Current Knowledge and Research Perspectives on Bryophytes in West Africa
Abstract
1. Introduction
2. Results
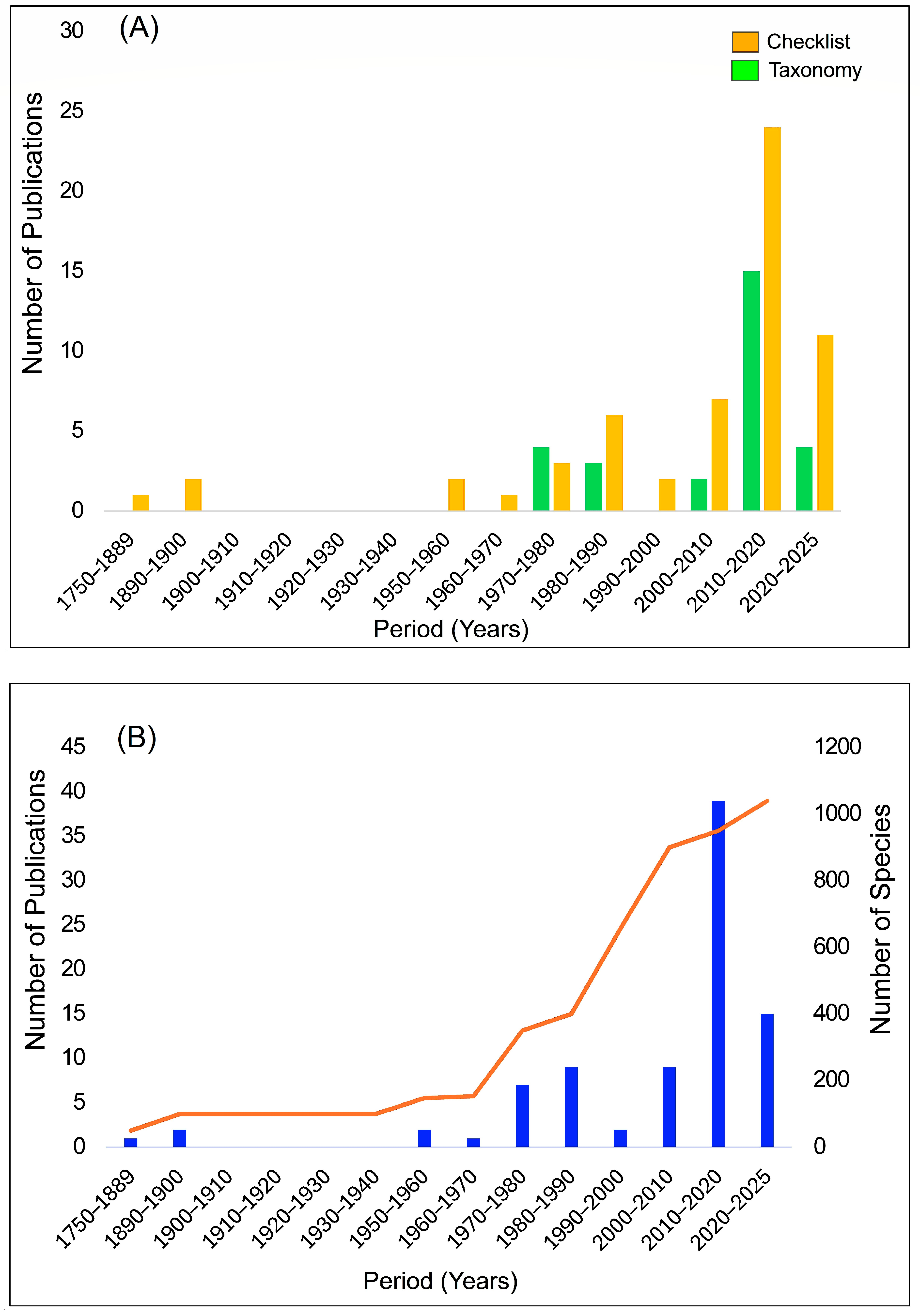
2.1. Historical and Contemporary Bryological Exploration in West Africa: A Synthesis of Literature
2.2. Research Bias
2.3. Spatial Distribution of Bryophyte Studies in West Africa over the Last Four Decades
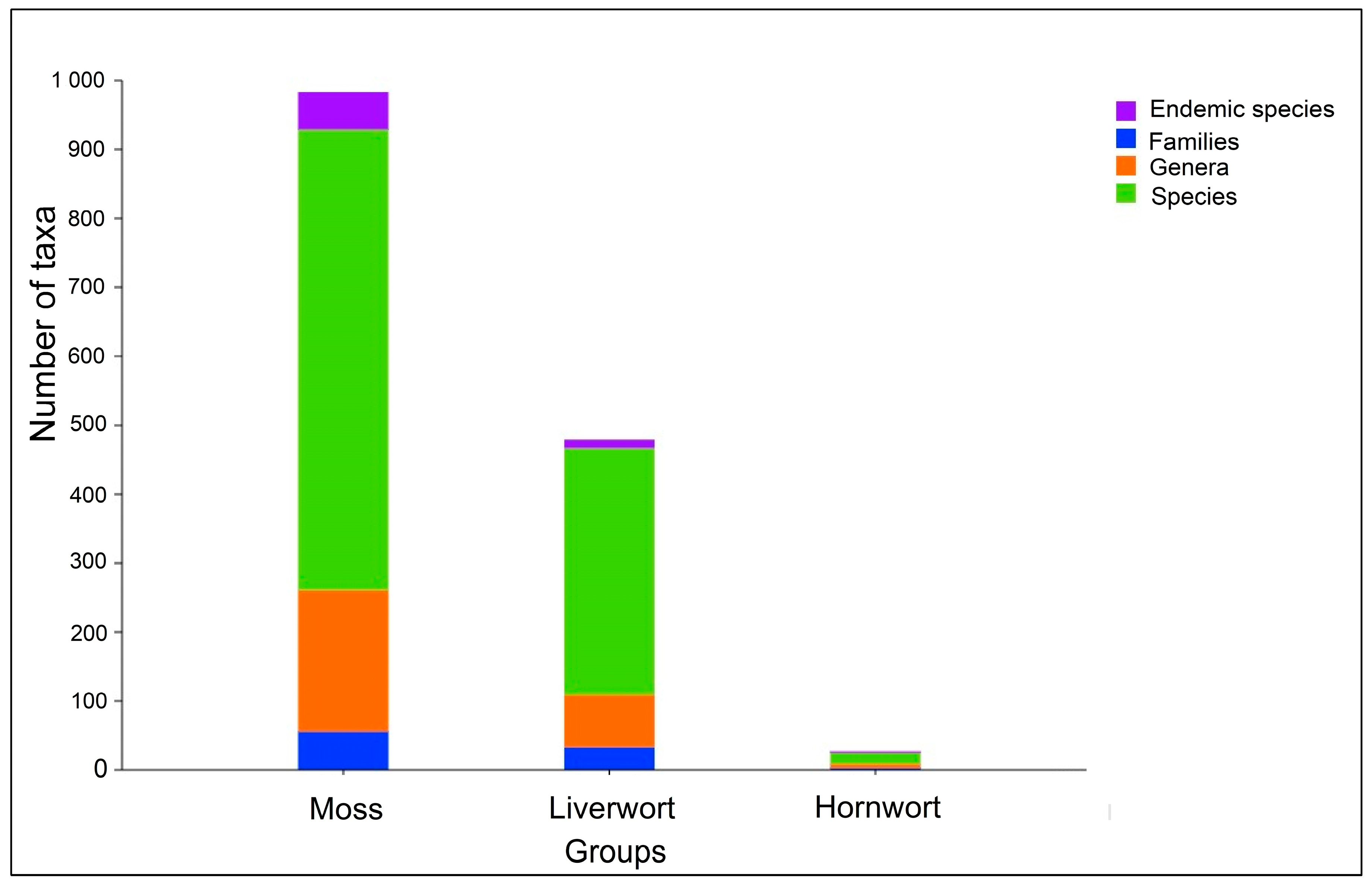
2.4. Bryophyte Flora of West Africa
2.4.1. Floristic Composition
2.4.2. Endemism of Bryophytes in West Africa
2.4.3. Identification Keys for Bryophytes of West Africa
3. Discussion
3.1. A Review of Literature and Bryological Exploration
3.2. Bryophyte Flora of West Africa
4. Future Direction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WA | West Africa |
| PhD | Doctor of Philosophy |
Appendix A
| Country | Hornworts | Mosses | Liverworts | Endemicity | Species |
|---|---|---|---|---|---|
| Benin | 2 | 43 | 39 | 2.63% | 84 |
| Bioko | 6 | 218 | 179 | 7.89% | 403 |
| Burkina Faso | 0 | 13 | 0 | 0 | 13 |
| Cape Verde | 1 | 188 | 62 | 13.16% | 251 |
| Gambia | 0 | 2 | 1 | 0 | 3 |
| Ghana | 4 | 143 | 172 | 7.89% | 319 |
| Guinea | 0 | 199 | 64 | 25% | 263 |
| Guinea-Bissau | 0 | 2 | 6 | 0 | 8 |
| Côte d’Ivoire | 1 | 203 | 111 | 9.21% | 315 |
| Liberia | 0 | 70 | 16 | 1.32% | 86 |
| Mali | 0 | 17 | 2 | 5.26% | 19 |
| Niger | 0 | 11 | 8 | 1.32% | 17 |
| Nigeria | 2 | 218 | 157 | 7.89% | 377 |
| São Tomé and Príncipe | 7 | 137 | 203 | 19.74% | 347 |
| Senegal | 0 | 80 | 36 | 2.63% | 116 |
| Sierra Leone | 6 | 110 | 143 | 5.58% | 259 |
| Togo | 1 | 89 | 57 | 3.95% | 147 |
References
- UNSD. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 18 August 2025).
- White, F. The Vegetation of Africa: A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa; Natural Resources Research No. 20; UNESCO: Paris, France, 1983; p. 356. [Google Scholar]
- Sayer, J. Benin and Togo. In The Conservation Atlas of Tropical Forests Africa; Springer: Berlin/Heidelberg, Germany, 1992; pp. 97–101. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- FAO. Évaluation des Ressources Forestières Mondiales 2020; FAO: Rome, Italy, 2021; ISBN 978-92-5-134306-7. [Google Scholar]
- Hallingbäck, T.; Tan, B.C. Towards a Global Action Plan for Endangered Bryophytes. An. Inst. Biol. Ser. Bot. 1996, 67, 213–221. [Google Scholar]
- Gignac, L.D. Bryophytes as Indicators of Climate Change. Bryologist 2001, 104, 410–420. [Google Scholar] [CrossRef]
- Ondo, I.; Dhanjal-Adams, K.L.; Pironon, S.; Silvestro, D.; Colli-Silva, M.; Deklerck, V.; Grace, O.M.; Monro, A.K.; Nicolson, N.; Walker, B.; et al. Plant Diversity Darkspots for Global Collection Priorities. New Phytol. 2024, 244, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, M.D.K.L. A Review of Bryophytes; Evolution, Value and Threats. Int. J. Sci. Res. Publ. 2019, 9, p8946. [Google Scholar] [CrossRef]
- Richards, P.W. The Bryologically Under-Worked Regions of the World, with Special Reference to West Africa and a Proposal for a Bryologia Africana. J. Hattori Bot. Lab. 1984, 55, 165–172. [Google Scholar]
- Dusén, P. New and Some Little Known Mosses from the West Coast of Africa. Kongl. Sv. Vet. Akademiens Handlingar. 1895, 28, 85. [Google Scholar]
- Schultze-Motel, W. Katalog Der Laubmoose von West-Afrika. Willdenowia 1975, 7, 473–535. [Google Scholar]
- De La Varde, R.P.; Richards, P.W. Notes on African Mosses. II. Fissidentaceae and Archifissidentaceae from Nigeria and the British Cameroons. Trans. Br. Bryol. Soc. 1956, 3, 85–97. [Google Scholar] [CrossRef]
- Richards, P.W. Ecological Notes on West African Vegetation: I. The Plant Communities of the Idanre Hills, Nigeria. J. Ecol. 1957, 45, 563–577. [Google Scholar] [CrossRef]
- Egunyomi, A.; Olarinmoye, S.O. Mosses from Nigeria. II. Collections from the Obudu Cattle Ranch. Bryologist 1979, 82, 76–77. [Google Scholar] [CrossRef]
- Frahm, J.-P.; Lindlar, A.; Sollman, P.; Fischer, E. Bryophytes from the Cape Verde Islands. Trop. Bryol. 1996, 12, 123–154. [Google Scholar]
- Dilg, C.; Frahm, J.-P. Ein Beitrag Zur Moosflora von Benin. Trop. Bryol. 2000, 19, 1–6. [Google Scholar]
- Frahm, J.-P.; Porembski, S. Moose von Inselbergen in Benin. Trop. Bryol. 1998, 14, 3–10. [Google Scholar]
- Garcia, C.; Sérgio, C.; Shevock, J.R. The Bryophyte Flora of São Tomé and Príncipe (Gulf of Guinea): Past, Present and Future. In Biodiversity of the Gulf of Guinea Oceanic Islands: Science and Conservation; Ceríaco, L.M.P., de Lima, R.F., Melo, M., Bell, R.C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 217–248. ISBN 978-3-031-06153-0. [Google Scholar]
- Sergio, C.; Garcia, C. Bryophyte Flora of Sao Tome e Principe Archipelago (West Africa): Annotated Catalogue. Cryptogam. Bryol. 2011, 32, 145–196. [Google Scholar] [CrossRef]
- Müller, F.; Pócs, T.; Shevock, J.R. Additions to the Liverwort and Hornwort Flora of São Tomé and Príncipe. Trop. Bryol. 2011, 33, 19–22. [Google Scholar]
- Hodgetts, N.G.; Ameka, G.; Agyei, R.; Dankwah, C. Additions and Corrections to the Bryophyte Flora of Ghana, Including a New Species of Cololejeunea (Spruce) Schiffn. (Lejeuneaceae, Marchantiophyta). J. Bryol. 2021, 43, 251–258. [Google Scholar] [CrossRef]
- Hodgetts, N.G.; Essilfie, M.K.; Adu-Gyamfi, A.; Akom, E.; Kumadoh, J.; Opoku, J. Bryophytes of Atewa Forest, Eastern Region, Ghana. J. Bryol. 2016, 38, 211–222. [Google Scholar] [CrossRef]
- Müller, F. New Bryophyte Records for West and Central African Countries. Acta Biol. Plant. Agriensis 2023, 11, 90–106. [Google Scholar] [CrossRef]
- Gyarmati, A. Hépatiques de Côte-d’Ivoire, récoltes du RP É. Assel. Cryptogam. Bryol. 2001, 22, 47–51. [Google Scholar] [CrossRef]
- Diop, D.; Diop, D.; Bruggeman-Nannenga, M.A.; Samba Mbaye, M. Bryophytes of Kédougou (Eastern Senegal), with a Key to the Fissidens of Senegal. J. Bryol. 2018, 40, 62–67. [Google Scholar] [CrossRef]
- Diop, D.; Diouf, N.; Ndour, S.; Diouf, J.; Dieng, B.; Diop, D.; Diop, S.; Camara, A.A.; Mbaye, M.S.; Noba, K. Bryophyta (Mosses) of Senegal: The Case of Mosses in Herbaria and Databases. GSC Biol. Pharm. Sci. 2021, 17, 177–185. [Google Scholar] [CrossRef]
- Abalo-Loko, G.A.; Guelly, K.A.; Wigginton, M.; Reeb, C. Checklist of Liverworts and Hornworts of Togo. Cryptogam. Bryol. 2019, 40, 233. [Google Scholar] [CrossRef]
- Sérgio, C.; Draper, D.; Porley, R.D. Three New Records of Anthocerotophyta for Western Africa (Sierra Leone) Based on Spore Ornamentation of a Specimen Collected by A. Harrington, with an Emphasis on Anthoceros Sect. Fusiformes Grolle. J. Bryol. 2020, 42, 160–168. [Google Scholar] [CrossRef]
- Abubakar, B.Y.; Abdullahi, S. Host Preference of Bryophytes Composition from Northern Nigeria. Acta Musei Silesiae Sci. Nat. 2012, 61, 213. [Google Scholar] [CrossRef]
- Bolaji, A.O.; Faluyi, J.O. Morphological, Anatomical and Cytological Studies of Some Moss Species from Nigeria. Not. Sci. Biol. 2017, 9, 404–413. [Google Scholar] [CrossRef]
- Ezukanma, I.O.; Tessler, M.; Salaam, A.M.; Chukwuka, K.S.; Ogunniran, A.J. Epiphytic Bryophytes of Urban Agroforests in Ibadan, Southwest Nigeria. J. Bryol. 2019, 41, 341–349. [Google Scholar] [CrossRef]
- Alia, B.M.H.; Reeb, C.; Tossou, M.G.; Bruggeman-Nannenga, M.A. Fissidens bassilae (Fissidentaceae, Musci), a New Species from Africa. Bryophyt. Divers. Evol. 2024, 47, 22–25. [Google Scholar] [CrossRef]
- Garcia, C.A.; Sérgio, C.; Martins, A.; Rodrigues, A.S.B.; Sim-Sim, M. A Contribution to the Knowledge of the Bryophytes of the Cape Verde Islands, with an Emphasis on Santo Antão and São Vicente. J. Bryol. 2021, 43, 122–128. [Google Scholar] [CrossRef]
- Sim-Sim, M.; Martins, A.; Garcia, C.A. Updated List of Bryophytes from Cape Verde Archipelago. Diversity 2024, 16, 217. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Duarte, M.C.; Francisco-Ortega, J.; Catarino, L.; Havik, P. Recovering Plant Data for Guinea-Bissau: Implications for Biodiversity Knowledge of West Africa. Diversity 2018, 10, 109. [Google Scholar] [CrossRef]
- Coelho, F.D.L. Duas Descrições Seiscentistas da Guiné; Académia Portuguesa da História: Lisbon, Portugal, 1953; p. 283. [Google Scholar]
- Santos, B.C.D. Memórias Do Ultramar: Os Escritos Sobre a “Guiné de Cabo-Verde” e a Influência Dos Processos de Crioulização. (Séc. XVI e XVII). Ph.D. Thesis, Universidade Federal de Juiz de Fora, Juiz de Fora, Brazil, 2017. [Google Scholar]
- Harris, S.A. Seventeenth-Century Plant Lists and Herbarium Collections: A Case Study from the Oxford Physic Garden. J. Hist. Collect. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Carteret, X. Michel Adanson in Senegal (1749–1754): A Great Naturalistic and Anthropological Journey of the Enlightenment. Rev. D’Hist. Sci. 2012, 65, 5–25. [Google Scholar]
- Hepper, F.N. The Niger and the Nile: Botanical Exploration around Two African Rivers. Ann. Mo. Bot. Gard. 1991, 78, 81–86. [Google Scholar] [CrossRef]
- William Mitten Papers (PP). Available online: https://www.nybg.org/library/finding_guide/archv/mitten_ppb.html (accessed on 21 September 2025).
- Von Brescius, M. German Science in the Age of Empire: Enterprise, Opportunity and the Schlagintweit Brothers, 1st ed.; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-108-57956-8. [Google Scholar]
- Van de Kerckhove, O.; Duckett, J.G.; Harrington, A.J.; Hodgetts, N.G.; Long, D.G.; O’Shea, B.J.; Perry, A.R.; Wigginton, M.J. EW Jones’s Liverwort and Hornwort Flora of West Africa; National Botanic Garden of Belgium: Meise, Belgium, 2004; Volume 30, p. i432. [Google Scholar]
- Infante, M.; Heras, P.; Buck, W.R. Bryophytes from the Republic of Equatorial Guinea (West-Central Africa) II. Bryophytes Collected by Emilio Guinea (1907–1985) in the Island of Bioco in 1947. Trop. Bryol. 1997, 13, 131–136. [Google Scholar]
- Pérez, P.H.; Sánchez, M.I. Bryophytes from the Republic of Equatorial Guinea (West-Central Africa) I. Introduction and Preliminary Checklist. Bryophyt. Divers. Evol. 1996, 12, 41–58. [Google Scholar] [CrossRef]
- Edwards, S.R. A Revision of West Tropical African Calymperaceae, I. Introduction and Calymperes. J. Bryol. 1980, 11, 49–93. [Google Scholar] [CrossRef]
- Olarinmoye, S.O. Ecology of Epiphyllous Liverworts: Growth in Three Natural Habitats in Western Nigeria. J. Bryol. 1974, 8, 275–289. [Google Scholar] [CrossRef]
- Egunyomi, A. Mosses from Nigeria III: New Records from the Middle-Belt Area. J. Hattori Bot. Lab. 1980, 48, 187–193. [Google Scholar]
- Egunyomi, A. Eight Mosses New to Nigeria. Bryologist 1981, 84, 533–535. [Google Scholar] [CrossRef]
- Egunyomi, A.; Olarinmoye, S.O. Syrrhopodon Obuduensis, a New Moss Species from Nigeria. Bryologist 1982, 85, 312–314. [Google Scholar] [CrossRef]
- Frahm, J.P.; Porembski, S.; Seine, R.; Barthlott, W. Bryophytes from Inselbergs in the Ivory Coast and Zimbabwe. Nova Hedwig. 1996, 62, 177–189. [Google Scholar] [CrossRef]
- Oyesiku, O.O. A Review of Nigerian Bryophytes: Past, Present and Future. Afr. J. Agric. Res. 2012, 7, 4352–4356. [Google Scholar] [CrossRef]
- Adebiyi, A.O.; Oyeyemi, S.D. Distribution of Mosses in Ekiti State, Nigeria. N. Y. Sci. J. 2013, 6, 23–25. [Google Scholar]
- Adu-Gyamfi, A.; Hodgetts, N. Bryophytes of Ghana. Biodivers. Inf. Sci. Stand. 2018, 2, e25879. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Cano, M.J. Didymodon caboverdeanus J.A. Jiménez & M.J. Cano (Pottiaceae, Musci), a New Species from the Cape Verde Archipelago. J. Bryol. 2017, 39, 171–176. [Google Scholar] [CrossRef]
- Martins, A.; Garcia, C.A.; Patino, J.; Sim-Sim, M. Exormotheca martins-loussaoae (Exormothecaceae, Hepaticae), a New Species from Cape Verde. Plant Biosyst. 2023, 157, 294–300. [Google Scholar] [CrossRef]
- Costa, D.P.; Garcia, C.A.; Sérgio, C. Bryophyte Flora of São Tomé and Príncipe: An Update of the Lejeuneaceae Family. Bryophyt. Divers. Evol. 2024, 47, 136–158. [Google Scholar] [CrossRef]
- Qian, H.; Zhou, Y.; Zhang, J.; Jin, Y.; Deng, T.; Cheng, S. A Synthesis of Botanical Informatics for Vascular Plants in Africa. Ecol. Inform. 2021, 64, 101382. [Google Scholar] [CrossRef]
- Becker, G. Où en est la philosophie de la botanique? Publ. Soc. Linnéenne Lyon 1958, 27, 188–192. [Google Scholar] [CrossRef]
- Frahm, J.-P.; Pócs, T.; O’shea, B.; Koponen, T.; Piipo, S.; Enroth, J.; Rao, P.; Fang, Y. Manual of Tropical Bryology. Trop. Bryol. 2003, 23, 1–200. [Google Scholar]
- Lindo, Z.; Gonzalez, A. The Bryosphere: An Integral and Influential Component of the Earth’s Biosphere. Ecosystems 2010, 13, 612–627. [Google Scholar] [CrossRef]
- Egbetokun, A.; Olofinyehun, A.; Ayo-Lawal, A.R.; Sanni, M.; Oluwatope, O.; Adeleke, Y.S. Doing Research in Nigeria. In Doing Research in Nigeria; National Centre for Technology Management & The Global Development Network: Ile-Ife, Nigeria, 2020; p. 144. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Chia, T.; Oyeniran, O.I.; Welcome, M.O.; Mangse, G.; Athar, H.-R.; Jellason, N.P. The Need for Nigerian Universities to Collaborate for Quality Research Output. In Innovations and Interdisciplinary Solutions for Underserved Areas; Mambo, A.D., Gueye, A., Bassioni, G., Eds.; Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer Nature: Cham, Switzerland, 2022; Volume 449, pp. 279–289. ISBN 978-3-031-23115-5. [Google Scholar]
- Shevock, J.R.; Pursell, R.A.; Garcia, C.A.; Bruggeman-Nannenga, M.A.; Sergio, C. The Genus Fissidens in the Republic of Sao Tome and Principe, Gulf of Guinea, West Africa. J. Bryol. 2013, 35, 197–205. [Google Scholar] [CrossRef]
- Potier de la Varde, R. Petite Contribution à La Flore Bryologique de Fernando Poo. Bull. L’Institut Fr. D’Afrique Noire 1953, 15, 483–486. [Google Scholar]
- Muller, F. Bryophytes of Bioko (Equatorial Guinea), Results of an Excursion in 2002. Trop. Bryol. 2006, 27, 9. [Google Scholar]
- Ellis, L.T.; Arrocha, C.; Benítez, Á.; Beyrouthy, M.; Chandini, V.K.; Czernyadjeva, I.V.; Deme, J.; Erzberger, P.; Fedosov, V.E.; Górski, P.; et al. New National and Regional Bryophyte Records, 71. J. Bryol. 2022, 44, 252–263. [Google Scholar] [CrossRef]
- Ellis, L.T.; Afonina, O.M.; Alataş, M.; Alia, H.B.M.; Alvarez, J.; Aponte Rojas, A.M.; Atwood, J.J.; Bacilliere, G.; Batan, N.; Biberdžić, V.; et al. New National and Regional Bryophyte Records, 76. J. Bryol. 2024, 46, 51–74. [Google Scholar] [CrossRef]
- Schultze-Motel, W. Die Laubmoose von Togo, West-Afrika. Lindbergia 1979, 89–92. [Google Scholar]
- Engel, M.S.; Ceríaco, L.M.P.; Daniel, G.M.; Dellapé, P.M.; Löbl, I.; Marinov, M.; Reis, R.E.; Young, M.T.; Dubois, A.; Agarwal, I.; et al. The Taxonomic Impediment: A Shortage of Taxonomists, Not the Lack of Technical Approaches. Zool. J. Linn. Soc. 2021, 193, 381–387. [Google Scholar] [CrossRef]
- Renzaglia, K.S.; Villarreal Aguilar, J.C.; Garbary, D.J. Morphology Supports—The Setaphyte Hypothesis: Mosses plus Liverworts Form a Natural Group. Bryophyt. Divers. Evol. 2018, 40, 11. [Google Scholar] [CrossRef]
- Pototsky, P.C.; Cresswell, W. Conservation Research Output in Sub-Saharan Africa Is Increasing, but Only in a Few Countries. Oryx 2021, 55, 924–933. [Google Scholar] [CrossRef]
- O’Shea, B.J. Checklist of the Mosses of Sub-Saharan Africa (Version 5, 12/06). Trop. Bryol. Res. Rep. 2006, 6, 1–152. [Google Scholar]
- Wigginton, M.J. Checklist and Distribution of the Liverworts and Hornworts of Sub-Saharan Africa, Including the East African Islands (4th ed., 25 June 2018). Trop. Bryol. Res. Rep. 2018, 9, 1–138. [Google Scholar]
- Goffinet, B.; Buck, W.R.; Shaw, A.J. Morphology, Anatomy, and Classification of the Bryophyta. In Bryophyte Biology; Goffinet, B., Shaw, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 55–138. [Google Scholar]
- Söderström, L.; Hagborg, A.; von Konrat, M.; Bartholomew-Began, S.; Bell, D.; Briscoe, L.; Brown, E.; Cargill, D.C.; da Costa, D.P.; Crandall-Stotler, B.J.; et al. World Checklist of Hornworts and Liverworts. PhytoKeys 2016, 59, 1–828. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Churchill, S.P.; Salazar-Allen, N. Guide to the Bryophytes of Tropical America; Memoirs of the New York Botanical Garden; New York Botanical Garden Press: Bronx, NY, USA, 2001; Volume 86, pp. 1–577. [Google Scholar]
- Ros, R.M.; Cano, M.J.; Guerra, J. Bryophyte Checklist of Northern Africa. J. Bryol. 1999, 21, 207–244. [Google Scholar] [CrossRef]
- El-Saadawi, W.; Shabbara, H.M.; El-Sakaty, S.I. Mosses of the Egyptian Conservation Areas: II. Omayed Protected Area. Cryptogam. Bryol. 2013, 34, 61–71. [Google Scholar] [CrossRef]
- El-Saadawi, W.; Shabbara, H.; Youssef, S.; Khalil, M. Bryophytes of Libya. II. Musci: An Annotated Checklist. Taeckholmia 2017, 37, 41–51. [Google Scholar] [CrossRef]
- Youssef, S. Bryophytes of Libya. I. Hepatophyta: An Annotated Checklist. Egypt. J. Bot. 2017, 57, 277–279. [Google Scholar] [CrossRef]
- Taha, M. Three New Pottiaceae Records to the Bryoflora of Libya. Egypt. J. Bot. 2019, 59, 15–28. [Google Scholar] [CrossRef]
- Osman, I.B.; Hugonnot, V.; Muller, S.D.; Daoud-Bouattour, A. A Contribution to the Study of Hornworts and Liverworts in Tunisia: A Checklist and Ecology of Kroumirian Species. Cryptogam. Bryol. 2019, 40, 271. [Google Scholar] [CrossRef]
- El-Saadawi, W.; Taha, M.A. Toward A Red List of Bryophytes of Egypt. I. Hepatics. Egypt. Acad. J. Biol. Sci. H Bot. 2020, 11, 23–27. [Google Scholar] [CrossRef]
- Van Rooy, J.; Bergamini, A.; Bisang, I. Fifty Shades of Red: Lost or Threatened Bryophytes in Africa. Bothalia 2019, 49. [Google Scholar] [CrossRef]
- Hallingbäck, T.; Hodgetts, N.G. Mosses, Liverworts, and Hornworts: Status Survey and Conservation Action Plan for Bryophytes; IUCN/SSC Bryophyte Specialist Group; IUCN: Grand, Switzerland; Cambridge, UK, 2000; pp. x + 106. ISBN 2-8317-0466-9. [Google Scholar]
- Moreira, H.; Kuipers, K.J.J.; Posthuma, L.; Zijp, M.C.; Hauck, M.; Huijbregts, M.A.J.; Schipper, A.M. Threats of Land Use to the Global Diversity of Vascular Plants. Divers. Distrib. 2023, 29, 688–697. [Google Scholar] [CrossRef]
- Ellis, L.T. Taxonomic Notes on Calymperes erosum Müll.Hal. (Calymperaceae), C. palisotii Schwägr. and Related West African Taxa. J. Bryol. 2018, 40, 333–341. [Google Scholar] [CrossRef]
- Jones, E.W. African Hepatics: X. Leptocolea and Cololejeunea. Trans. Br. Bryol. Soc. 1954, 2, 408–438. [Google Scholar] [CrossRef]
- Ochi, H. Revision of African Bryoideae, Musci (First Part). J. Fac. Educ. Tottori Univ. 1972, 23, 125. [Google Scholar]
- Richards, P.W.; Edwards, S.R. Notes on African Mosses—V. J. Bryol. 1972, 7, 47–60. [Google Scholar] [CrossRef]
- Jones, E.W. African Hepatics XXIll. Some Species of Lejeunea. J. Bryol. 1972, 7, 23–45. [Google Scholar] [CrossRef]
- Petit, E. Clefs Pour la Détermination des Familles et des Genres des Mousses Pleurocarpes (Musci) d’Afrique. Bull. Jard. Bot. Natl. Belg. Bull. Natl. Plantentuin Van Belg. 1978, 48, 135–181. [Google Scholar] [CrossRef]
- Vanden Berghen, C. Le Genre Lopholejeunea (Spruce) Schiffn. (Lejeuneaceae, Hepaticae) En Afrique. Bull. Jard. Bot. Natl. Belg. Bull. Natl. Plantentuin Van Belg. 1984, 54, 393–464. [Google Scholar] [CrossRef]
- Perold, S.M. Studies in the Ricciaceae of Sub-Saharan Africa: A Provisional Key to the Currently Known Species. Bothalia 1996, 26, 95–123. [Google Scholar] [CrossRef]
- So, M.L. Metzgeria (Metzgeriaceae, Marchantiophyta) in Africa. N. Zldn. J. Bot. 2004, 42, 271–292. [Google Scholar] [CrossRef]
- Malombe, I. Systematics of Cheilolejeunea (Spruce) Schiffn. (Lejeuneaceae) in Continental Africa and Its Ecological Significance in Conservation of Kakamega and Budongo Rainforests. Ph.D. Thesis, University of Koblenz and Landau, Mainz, Germany, 2009. [Google Scholar]
- Bruggeman-Nannenga, M.A. Subgenus Fissidens in Tropical Eastern Africa with Emphasis on the Tanzanian Collections by Tamás Pócs. Pol. Bot. J. 2013, 58, 369–417. [Google Scholar] [CrossRef]
- Phephu, N.; Van Rooy, J.; Van Wyk, A.E. New Combinations and a Key to the Species of Pelekium (Thuidiaceae) in Sub-Saharan Africa and the East African Islands. Phytotaxa 2013, 84, 60–64. [Google Scholar] [CrossRef]
- Phephu, N. A Taxonomic Revision of Thuidiaceae (Bryophyta) in Africa and the East African Islands. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2013. [Google Scholar]
- Alonso, M.; Jiménez, J.A.; Cano, M.J. A New Species of Chionoloma (Pottiaceae) from Central and South America with a Key to Neotropical Species of the Genus. Bryologist 2017, 120, 340–346. [Google Scholar] [CrossRef]
- Bruggeman-Nannenga, M.A. Fissidens subgenus aloma (Bryophyta) in Tropical Africa I. the Large-Celled Costate and Ecostate Species. Pol. Bot. J. 2017, 62, 139–168. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Ilkiu-Borges, A.L.; Oliveira-Da-silva, F.R. The Genus Radula Dumort. (Marchantiophyta: Radulaceae) in Madagascar, with a Key to the Tropical African Species. Nova Hedwig. 2022, 115, 349–382. [Google Scholar] [CrossRef]
- Bruggeman-Nannenga, M.A. Subgenus Polypodiopsis (Fissidens, Bryophyta) in Tropical Africa II. The Completely Limbate Species with Small to Medium Sized, Pluripapillose or Mammillose Laminal Cells, Including F. latelimbatus sp. nov. Lindbergia 2024, 2024, 4–19. [Google Scholar] [CrossRef]
- Järvenpää, S.; Kytöviita, M.-M.; Pitkämäki, T.; Lampinen, J. Contrasting Responses of Vascular Plants and Bryophytes to Present and Past Connectivity in Unmanaged Grasslands. Biodivers. Conserv. 2023, 32, 139–162. [Google Scholar] [CrossRef]
- Marline, L.; Ranaivoson, N.A.S.; Smith, R.J.; Ah-Peng, C.; Hedderson, T.A.; Wilding, N.; Antonelli, A. Advancing Bryophyte Research and Conservation: A Case Study on Madagascar. Ann. Bot. 2025, 136, mcaf035. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Wilding, N.; Kluge, J.; Descamps-Julien, B.; Bardat, J.; Chuah-Petiot, M.; Strasberg, D.; Hedderson, T.A. Bryophyte Diversity and Range Size Distribution along Two Altitudinal Gradients: Continent vs. Island. Acta Oecolog. 2012, 42, 58–65. [Google Scholar] [CrossRef]
- Marline, L.; Ah-Peng, C.; Hedderson, T.A.J. Epiphytic Bryophyte Diversity and Range Distributions along an Elevational Gradient in Marojejy, Madagascar. Biotropica 2020, 52, 616–626. [Google Scholar] [CrossRef]
- Marline, L.; Andriamiarisoa, R.L.; Bardat, J.; Chuah-Petiot, M. Checklist of the Bryophytes of Madagascar. Cryptogam. Bryol. 2012, 33, 199–255. [Google Scholar] [CrossRef]
- Phephu, N.; Witkowski, E.T.F.; Rooy, J.V.; Sim-Sim, M. An Updated Checklist of the Liverworts and Hornworts of Southern Africa. Phytotaxa 2025, 725, 231–270. [Google Scholar] [CrossRef]
- Malcolm, B.; Malcolm, N.; Shevock, J. New Zealand Mosses: An Il Lustrated Key; Version xii; New Zealand Plant Conservation Network: Wellington, New Zealand, 2020; Available online: https://www.nzpcn.org.nz/publications/documents/new-zealand-mosses-an-illustrated-key-version-xii-2020 (accessed on 1 October 2025).
- Thouvenot, L.; Müller, F. Contribution to the Bryophyte Flora of New Caledonia IV. Species New to the Country, New Localities Together with Taxonomic Notes. Cryptogam. Bryol. 2021, 42, 181–196. [Google Scholar] [CrossRef]
- Hedderson, T.A.; Cox, C.J.; Gibbings, J.G. Phylogenetic Relationships of the Wardiaceae (Musei); Evidence from 18s rRNA and Rps4 Gene Sequences. Bryologist 1999, 102, 26–31. [Google Scholar] [CrossRef]
- Shaw, A.J.; Goffinet, B. Bryophyte Biology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kpetikou, C.G.; Agoundé, G.; Dassou, G.H.; Salako, K.V.; Tossou, G.M.; Hedderson, T. Current Knowledge and Research Perspectives on Bryophytes in West Africa. Diversity 2025, 17, 807. https://doi.org/10.3390/d17120807
Kpetikou CG, Agoundé G, Dassou GH, Salako KV, Tossou GM, Hedderson T. Current Knowledge and Research Perspectives on Bryophytes in West Africa. Diversity. 2025; 17(12):807. https://doi.org/10.3390/d17120807
Chicago/Turabian StyleKpetikou, Chabi Ghyslain, Gafarou Agoundé, Gbèwonmèdéa Hospice Dassou, Kolawolé Valère Salako, Gbèkponhami Monique Tossou, and Terry Hedderson. 2025. "Current Knowledge and Research Perspectives on Bryophytes in West Africa" Diversity 17, no. 12: 807. https://doi.org/10.3390/d17120807
APA StyleKpetikou, C. G., Agoundé, G., Dassou, G. H., Salako, K. V., Tossou, G. M., & Hedderson, T. (2025). Current Knowledge and Research Perspectives on Bryophytes in West Africa. Diversity, 17(12), 807. https://doi.org/10.3390/d17120807






