Diversity and Environmental Challenges in the Ecuadorian Amazon: Integrating Agriculture and Conservation in the Face of Deforestation
Abstract
1. Introduction
2. Shifting Agriculture and Its Relationship to Tropical Forests
3. Divergence Between Biodiversity Conservation and Food Security in the Ecuadorian Amazon
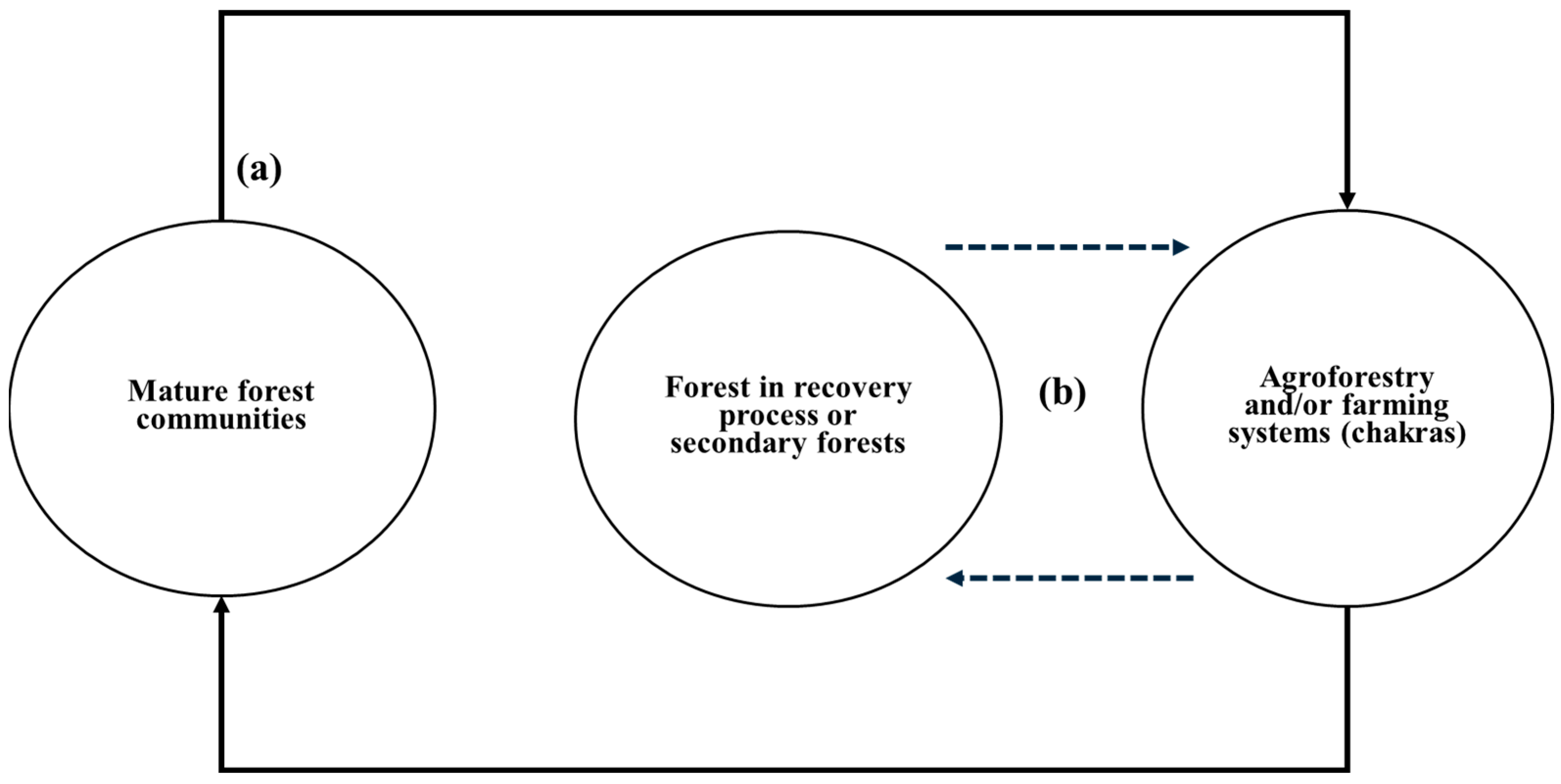
4. The Chakra System: Structure, Dynamics, and Ecological Advantages
5. Cacao Chakras: Socioeconomic and Environmental Advantages and Challenges
6. General Considerations: Advantages, Disadvantages, and Broader Implications of Chakras Agroforestry
| Ecosystem Process/Aspect | Chakra AFS | Conventional SA |
|---|---|---|
| Biodiversity | A: High floristic and structural diversity. Integrates native trees, shrubs, and crops; provides habitat continuity and promotes pollinator networks [4,61,66,71]. D: More management effort and ecological knowledge to maintain species diversity over time [60,66]. | A: Natural regeneration during fallow can restore species richness if cycles are long [33,35,36]. D: Periodic land clearing causes habitat loss; shortened fallow reduces recovery capacity [34,35,37]. |
| Soil Fertility | A: Enhance nutrient cycling and soil organic matter through; prevent erosion and nutrient leaching [55,78,81,85]. D: Decline in fertility possible under poor management or short rotations [78,86]. | A: Initial ash from burning provides temporary nutrient enrichment [34]. D: Rapid nutrient loss, erosion, and reduced fertility with repeated cultivation and shortened fallow [36,37,40]. |
| Carbon Sequestration | A: Store substantial above- and below-ground carbon, comparable to secondary forests [30,31,73]. D: Lower carbon storage when large trees are removed during establishment [74]. | A: Temporary carbon storage during regrowth phases [33,73]. D: Carbon released during burning; reduced recovery under shorter cycles [91,93]. |
| Water Regulation | A: Maintain soil moisture, reduce runoff, and enhance infiltration via multi-layer canopy and root diversity [55,98]. D: Water use may increase under high-density planting of shade trees [55,99]. | A: Partial hydrological recovery possible during extended fallow [33]. D: Deforestation increases runoff, sedimentation, and watershed degradation [4,87]. |
| Climate Regulation | A: Moderate local temperature and humidity through shading and evapotranspiration; reduce greenhouse gas emissions by maintaining tree cover [30,31,76]. | D: Short-term emissions during biomass decomposition and burning; contribute to microclimatic warming and variability [87,89]. |
| Food Security & Livelihood | A: Provide diverse food and income sources (cacao, cassava, maize, fruits); improve resilience to market or climatic fluctuations [62,63,96,97]. D: Depend on stable markets and labor availability; profitability can vary with prices [97,103]. | A: Support subsistence food production during cultivation period [33,40]. D: Limited long-term income; vulnerable to yield decline and climatic variability [38]. |
| Cultural & Social Value | A: Preserve Indigenous ecological knowledge, cultural identity, and traditional land stewardship (e.g., Kichwa chakras) [60,61,66,99]. D: Transmission of knowledge depends on generational continuity and community cohesion [60,102]. | A: May maintain some traditional practices if managed communally [33]. D: Often driven by short-term needs; loss of Indigenous management systems [104,105]. |
| Land Use Pressure | A: Reduce expansion into primary forests by maintaining long-term productive plots; optimize use of cleared land [66,75]. D: Require secure land tenure and long-term planning [104,105] | A: Can support small populations sustainably when cycles are long [34,35]. D: Major driver of deforestation when population or market pressure shortens cycles [54,55,56]. |
| Resilience to Disturbance | A: High ecological resilience due to multi-strata vegetation and soil protection [4,66,78]. | D: Low resilience under intensive use; repeated clearing degrades ecosystem recovery [34,37,79]. |
| Overall Limitations | Depend on secure land tenure, local labor, and stable markets (e.g., fair-trade initiatives such as Kallari) for long-term viability [76,103]. | Rapid soil degradation, deforestation, and declining yields under intensified cycles [78,79,80]. |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pacifici, M.; Visconti, P.; Butchart, S.H.; Watson, J.E.; Cassola, F.M.; Rondinini, C. Species’ traits influenced their response to recent climate change. Nat. Clim. Change 2017, 7, 205–208. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Ulrich, W.; Batáry, P.; Baudry, J.; Beaumelle, L.; Bucher, R.; Čerevková, A.; Felipe-Lucia, M.R.; Gallé, R.; Kesse-Guyot, E.; et al. From functional diversity to human well-being: A concepttual framework for agroecosystem sustainability. Agric. Syst. 2023, 208, 103659. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Global Forest Resources Assessment 2020: Key Findings. 2020. Available online: http://www.fao.org/3/CA8753EN/CA8753EN.pdf (accessed on 15 October 2025).
- Porro, R.; Miller, R.P.; Tito, M.R.; Donovan, J.A.; Vivan, J.L.; Trancoso, R.; Van Kanten, R.F.; Grijalva, J.E.; Ramirez, B.L.; Gonçalves, A.L. Agroforestry—The Future of Global Land Use; Nair, P.K.R., Garrity, D., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Homeier, J.; Werner, F.A.; Gawlik, J.; Peters, T.; Diertl, K.H.J.; Richter, M. Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador. In Plant Diversity and Its Relevance for the Provision of Ecosystem Services; Bendix, J., Beck, E., Bräuning, A., Makeschin, F., Mosandl, R., Scheu, S., Wilcke, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 93–106. [Google Scholar]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; de la Cruz, M. Deforestation and Forest Fragmentation in South Ecuador since the 1970s–Losing a Hotspot of Biodiversity. PLoS ONE 2015, 10, e0133701. [Google Scholar] [CrossRef]
- Esquivel, M.J.; Vilchez-Mendoza, S.; Harvey, C.A.; Ospina, M.A.; Somarriba, E.; Deheuvels, O.; Filho, E.d.M.V.; Haggar, J.; Detlefsen, G.; Cerdan, C.; et al. Patterns of shade plant diversity in four agroforestry systems across Central America: A meta-analysis. Sci. Rep. 2023, 13, 8538. [Google Scholar] [CrossRef] [PubMed]
- Visscher, A.M.; Meli, P.; Fonte, S.J.; Bonari, G.; Zerbe, S.; Wellstein, C. Agroforestry enhances biological activity, diversity and soil-based ecosystem functions in mountain agroecosystems of Latin America: A meta-analysis. Glob. Change Biol. 2024, 30, e17036. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, M.; Tobin, P.C.; McGuire, A.D.; Reich, P.B. Biodiversity influences plant productivity through niche–efficiency. Proc. Natl. Acad. Sci. USA 2015, 112, 5738–5743. [Google Scholar] [CrossRef]
- Sari, R.R.; Priyadarshini, R.; Rozendaal, D.M.; Saputra, D.D.; Hairiah, K.; Van Noordwijk, M. Tree diversity and social–ecological resilience of agroforestry after volcanic ash deposition in Indonesia. Sustain. Sci. 2023, 18, 2735–2753. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; and Woodfin, R.M. Declining biodiversity can alter the performance of ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Tilman, D. Distinguishing between the effects of species diversity and species composition. Oikos 1997, 80, 185. [Google Scholar] [CrossRef]
- Hector, A. Diversity favours productivity. Nature 2011, 472, 45–46. [Google Scholar] [CrossRef]
- Coelho, A.J.P.; Teixeira, H.M.; Verweij, P.; Matos, F.A.R.; Villa, P.M.; Meira-Neto, J.A.A. Functional richness mediates landscape and management effects on tree biomass and soil fertility during secondary forest succession. Ecol. Indic. 2024, 162, 112029. [Google Scholar] [CrossRef]
- Isaac, M.E.; Gagliardi, S.; Ordoñez, J.C.; Sauvadet, M. Shade tree trait diversity and functions in agroforestry systems: A review of which traits matter. J. Appl. Ecol. 2024, 61, 1159–1173. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Ammer, C. Diversity and forest productivity in a changing climate. New Phytol. 2019, 221, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Heredia-R, M.; Torres, B.; Cayambe, J.; Ramos, N.; Luna, M.; Diaz-Ambrona, C.G. Sustainability assessment of smallholder agroforestry indigenous farming in the Amazon: A case study of Ecuadorian Kichwas. Agronomy 2020, 10, 1973. [Google Scholar] [CrossRef]
- Duivenvoorden, J.F.; Svenning, J.C.; Wright, S.J. Beta diversity in tropical forests. Science 2002, 295, 636–637. [Google Scholar] [CrossRef]
- Bhat, Y.; Nandy, S.; Das, K.; Tamang, M.; Padalia, H.; Nath, A.J.; Majumdar, K.; Pebam, R.; Thongni, P.; Kurmi, B.; et al. Vegetation disturbance and regrowth dynamics in shifting cultivation landscapes. Sci. Rep. 2024, 14, 28324. [Google Scholar] [CrossRef]
- MEA. Millennium Ecosystem Assessment. In Ecosystems and Human Well-being: Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Polania, C.; Pla, L.; Casanoves, F. Diversidad Funcional y Servicios Ecosistémicos; Valoración y Análisis de la Diversidad Funcional y su Relación con los Servicios Ecosistémicos; Casanoves, F., Pla, L., Di Rienzo, J., Eds.; CATIE: Cartago, Costa Rica, 2011; pp. 5–8. [Google Scholar]
- Mathieu, A.; Martin-Guay, M.O.; Rivest, D. Enhancement of Agroecosystem Multifunctionality by Agroforestry: A Global Quantitative Summary. Glob. Change Biol. 2025, 31, e70234. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Wardle, D.A. Is “sampling effect” a problem for experiments investigating biodiversity-ecosystem function relationships? Oikos 1999, 87, 403–407. [Google Scholar] [CrossRef]
- Gasparatos, A.; Stromberg, P.; Takeuchi, K. Biofuels, ecosystem services and human wellbeing: Putting biofuels in the ecosystem services narrative. Agric. Ecosyst. Environ. 2011, 142, 111–128. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Lopez-Piñeiro, A.; Torres, B.; Bravo-Medina, C. Biodiversity and carbon sequestration in chakra-type agroforestry systems and humid tropical forests of the Ecuadorian Amazon. Forests 2024, 15, 557. [Google Scholar] [CrossRef]
- Álava-Núñez, P.; Torres, B.; Castro, M.; Robles, M. AGB carbon stock analysis in the Indigenous agroforestry of the Ecuadorian Amazon: Chakra and Aja as Natural Climate Solutions. Front. For. Glob. Change 2025, 8, 1513140. [Google Scholar] [CrossRef]
- Loreau, M. Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos 2000, 91, 3–17. [Google Scholar] [CrossRef]
- Kapp, G.; Manning, D.B. Land Management Systems at the Interface Between Forestry and Agriculture; Forests and Rural Development; Pretzsch, J., Darr, D., Uibrig, H., Auch, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 85–110. [Google Scholar]
- Delang, C.O.; Li, W.M. Ecological Succession on Fallowed Shifting Cultivation Fields: A Review of the Literature; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Uhl, C. Factors controlling succession following slash-and-burn agriculture in Amazonia. J. Ecol. 1987, 75, 377–407. [Google Scholar] [CrossRef]
- Kennard, D.K. Secondary forest succession in a tropical dry forest: Patterns of development across a 50-year chronosequence in lowland Bolivia. J. Trop. Ecol. 2002, 18, 53–66. [Google Scholar] [CrossRef]
- Nye, P.H.; Greenland, D.J. The Soil Under Shifting Cultivation; Technical Communication; Commonwealth Agricultural Bureaux: London, UK, 1960. [Google Scholar]
- Mertz, O. The relationship between length of fallow and crop yields in shifting cultivation: A rethinking. Agrofor. Syst. 2002, 55, 149–159. [Google Scholar] [CrossRef]
- Dalle, S.P.; de Blois, S. Shorter fallow cycles affect the availability of noncrop plant resources in a shifting cultivation system. Ecol. Soc. 2006, 11, 1–26. [Google Scholar] [CrossRef]
- Hoffmann, U. Agriculture at the crossroads: Assuring food security in developing countries under the challenge of global warming. Trade Environ. Rev. 2013, 1, 2–8. [Google Scholar]
- Guiracocha, G.; Harvey, C.; Somarriba, E.; Krauss, U.; Carrillo, E. Conservación de la biodiversidad en sistemas agroforestales con cacao y banano en Talamanca, Costa Rica. Agroforestería En. Las. Américas 2001, 8, 7–11. [Google Scholar]
- Ashley, R.; Russell, D.; and Swallow, B. The policy terrain in protected area landscapes: Challenges for agroforestry in integrated landscape conservation. Biodivers. Conserv. 2006, 15, 663–689. [Google Scholar] [CrossRef]
- ECLAC. Economic Commission for Latin America and the Caribbean. Poverty in Latin America Remained Steady in 2017, but Extreme Poverty Increased to the Highest Level Since 2008, While Inequality Has Fallen Notably Since 2000. 2019. Available online: https://www.cepal.org/en/pressreleases/poverty-latin-america-remained-steady-2017-extreme-poverty-increased-highest-level (accessed on 15 October 2025).
- Reitsma, R.; Parrish, J.D.; McLarney, W. The role of cacao plantations in maintaining forest avian diversity in southeastern Costa Rica. Agrofor. Syst. 2001, 53, 185–193. [Google Scholar] [CrossRef]
- Schroth, G.; Harvey, C.A.; da Fonseca, G.A.; Vasconcelos, H.L.; Gascon, C.; Izac, A.M.N. (Eds.) Agroforestry and Biodiversity Conservation in Tropical Landscapes; Island Press: London, UK, 2004. [Google Scholar]
- Harvey, C.A.; Gonzalez, J.; and Somarriba, E. Dung beetle and terrestrial mammal diversity in forests, indigenous agroforestry systems and plantain monocultures in Talamanca, Costa Rica. Biodivers. Conserv. 2006, 15, 555–585. [Google Scholar] [CrossRef]
- Sierra, R.; Campos, F.; and Chamberlin, J. Assessing biodiversity conservation priorities: Ecosystem risk and representativeness in continental Ecuador. Landsc. Urban. Plan. 2002, 59, 95–110. [Google Scholar] [CrossRef]
- Finer, M.; Jenkins, C.N.; Pimm, S.L.; Keane, B.; Ross, C. Oil and gas projects in the western Amazon: Threats to wilderness, biodiversity, and indigenous peoples. PLoS ONE 2008, 3, e2932. [Google Scholar] [CrossRef]
- Bass, M.S.; Finer, M.; Jenkins, C.N. Global conservation significance of Ecuador’s Yasuní National Park. PLoS ONE 2010, 5, e8767. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.C.; Hernández, C.; Romoleroux, K.; Losos, E.; Magard, E.; and Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Vallejo, M.I.; Samper, C.; Mendoza, H.; Otero, J.T. La Planada Forest Dynamics Plot, Colombia. In Tropical Forest Diversity and Dynamism: Findings from a Large-Scale Plot Network; Losos, E.C., Leigh, E.G., Jr., Eds.; University of Chicago Press: Chicago, IL, USA, 2004; pp. 517–526. [Google Scholar]
- Bunyavejchewin, S.; Baker, P.J.; LaFrankie, J.V.; Ashton, P.S. HuaiKha Khaeng Forest Dynamics Plot, Thailand. In Tropical Forest Diversity and Dynamism: Findings from a Large-Scale Plot Network; Losos, E.C., Leigh, E.G., Jr., Eds.; University of Chicago Press: Chicago, IL, USA, 2004; pp. 482–491. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Nepstad, D.; Schwartzman, S.; Bamberger, B.; Santilli, M.; Ray, D.; Schlesinger, P.; Lefebvre, P.; Alencar, A.; Prinz, E.; Fiske, G.; et al. Inhibition of Amazon deforestation and fire by parks and indigenous lands. Conserv. Biol. 2006, 20, 65–73. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015: How Are the World’s Forests Changing? Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/3/a-i4808e.pdf (accessed on 15 October 2025).
- Mena, C.F. Trajectories of Land-use and Land-cover in the Northern Ecuadorian Amazon. Photogramm. Eng. Remote Sens. 2008, 74, 737–751. [Google Scholar] [CrossRef]
- Veas, N.; Moncayo, P. Mapa de Carbón. In Evaluación Forestal Nacional-Resultados; Ministerio del Ambiente: Quito, Ecuador, 2014; pp. 127–143. [Google Scholar]
- MAE. Áreas Protegidas del Ecuador Socio Estratégico para el Desarrollo. Ministerio del Ambiente. 2016. Available online: https://www.scribd.com/document/471108580/Areas-protegidas-del-Ecuador-socio-estrategico-para-el-desarrollo (accessed on 15 September 2025).
- INEC. Instituto Nacional de Estadística y Censos. Censo de Población y Vivienda 2010. 2010. Available online: http://www.ecuadorencifras.gob.ec/ (accessed on 10 October 2025).
- Arévalo, V. Chakras, Bosques y Ríos: El Entramado de la Biocultura Amazónica; Editorial Abya-Yala: Quito, Ecuador, 2009. [Google Scholar]
- Perreault, T. Why chacras (swidden gardens) persist: Agrobiodiversity, food security, and cultural identity in the Ecuadorian Amazon. Hum. Organ. 2005, 64, 327–339. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Schroth, G.; Harvey, C.A. Biodiversity conservation in cocoa production landscapes: An overview. Biodivers. Conserv. 2007, 16, 2237–2244. [Google Scholar] [CrossRef]
- Cerda, R.; Deheuvels, O.; Calvache, D.; Niehaus, L.; Saenz, Y.; Kent, J.; Vilchez, S.; Villota, A.; Martinez, C.; Somarriba, E. Contribution of cocoa agroforestry systems to family income and domestic consumption: Looking toward intensification. Agrofor. Syst. 2014, 88, 957–981. [Google Scholar] [CrossRef]
- Abebe, T. Diversity in Homegarden Agroforestry Systems of Southern Ethiopia. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005. [Google Scholar]
- Vera, V.R.R.; Cota-Sánchez, J.H.; Grijalva Olmedo, J.E. Biodiversity, dynamics, and impact of chakras on the Ecuadorian Amazon. J. Plant Ecol. 2019, 12, 34–44. [Google Scholar] [CrossRef]
- Valencia, R.; Balslev, H.; Miño, G.P.Y. High tree alpha-diversity in Amazonian Ecuador. Biodivers. Conserv. 1994, 3, 21–28. [Google Scholar] [CrossRef]
- Palacio, W.; Jaramillo, N. Riqueza florística y forestal de los bosques tropicales húmedos del Ecuador e implicaciones para su manejo. Recur. Nat. Y Ambiente 2001, 36, 46–50. [Google Scholar]
- Asase, A.; Tetteh, D.A. The role of complex agroforestry systems in the conservation of forest tree diversity and structure in southeastern Ghana. Agrofor. Syst. 2010, 79, 355–368. [Google Scholar] [CrossRef]
- Ramírez-Meneses, A.; García-López, E.; Obrador-Olán, J.J.; Ruiz-Rosado, O.; Camacho-Chiu, W. Diversidad florística en plantaciones agroforestales de cacao en Cárdenas, Tabasco, México. Ecosistemas Y Recur. Agropecu. 2014, 29, 215–230. [Google Scholar]
- Jones, K. Review of sangre de drago (Croton lechleri)-a South American tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: Traditional uses to clinical research. J. Altern. Complement. Med. 2003, 9, 877–896. [Google Scholar] [CrossRef]
- Suarez, S.A. Diet and travel costs for spider monkeys in a nonseasonal, hyperdiverse environment. Int. J. Primatol. 2006, 27, 411–436. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: Legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Letcher, S.G.; van Breugel, M.; Martínez-Ramos, M.; Bongers, F.; Finegan, B. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Jadán, O.; Torres, B.; Selesi, D.; Peña, D.; Rosales, C.; Günter, S. Diversidad florística y estructura en cacaotales tradicionales y bosque natural (Sumaco, Ecuador). Colomb. For. 2016, 19, 5–18. [Google Scholar] [CrossRef][Green Version]
- Torres, B.; Maza, O.J.; Aguirre, P.; Hinojosa, L.; Günter, S. Contribution of Traditional Agroforestry to Climate Change Adaptation in the Ecuadorian Amazon: The Chakra System. In Handbook of Climate Change Adaptation; Filho, W.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1973–1994. [Google Scholar][Green Version]
- Loo, L.C.; Song, G.Z.M.; Chao, K.J. Characteristics of tropical human-modified forests after 20 years of natural regeneration. Bot. Stud. 2017, 58, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Vera-Vélez, R.; Grijalva, J.; Cota-Sánchez, J.H. Cocoa agroforestry and tree diversity in relation to past land use in the Northern Ecuadorian Amazon. New For. 2019, 50, 891–910. [Google Scholar] [CrossRef]
- Gaglio, M.; Aschonitis, V.G.; Mancuso, M.M.; Puig, J.P.R.; Moscoso, F.; Castaldelli, G.; Fano, E.A. Changes in land use and ecosystem services in tropical forest areas: A case study in Andes mountains of Ecuador. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 13, 264–279. [Google Scholar] [CrossRef]
- Zurita-Benavides, M.G. Cultivando las plantas y la sociedad waorani. Boletim do Museu Paraense Emílio Goeldi. Ciências Humanas 2018, 12, 495–516. [Google Scholar]
- Jakovac, C.C.; Peña-Claros, M.; Mesquita, R.C.; Bongers, F.; Kuyper, T.W. Swiddens under transition: Consequences of agricultural intensification in the Amazon. Agric. Ecosyst. Environ. 2016, 218, 116–125. [Google Scholar] [CrossRef]
- Cairns, M.F. Shifting Cultivation and Environmental Change: Indigenous People, Agriculture and Forest Conservation; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Legendre, P.; Borcard, D.; Peres-Neto, P.R. Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr. 2005, 75, 435–450. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef]
- Russell, A.; Kivlin, S.; Hawkes, C. Tropical tree species effects on soil pH and biotic factors and the consequences for macroaggregate dynamics. Forests 2018, 9, 184. [Google Scholar] [CrossRef]
- Powers, J.S.; Kalicin, M.H.; Newman, M.E. Tree species do not influence local soil chemistry in a species-rich Costa Rica rain forest. J. Trop. Ecol. 2004, 20, 587–590. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Lefebvre, P.; da Silva, U.L.; Tomasella, J.; Schlesinger, P.; Solórzano, L.; Moutinho, P.; Ray, D.; Benito, J. Amazon drought and its implications for forest flammability and tree growth: A basin-wide analysis. Glob. Change Biol. 2004, 10, 704–717. [Google Scholar] [CrossRef]
- Nobre, C.A.; Borma, L.D.S. Tipping points’ for the Amazon forest. Curr. Opin. Environ. Sustain. 2009, 1, 28–36. [Google Scholar] [CrossRef]
- Malhi, Y.; Aragão, L.E.; Galbraith, D.; Huntingford, C.; Fisher, R.; Zelazowski, P.; Sitch, S.; McSweeney, C.; Meir, P. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl. Acad. Sci. USA 2009, 106, 20610–20615. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, M.A.; Barber, C.P. Climate change, human land use and future fires in the Amazon. Glob. Change Biol. 2009, 15, 601–612. [Google Scholar] [CrossRef]
- Silva, J.M.N.; Carreiras, J.M.B.; Rosa, I.; Pereira, J.M.C. Greenhouse gas emissions from shifting cultivation in the tropics, including uncertainty and sensitivity analysis. J. Geophys. Res. Atmos. 2011, 116, 1–21. [Google Scholar] [CrossRef]
- Ayanu, Y.Z.; Nguyen, T.T.; Marohn, C.; Koellner, T. Crop production versus surface-water regulation: Assessing tradeoffs for land-use scenarios in the Tat Hamlet Watershed, Vietnam. International Journal of Biodiversity Science, Ecosystem Services and Management 2011, 7, 231–244. [Google Scholar] [CrossRef]
- Ellen, R. Studies of swidden agriculture in Southeast Asia since 1960: An overview and commentary on recent research and syntheses. Asia Pac. World 2012, 3, 18–38. [Google Scholar] [CrossRef]
- Heinimann, A.; Mertz, O.; Frolking, S.; Christensen, A.E.; Hurni, K.; Sedano, F.; Chini, L.P.; Sahajpal, R.; Hansen, M.; Hurtt, G. A global view of shifting cultivation: Recent, current, and future extent. PLoS ONE 2017, 12, eD184479. [Google Scholar]
- Henley, D. Swidden farming as an agent of environmental change: Ecological myth and historical reality in Indonesia. Environ. Hist. 2011, 17, 525–554. [Google Scholar] [CrossRef]
- Watanabe, R. Analyzing Food Sources and Food Insecurity of Kichwa Farming Families in the Ecuadorian Amazon. Bachelor’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2024. [Google Scholar]
- Torres, B.; Luna, M.; Tipán-Torres, C.; Ramírez, P.; Muñoz, J.C.; García, A. A simplified integrative approach to assessing productive sustainability and livelihoods in the “amazonian chakra” in Ecuador. Land 2024, 13, 2247. [Google Scholar] [CrossRef]
- zur Lage, R.B.; Peña-Claros, M.; Rios, M. Management of trees and palms in swidden fallows by the Kichwa people in the Ecuadorian Amazon. Environ. Dev. 2023, 46, 100855. [Google Scholar] [CrossRef]
- Warren-Thomas, E.; Nelson, L.; Juthong, W.; Bumrungsri, S.; Brattström, O.; Stroesser, L.; Chambon, B.; Penot, É.; Tongkaemkaew, U.; Edwards, D.P.; et al. Rubber agroforestry in Thailand provides some biodiversity benefits without reducing yields. J. Appl. Ecol. 2020, 57, 17–30. [Google Scholar]
- Gunawan, H.; Yeny, I.; Karlina, E.; Suharti, S.; Murniati; Subarudi; Mulyanto, B.; Ekawati, S.; Garsetiasih, R.; Pratiwi; et al. Integrating social forestry and biodiversity conservation in Indonesia. Forests 2022, 13, 2152. [Google Scholar] [CrossRef]
- Salafsky, N. Forest gardens in the Gunung Palung region of West Kalimanta, Indonesia: Defining a locally-developed, market-oriented agroforestry system. Agrofor. Syst. 1994, 28, 237–268. [Google Scholar] [CrossRef]
- Wiegel, J.; Del Río, M.; Gutiérrez, J.F.; Claros, L.; Sánchez, D.; Gómez, L.; González, C.; Reyes, B. Coffee and cacao market systems in the Americas: Opportunities for supporting renovation and rehabilitation. Int. Cent. Trop. Agric. 2020. Available online: https://hdl.handle.net/10568/108108 (accessed on 15 October 2025).
- Kallari. KALLARI. 2024. Available online: https://kallari.com.ec/ (accessed on 31 October 2025).
- Padoch, C.; Sunderland, T. Managing landscapes for greater food security and improved livelihoods. Unasylva 2013, 64, 3–13. [Google Scholar]
- Montagnini, F.; Nair, P.R. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2024, 61, 281–295. [Google Scholar]
- Van Noordwijk, M.; Ekadinata, A.; Leimona, B.; Catacutan, D.; Martini, E.; Tata, H.L.; Öborn, I.; Hairiah, K.; Wangpakapattanawong, P.; Mulia, R.; et al. Agroforestry options for degraded landscapes in Southeast Asia. In Agroforestry for Degraded Landscapes: Recent Advances and Emerging Challenges; Springer: Singapore, 2020; Volume 1, pp. 307–347. [Google Scholar]
- Coq-Huelva, D.; Higuchi, A.; Alfalla-Luque, R.; Burgos-Morán, R.; Arias-Gutiérrez, R. Co-evolution and bio-social construction: The Kichwa agroforestry systems (chakras) in the Ecuadorian Amazonia. Sustainability 2017, 9, 1920. [Google Scholar] [CrossRef]
- Corna, S. Indigenous Agroforestry Systems Empowerment as Participatory Sustainable Development Strategy for the Amazon Basin: Chakra Kichwa Amazónica of Ecuador. Master’s Thesis, Universidade NOVA de Lisboa, Lisboa, Portugal, 2022. [Google Scholar]
- Pinheiro Edelstein, C. Effect of functional diversity on ecosystem services in cocoa agroforestry systems. Maest. En. Agroforestería Y Agric. Sosten. 2023. [Google Scholar] [CrossRef]
- Swamy, L.; Drazen, E.; Johnson, W.R.; Bukoski, J.J. The future of tropical forests under the United Nations Sustainable Development Goals. J. Sustain. For. 2018, 37, 221–256. [Google Scholar] [CrossRef]
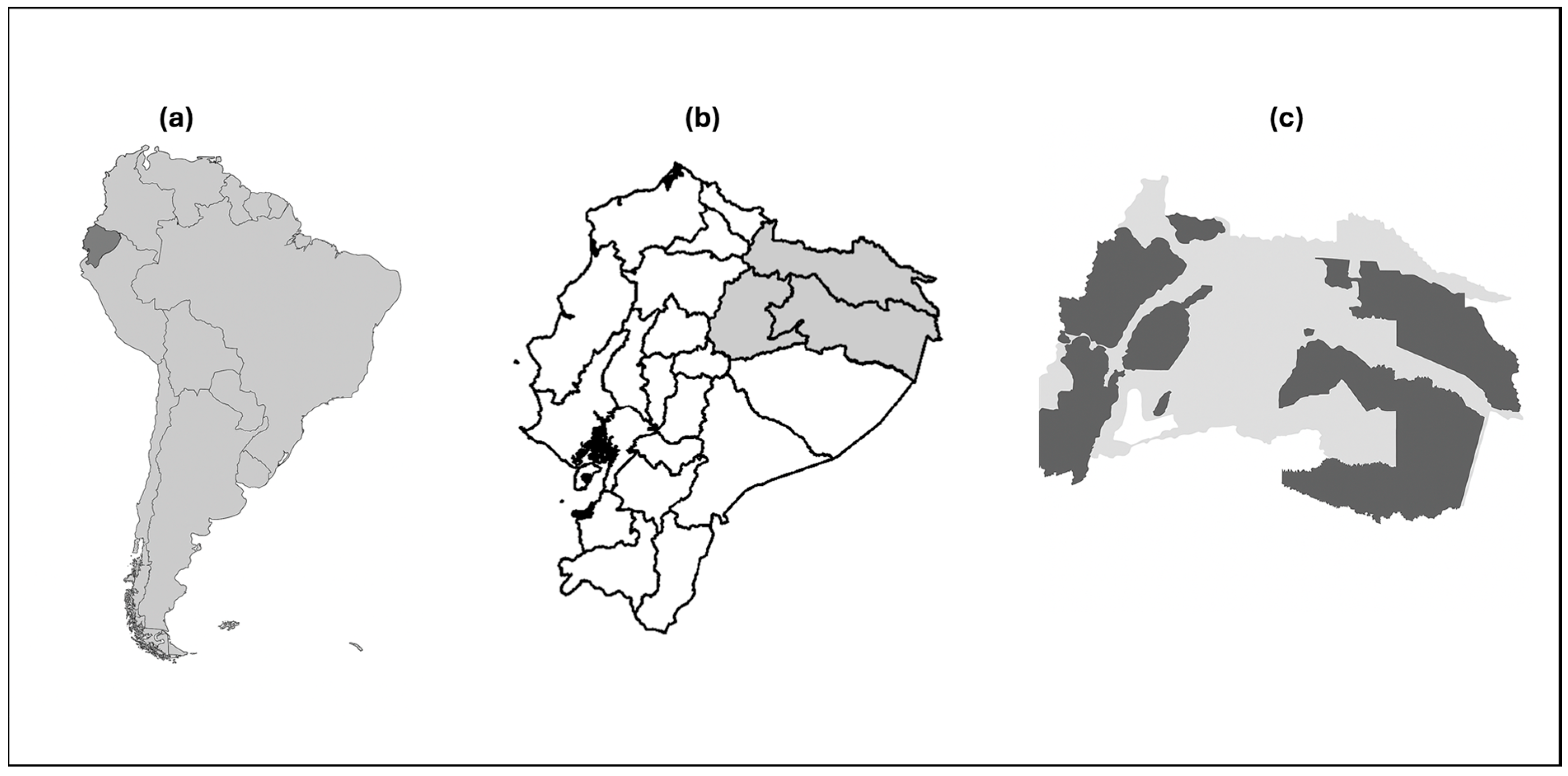

| Organisms | # of Species | Amazon Basin (%) |
|---|---|---|
| Area of the NEA * | 9820 km2 * | 0.15% * |
| Amphibians | 150 | 28% |
| Reptiles | 121 | 33% |
| Birds | 596 | 34% |
| Mammals | 169–204 | 27–33% |
| Fish | 382–499 | 12–16% |
| Vascular plants | 2704–4000 | 7–10% |
| Land Use | Total Area (ha) | Northern EA (%) | Southern EA (%) |
|---|---|---|---|
| Cocoa | 58,965 | 95.4 | 4.6 |
| Coffee | 22,164 | 95.3 | 4.7 |
| Plantain | 25,380 | 59.5 | 40.5 |
| Corn | 21,534 | 90.8 | 9.2 |
| Cassava | 9386 | 30.0 | 70.0 |
| Grasslands | 361,730 | 39.9 | 60.1 |
| Forest | 2,911,341 | 46.7 | 53.3 |
| Fallow | 7370 | 84.8 | 15.2 |
| Moors | 55,938 | 57.1 | 42.9 |
| Other | 154,048 | 57.1 | 42.9 |
| Total | 3,627,856 | 46 | 54 |
| Components | Description |
|---|---|
| Main crops cultivated | Cacao, cassava, maize, rice, and other subsistence crops |
| Tree management | Multi-species canopy structure with varying shade levels |
| Technology used | Traditional practices; reliance on small tools |
| Labor force | Family-based, individual and household members |
| Fertilization | None |
| Pesticide use | None or minimal |
| Weed management | Manual |
| Producer profile | Small-scale Kichwa family |
| Production objective | Personal food and income resource |
| Production management | Communities |
| System | # Plant Families | # Genera | # Species |
|---|---|---|---|
| Manihot esculenta (cassava) | 18 | 20 | 20 ± 1.54 |
| Zea mays (corn) | 21 | 30 | 32 ± 2.12 |
| Theobroma cacao (cocoa) | 33 | 57 | 62 ± 2.37 |
| Secondary forest | 31 | 52 | 54 ± 1.79 |
| Mature forest | 38 | 74 | 81 ± 1.48 |
| Total * | 43 | 96 | 109 ± 9.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Velez, R.; Ramos-Veintimilla, R. Diversity and Environmental Challenges in the Ecuadorian Amazon: Integrating Agriculture and Conservation in the Face of Deforestation. Diversity 2025, 17, 792. https://doi.org/10.3390/d17110792
Vera-Velez R, Ramos-Veintimilla R. Diversity and Environmental Challenges in the Ecuadorian Amazon: Integrating Agriculture and Conservation in the Face of Deforestation. Diversity. 2025; 17(11):792. https://doi.org/10.3390/d17110792
Chicago/Turabian StyleVera-Velez, Roy, and Raúl Ramos-Veintimilla. 2025. "Diversity and Environmental Challenges in the Ecuadorian Amazon: Integrating Agriculture and Conservation in the Face of Deforestation" Diversity 17, no. 11: 792. https://doi.org/10.3390/d17110792
APA StyleVera-Velez, R., & Ramos-Veintimilla, R. (2025). Diversity and Environmental Challenges in the Ecuadorian Amazon: Integrating Agriculture and Conservation in the Face of Deforestation. Diversity, 17(11), 792. https://doi.org/10.3390/d17110792






