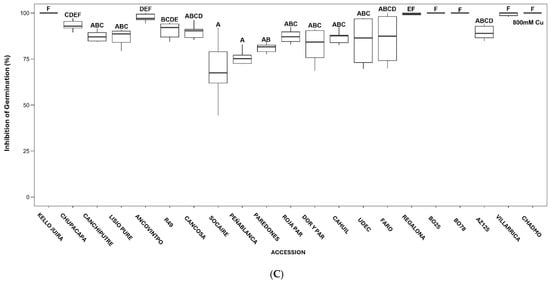
Figure 1.
Boxplot of effects of 400 mM (A), 600 mM (B), and 800 mM CuSO4·5H2O (C) on Inhibition of Germination (%) of quinoa accessions. Different letters denote significant differences among accessions.
At increasing Cu concentrations, lower IG values were observed in Peñablanca, Socaire, Paredones, Roja Paredones, and Dorado Paredones. Higher IG values were observed in Kello Juira, Regalona, BO-25, BO-78, Villarrica, and Chadmo (with total inhibition of germination at 800 mM Cu), underscoring their sensitivity to Cu stress (Figure 1A–C).
In subsequent growth experiments, accession Ancovinto was not included, owing to low seed germination percentage.
3.2. Effect of Cu on Early Seedling Growth in Petri Dishes
Early seedling growth was assessed at low Cu concentrations (5 µM and 12.5 µM of CuSO4·5H2O). While 5 µM of Cu had no significant effect on root growth, 12.5 µM of Cu significantly reduced the root length in accessions Chucapaca, Kello Juira, Lisio Putre, Cancosa, Peñablanca, Roja Paredones, Dorado Paredones, Regalona, BO-25, BO-78, AZ-125, and Chadmo. Conversely, accessions like Canchi Putre, R49, Paredones, Cahuil, UdeC9, Faro, and Villarrica showed no significant reduction in root length, indicating higher Cu tolerance (Figure 2A). The relative root length (RRL), a more reliable metric for comparing between accessions, further highlights these differences, with R49, Faro, and Paredones exhibiting the highest RRL values, while Chucapaca, BO-78, and Chadmo showed the lowest RRL values under Cu stress (Figure 2B). These results suggest that the relative root length of early seedlings is a reliable indicator of Cu tolerance in quinoa.
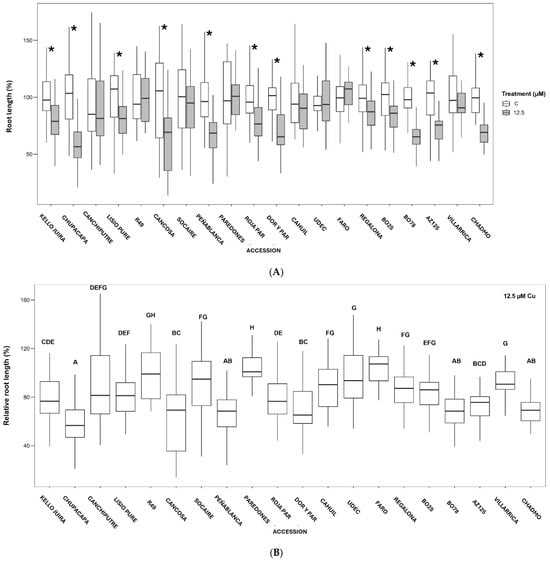
Figure 2.
Boxplot of effects of Cu treatments on radicle length (A) and relative root length (B) of quinoa seedlings (C, length in nutrient solution in the absence of supplemented Cu; 12.5, length in nutrient solution supplemented with 12.5 µM of CuSO4·5H2O). Asterisks indicate significant statistical differences between C and 12.5 µM treatments for each accession (A). Different letters denote significant differences between accessions (B).
3.3. Effect of Cu on Plantlet Growth in Hydroponic Systems
In hydroponic systems, exposure to 350 µM of CuSO4·5H2O for 10 days revealed significant differences in biomass production among accessions.
In accessions R49 and Dorado Paredones, no significant effects of 350 µM of Cu on root fresh weight (RFW), root dry weight (RDW), shoot fresh weight (SFW), and shoot dry weight (SDW) were observed (Table 1). Significant effects of high Cu on RFW were observed in AZ-125. In Canchi Putre, Socaire, Peñablanca, and Roja Paredones, 350 µM of Cu induced significant changes in fresh biomass (RFW and SFW). Significant effects of 350 µM of Cu on RFW, RDW, and SFW (but not in SDW) were observed in Chucapaca, Cahuil, Regalona, Villarrica, and Chadmo. In Kello Juira and Paredones, 350 µM of Cu induced a significant effect on RFW, SFW, and SDW, but not RDW. All growth parameters were significantly affected in the presence of 350 µM of Cu in accessions Lisio Putre, Cancosa, Faro, UdeC9, BO-25, and BO-78 (Table 1).

Table 1.
Effect of 350 µM of CuSO4·5H2O in relative root and shoot fresh and dry weights in quinoa accessions.
Analysis of relative growth parameters (RRFW, RRDW, RSFW, RSDW) revealed significant differences among accessions (Table 1). The highest relative values were observed in R49 and Dorado Paredones, whereas Chucapaca, UdeC9, and Faro exhibited the lowest (Table 1).
A Principal Component Analysis (PCA) of the relative growth parameters identified two principal components explaining 90.3% of the variance (PC1 = 79.8%, PC2 = 10.5%). The PCA plot effectively separated accessions into distinct groups based on their Cu tolerance (Figure 3).
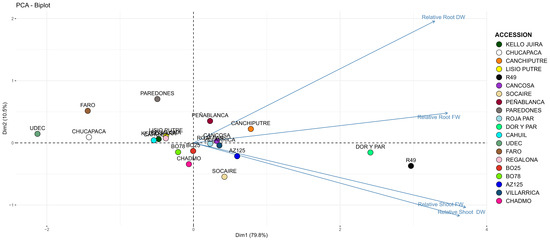
Figure 3.
Scatter plot of the two components obtained from PCA that differentiate accessions and the relative parameters according to the eigenvalues.
A Permutational Multivariate Analysis of Variance (PERMANOVA) confirmed significant differences among accessions (p < 0.001), with pairwise comparisons showing R49, Dorado Paredones, Chucapaca, Paredones, Faro, and UdeC9 to be significantly distinct (p < 0.05, Figure 3).
When considering only Chilean accessions, a significant effect of geographical origin was observed (p = 0.0015). Central accessions significantly differed from both Altiplano and southern accessions (Figure 4), suggesting that local adaptation may play a role in Cu tolerance.
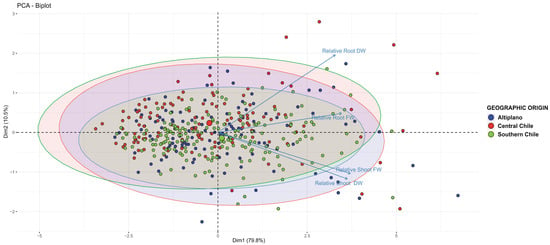
Figure 4.
Scatter plot of the PERMANOVA of the effects of the geographical origin in relative growth parameters of quinoa accessions.
4. Discussion
Copper (Cu) contamination is a major environmental concern, affecting agriculture and ecosystems in Chile and worldwide. While Cu is essential for plant development as an enzymatic cofactor, excessive concentrations disrupt physiological functions, leading to oxidative stress, metabolic impairment, reduced growth, and even death [5,6]. Identifying Cu-tolerant crops is crucial for sustainable agriculture. Quinoa, a plant with important nutritional and health benefits and with high adaptability to contrasting environments [33] is an interesting candidate as Cu-tolerant crop. Our study evaluated the Cu tolerance of twenty-one accessions of quinoa (of which nineteen are accessions from northern, central and southern Chile) by assessing germination, early seedling growth, and biomass under varying Cu concentrations.
In our assays, the germination of quinoa accessions was inhibited in the presence of high concentrations of Cu (Tables S2 and S3, Figure 1). Inhibitory effects of Cu on germination have been observed in other important crop species [4]. High levels of Cu can interfere with water uptake during seed imbibition and with enzyme activity involved in reserve mobilization during germination, inhibiting this process [43,44]. It is interesting to note that quinoa germination parameters are only significantly affected at 600 and 800 mM Cu in most accessions, which are higher concentrations than those reported to affect germination in other plant species (for example, 100 µM of Cu in Lactuca sativa, 200 µM in Pisum sativum, 350 µM in Triticum aestivum, 2 mM in Zea mays, 4 mM in the Chenopoidaceae Salsola vermiculata [4,44,45]). Our results indicate that, compared to other plants, quinoa is highly tolerant to Cu stress during germination. Moreover, all tested accessions could germinate at Cu concentrations usually found in contaminated sites (from 100 to even 2000 mg/kg, [17,18,19,20,46].
Although germination was affected by copper in all quinoa accessions studied, notable differences between accessions from different locations were found. Central Chilean accessions (e.g., Peñablanca, Roja Paredones, and Dorado Paredones) exhibited the highest tolerance, maintaining relatively high GP and GI and low MGT and inhibition (IG) values (Tables S2 and S3, Figure 1). In contrast, southern Chilean accessions (e.g., BO25 and BO78, Villarrica and Chadmo) were more sensitive, showing complete inhibition at 800 mM Cu. Interestingly, northern Chilean accessions displayed a mixed response (Tables S2 and S3, Figure 1).
Early seedling growth assays showed that quinoa accessions are differentially affected under 12.5 µM of Cu (Figure 2A,B), a concentration similar to those found as soluble Cu in some polluted agricultural soils in central Chile [20,47]. Results highlighted differences in tolerance of accessions from different locations. Central accessions, and a northern accession (R49, Peñablanca, Paredones, Faro) exhibited high relative root length (RRL) values, suggesting greater Cu resilience (Figure 2B). In contrast, southern accessions (BO-78, Chadmo) displayed significantly reduced growth, reinforcing their sensitivity.
In the hydroponic experiment, growth parameters of quinoa are differentially affected in the presence of 350 µM of Cu (Table 1). A decrease in growth and biomass production was observed in other plant species in the presence of different Cu concentrations (e.g., in hydroponic experiments: 10 µM in Cucumis sativus, 50 µM in Lolium perenne, 200 µM in Pisum sativum, 350 µM in Triticum aestivum and Zea mays [45,48,49,50]). In comparison, quinoa can be considered a relatively tolerant species. In some works, it was observed that Cu phytotoxicity in plants involved ROS generation, changes in antioxidant enzymatic activities, nutrient imbalance, and decline in chlorophyll and pigment content [38,49,51].
Comparing quinoa accessions in hydroponic experiments, a pattern similar to those observed in germination and early seedling growth experiments emerged: central accessions (and a northern accession) (e.g., R49, Dorado Paredones) maintained biomass production, while southern accessions suffered substantial reductions in fresh and dry weight (Table 1, Figure 3). PERMANOVA results (Figure 4) further confirmed that geographical origin significantly influenced Cu tolerance (p = 0.0015). Specifically, central accessions differed from both Altiplano and southern accessions, reinforcing the idea of local adaptation to Cu-rich soils. This observation is consistent with reports that natural Cu levels are higher in northern and central Chile, while southern Chilean soils are comparatively less Cu-enriched [52,53].
The differential responses of quinoa accessions to Cu stress highlight the species’ remarkable genetic diversity and adaptability, as well as the influence of the origin of the accessions regarding Cu tolerance.
Variations in Cu tolerance among varieties have been observed in other crop species, such as Brassica napus, Nicotinana tabacum, and Raphanus sativus. In that work, it was suggested that this differential tolerance is not due to differential Cu exclusion capacity, and that nutrient status and lignin and saturated fatty acid contents could be involved in tolerance [54]. Jeon (2024) observed differential tolerance to Cu in callus of three apple varieties, probably due to genetic variability [55].
In this work, a systematic comparison of quinoa germplasms from different geographical origins for tolerance under Cu stress was performed for the first time. The observed variability in Cu tolerance among quinoa accessions is consistent with findings in other plant species, where intra-specific differences in metal tolerance were linked to genetic adaptation and environmental exposure. For instance, Juncus acutus populations from Cu-polluted sites exhibited higher germination rates and seedling survival under Cu stress compared to those from non-polluted areas [56]. Similarly, Armeria maritima accessions from metalliferous soils showed greater tolerance than those from non-metalliferous habitats [57]. These parallels highlight the importance of genetic diversity in metal tolerance and the potential for selective breeding of quinoa for sustainable agriculture.
There are no reports on mechanisms of Cu tolerance in quinoa. In Regalona plants stressed by other heavy metals, such as cromium, ROS production, oxidative stress, and osmolyte and tocopherol accumulation were observed [58]. An increase in antioxidant enzymatic activities was observed in other quinoa species growing in polluted soils from mining areas containing high concentrations of Cu [59]. Future research should focus on elucidating the mechanisms involved in Cu tolerance in C. quinoa. These mechanisms may be related to enzymatic and non-enzymatic antioxidant activity, Cu chelation and sequestration by organic acids and metal-binding molecules, and regulation of ion homeostasis by ion transporters, chaperones, and regulatory genes. Exploring these mechanisms could provide strategies for mitigation of Cu toxicity in crop plants.
5. Conclusions
Quinoa is an important plant with higher nutritive value than traditional cereals, and it is a promising cultivar for human nutrition worldwide. It is of interest to characterize the effects of Cu pollution on this crop in order to evaluate possible consequences on yield and quality of quinoa in crop fields and to manage suitable cultivation strategies in case of pollution.
We have evaluated the effects of Cu on germination and early seedling growth of 21 accessions of C. quinoa. Significant differences between accessions were observed. Our study highlights the genetic diversity in Cu tolerance among quinoa accessions and reveals a clear regional pattern, where central accessions exhibit superior Cu tolerance, southern accessions are more sensitive, and northern accessions fail to germinate under high Cu levels but show strong seedling growth when germinated.
Our results suggest that all tested quinoa accessions could successfully germinate at Cu concentrations usually found in polluted soils. On the other hand, early seedling growth is more sensitive to Cu, being differentially affected by Cu at concentrations found in polluted soils, which may potentially affect their successful establishment and/or growth in Cu-contaminated sites. Identified tolerant accessions (such as R49, Peñablanca, and Dorado Paredones from northern and central Chile) may have a better performance in copper-affected soils, while more sensitive accessions (such as BO-25, BO-78, and Chadmo from southern Chile) may require stricter copper management practices.
More studies are needed to characterize the response of these accessions to Cu pollution. Biochemical, metabolomic, and molecular characterization comparing selected tolerant and sensitive accessions will allow for a comprehensive and integrative understanding of the underlying mechanisms involved in Cu stress and tolerance in quinoa. This will allow us to design toxicity mitigation strategies. In addition, field experiments in agricultural settings with plants at later stages will be required to support present findings and to evaluate effects of Cu contamination in quinoa growth, yield, seed quality, and food safety.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17040229/s1: Table S1: List of C. quinoa accessions; Table S2: Effect of Cu concentrations on germination of 21 quinoa accessions; Table S3: Effect of Cu concentrations on Mean Germination Time, Germination Index and Inhibition of Germination of 21 quinoa accessions.
Author Contributions
T.C.d.l.P. conceptualized the research. T.C.d.l.P. and C.B.Á. designed the assays. C.B.Á., I.P.P. and E.Z.-C. performed the assays and measurements. C.B.Á. performed the data analysis. T.C.d.l.P., E.O.-G. and L.B.-G. discussed the results, wrote, revised, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Chile’s Ministry of Science. ANID FONDECYT REGULAR 1220589.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the National Seed Bank of Chile managed by the Genetic Resources section of the National Institute of Agriculture Research (INIA-Intihuasi, Vicuña, Chile), the U.S. Department of Agriculture (USDA) and the Department of Botany of the Universidad de Concepción (Chile) for providing us with the C. quinoa accessions.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GP | Germination Percentage |
| MGT | Mean Germination Time |
| GI | Germination Index |
| IG | Percent Inhibition of Germination |
| RRL | Relative Root Length |
| RRFW | Relative Root Fresh Weight |
| RRDW | Relative Root Dry Weight |
| RSFW | Relative Shoot Fresh Weight |
| RSDW | Relative Shoot Dry Weight |
| PCA | Principal Component Analysis |
References
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in Biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [PubMed]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soils. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef]
- Rheman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Wairich, A.; De Conti, L.; Lamb, T.I.; Keil, R.; Neves, L.O.; Brunetto, G.; Sperotto, R.A.; Ricachenevsky, F.K. Throwing copper around: How Plants Control Uptake, distribution, and accumulation of copper. Agronomy 2022, 12, 994. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [PubMed]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular mechanisms of plant responses to copper: From deficiency to stress. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [PubMed]
- Miotto, A.; Ceretta, C.A.; Girotto, E.; Trentin, G.; Kaminski, J.; De Conti, L.; Moreno, T.; Elena, B.; Brunetto, G. Copper accumulation and availability in Sandy, acid, vineyard soils. Commun. Soil Sci. Plant Anal. 2017, 48, 1167–1183. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS Soil Survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar]
- Sereni, L.; Guenet, B.; Lamy, I. Mapping risks associated with soil copper contamination using availability and bio-availability proxies at the European scale. Environ. Sci. Pollut. Res. 2023, 30, 19828–19844. [Google Scholar]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-Ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.; Zhang, T.; Stoffella, P. Heavy metal contamination in soils: Sources, indicators and assessment. J. Environ. Indic. 2015, 9, 17–18. [Google Scholar]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Mantanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, A.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper accumulation in agricultural soils: Risks for the food chain and soil microbial populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef]
- Verdejo, J.; Ginocchio, R.; Sauvé, S.; Salgado, E.; Neaman, A. Thresholds of copper phytotoxicity in field-collected agricultural soils exposed to copper mining activities in Chile. Ecotoxicol. Environ. Saft. 2015, 122, 171–177. [Google Scholar]
- Schoffer, J.T.; Aponte, H.; Neaman, A.; de la Fuente, L.M.; Arellano, E.C.; Gil, P.M.; Ginocchio, R. Copper content in soils and litter from fruit orchards in Central Chile and its relationship with soil microbial activity. Plant Soil Environ. 2022, 68, 115–128. [Google Scholar] [CrossRef]
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. The Soils of Chile; Hartemink, A.E., Ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Angon, P.D.; Islam, M.S.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev. Int. 2006, 19, 99–109. [Google Scholar]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.A.; Veas, E.; Jorquera, C.; San Martín, R.; Jara, P. Re-Introduction of quinoa into Arid Chile: Cultivation of two lowland races under extremely low irrigation. J. Agron. Crop Sci. 2009, 195, 1–10. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Stolen, O. Quinoa—Morphology, phenology and prospects for its production in Europe. Eur. J. Agron. 1993, 2, 19–29. [Google Scholar] [CrossRef]
- Jacobsen, S.E. Adaptation of quinoa (Chenopodium quinoa) to Northern European agriculture: Studies on developmental pattern. Euphytica 1997, 96, 41–48. [Google Scholar] [CrossRef]
- Bendevis, M.A.; Sun, Y.; Rosenqvist, E.; Shabala, S.; Liu, F.; Jacobsen, S.E. Photoperiodic effects on short-pulse 14C assimilation and overall carbon and nitrogen allocation patterns in contrasting quinoa cultivars. Environ. Exp. Bot. 2014, 104, 9–15. [Google Scholar] [CrossRef]
- Bazile, D.; Bertero, D.; Nieto, C. (Eds.) Estado del Arte de la Quinua en El Mundo en 2013; FAO: Santiago de Chile, Chile; CIRAD: Montpellier, France, 2014; p. 724. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Quinoa 2013 International Year. Available online: https://www.fao.org/quinoa-2013/en/ (accessed on 17 December 2024).
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Amjad, M.; Iqbal, M.; Abbas, G.; Farooq, A.B.U.; Naeem, M.A.; Imran, M.; Murtaza, B.; Nadeem, M.; Jacobsen, S.E. Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation and human health. Environ. Geochem. Health 2022, 44, 1487–1500. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Rajan, S.; Ohri, D. Genetic diversity for morphological and quality traits in quinoa (Chenopodium quinoa Willd.) germplasm. Genet. Resour. Crop Evol. 2007, 54, 167–173. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1981, 45, 13–30. [Google Scholar] [CrossRef]
- Brenchley, J.L.; Probert, R.J. Seed germination responses to some environmental factors in the seagrass Zostera capicorni from Eastern Australia. Aquat. Bot. 1998, 62, 177–188. [Google Scholar] [CrossRef]
- Baruah, N.; Mondal, S.C.; Farooq, M.; Gogoi, N. Influence of heavy metals on seed germination and seedling growth of wheat, pea and tomato. Water Air Soil Pollut. 2019, 230, 273. [Google Scholar]
- Sarma, B.; Devi, P.; Gogoi, N.; Devi, Y.M. Effects of cobalt induced stress in Triticum aestivum L. crop. AJAB 2014, 2, 137–147. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; Circular 347; University of California, College of Agriculture, Agricultural Experiment Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. Centro de Transferencia InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 17 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 7 January 2025).
- Kranner, I.; Colville, I. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 23, 93–105. [Google Scholar]
- Sanjosé, I.; Navarro-Roldán, F.; Infante-Izquierdo, M.D.; Martínez-Sagarra, G.; Devesa, J.A.; Polo, A.; Ramirez-Acosta, S.; Sánchez-Guillón, E.; Jiménez-Nieva, F.J.; Muñoz-Rodríguez, A.F. Accumulation and effects of heavy metals on the germination and growth of Salsoa vermiculata L. seedlings. Diversity 2021, 13, 539. [Google Scholar] [CrossRef]
- Vasilachi-Mitoresu, I.C.; Stoleru, V.; Gavrilescu, M. Integrated assessment of Pb(II) and Cu(II) metal ion phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. plants: Insights into germination inhibition, seedling development, and ecosystem health. Plants 2023, 12, 3754. [Google Scholar] [CrossRef]
- Tapia-Gatica, J.; Selles, I.; Bravo, M.A.; Tessini, C.; Barros-Parada, W.; Novoselov, A.; Neaman, A. Global issues in setting legal limits on soil metal contamination: A case study of Chile. Chemosphere 2022, 290, 133404. [Google Scholar]
- Mondaca, P.; Catrin, J.; Verdejo, J.; Sauvé, S.; Neaman, A. Advances on the determination of thresholds of Cu phytotocity in field-contaminated soils in Central Chile. Environ. Pollut. 2017, 223, 146–152. [Google Scholar]
- Bosnic, D.; Bosnic, P.; Nikolic, D.; Nikolic, M.; Samardzic, J. Silicon and iron differently alleviate copper toxicity in cucumber leaves. Plants 2019, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Ben Massoud, M.; Sakohui, L.; Chaoui, A. Effects of plant growth regulators, calcium and citric acid on copper toxicity on pea seedlings. J. Plant Nutr. 2019, 42, 1230–1242. [Google Scholar]
- De Conti, L.; Cesco, S.; Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Melo, G.W.B.; Ceretta, C.A.; Trentin, E.; Marques, A.C.R.; Brunetto, G. Iron fertilization to enhance tolerance mechanisms to copper toxicity of ryegrass plants used as cover crop in vineyards. Chemosphere 2020, 243, 125298. [Google Scholar] [PubMed]
- Yadav, P.; Kaur, R.; Kohli, S.K.; Sirhindi, G.; Bhardwaj, R. Castasterone assisted accumulation of polyphenols and antioxidant to increase tolerance of B. juncea plants towards copper toxicity. Cogent. Food Agric. 2016, 2, 1276821. [Google Scholar]
- Tapia, J.; Mulet, J.; Poblete, N.; Rodríguez, C. Catastro de estudios de geoquímica Ambiental de suelos y sedimentos de Chile. In Proceedings of the XIV Congreso Geológico Chileno, La Serena, Chile, 4–8 October 2015. [Google Scholar]
- Informe País. Estado del Medio Ambiente en Chile. 2018. Universidad de Chile. Instituto de Asuntos Públicos. Centro de Análisis de Políticas Públicas. Available online: https://repositorio.uchile.cl/bitstream/handle/2250/179262/Capitulo5-Suelos.pdf?sequence=1 (accessed on 7 October 2024).
- Novello, N.; Ferfuia, C.; Pasković, I.; Fabris, A.; Baldini, M.; Schat, H.; Pošćić, F. Independent variation in copper tolerance and copper accumulation among crop species and varieties. Plant Physiol. Biochem. 2020, 156, 538–551. [Google Scholar]
- Jeon, D. Evaluating the impact of copper concentrations on apple variety performance: Insights from a callus media system. Sustainability 2024, 16, 9741. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Pérez-Romero, J.A.; Mesa-Marín, J.; López-Jurado, J.; Redondo-Gómez, S. Inter-population differences tolerance to Cu excess during the initial phases of Juncus acutus life cycle: Implications for the design of metal restoration strategies. Int. J. Phytoremediation 2019, 21, 550–555. [Google Scholar]
- Purmale-Trasune, L.; Jēkabsone, A.; Andersone, U.; Karlsons, A.; Osvalde, A.; Levins, G. Comparison of in vitro and in planta heavy metal tolerance and accumulation potential of different Armeria maritima accessions from a dry Coastal Meadow. Plants 2022, 11, 2104. [Google Scholar] [CrossRef]
- Scoccianti, V.; Bucchini, A.E.; Iacobucci, M.; Ruiz, K.B.; Biondi, S. Oxidative stress and antioxidant responses to increasing concentrations of trivalent chromium in the Andean crop species Chenopodium quinoa Willd. Ecotoxicol. Environ. Saf. 2016, 133, 25–35. [Google Scholar]
- Gao, T.; Wang, H.; Li, C.; Zuo, M.; Wang, X.; Liu, Y.; Yang, Y.; Xu, D.; Liu, Y.; Fang, X. Effects of heavy metal stress on physiology, hydraulics, and anatomy of three desert plants in the Jinchang mining area, China. Int. J. Environ. Res. Public Health 2022, 19, 15873. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).