Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys Polyactis) in the Southern Yellow Sea
Abstract
1. Introduction
2. Materials and Methods
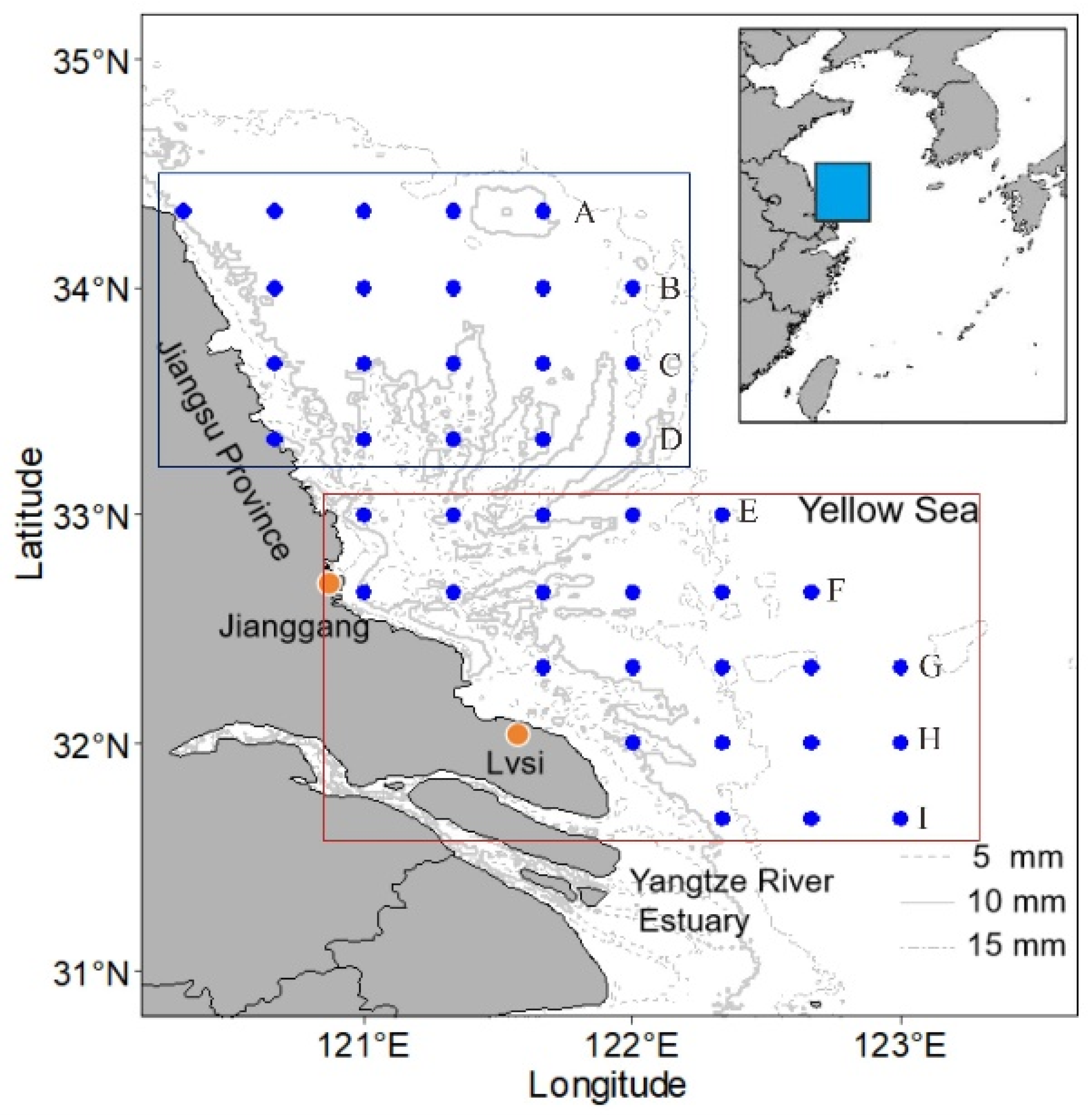
2.1. Study Area
2.2. Data Sampling and Processing
2.3. Data Analysis
2.3.1. Statistical Model
2.3.2. Model Adequacy Diagnosis and Selection
3. Results
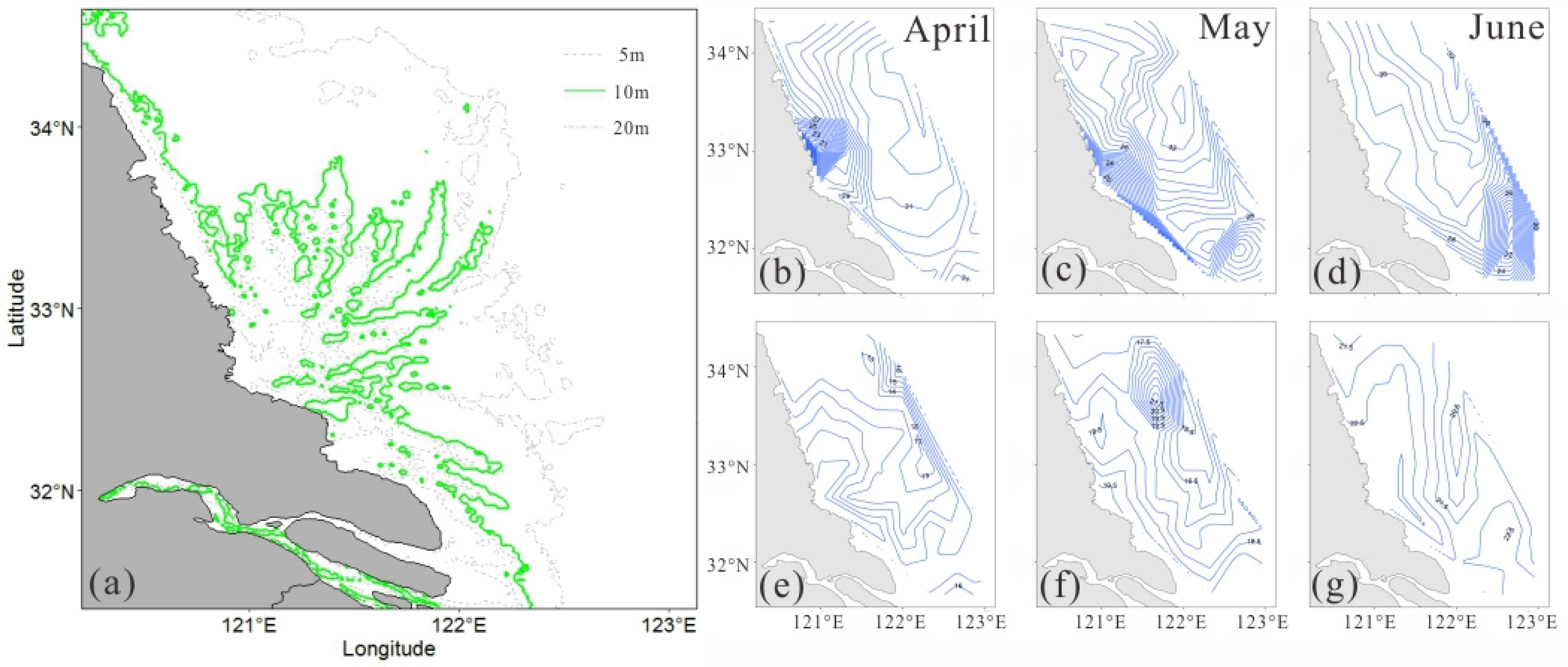
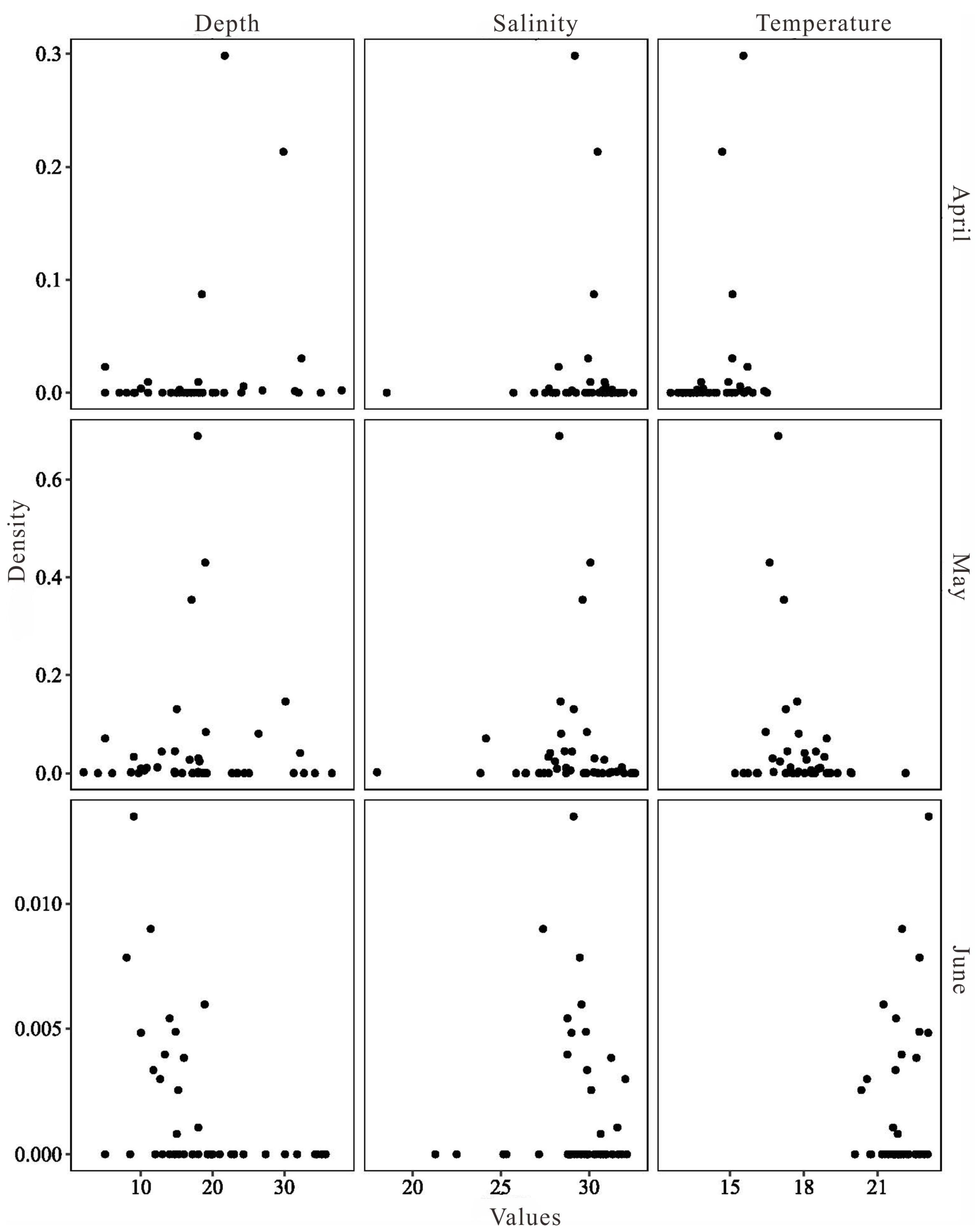
3.1. Characteristics of Depth, Temperature, and Salinity
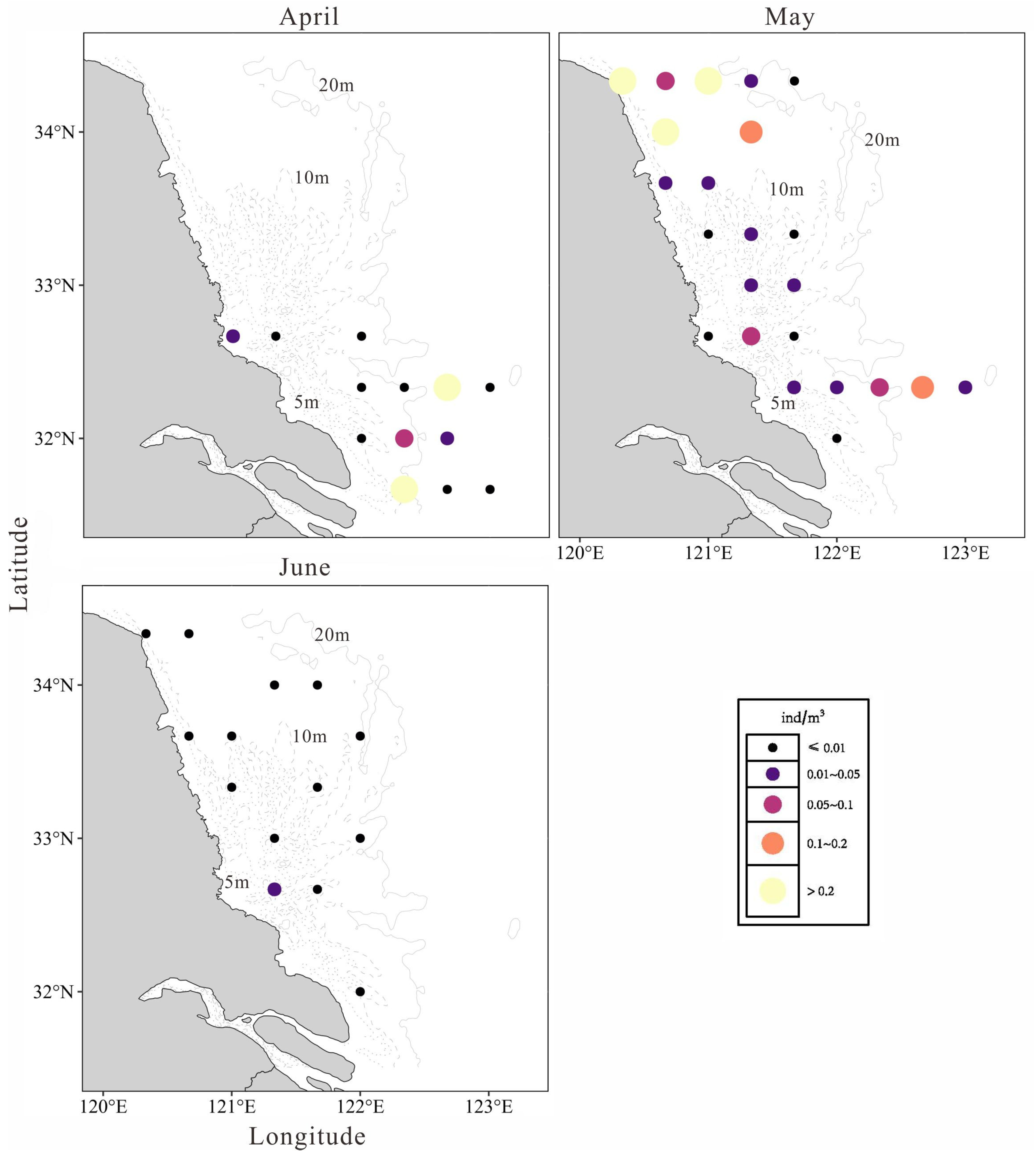
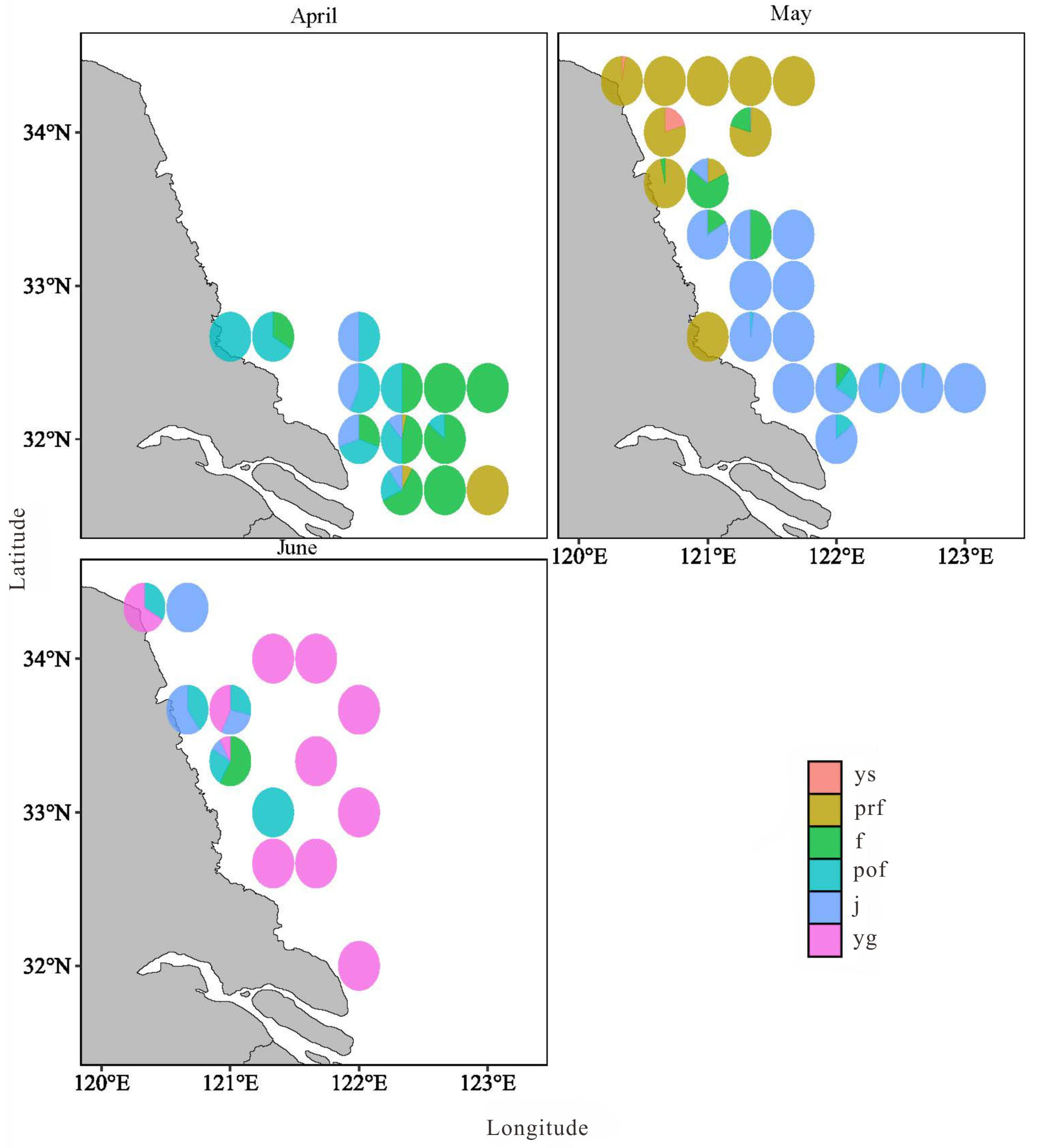
3.2. Temporal and Spatial Distribution
3.3. Species Distribution Model
3.3.1. Model Evaluation
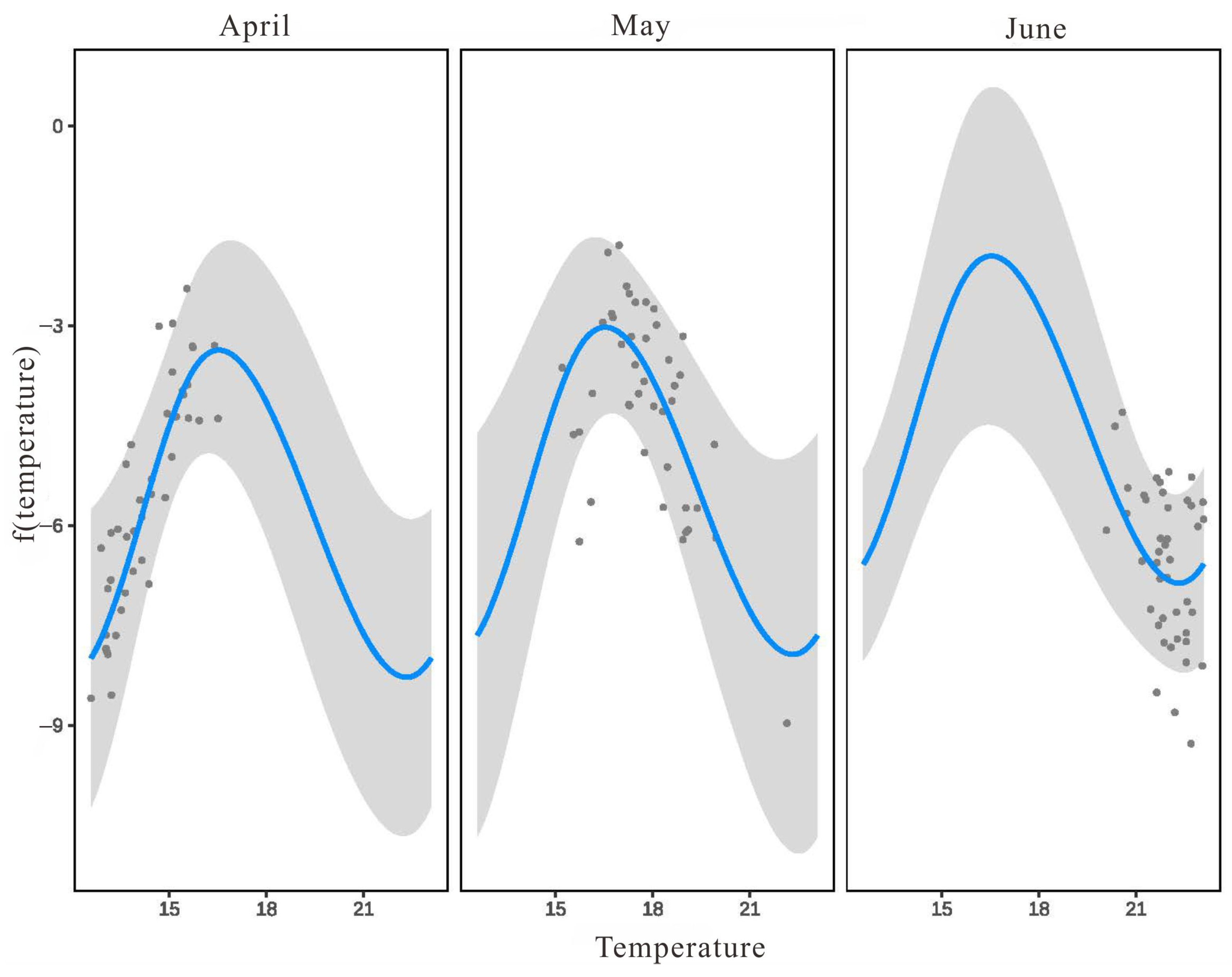
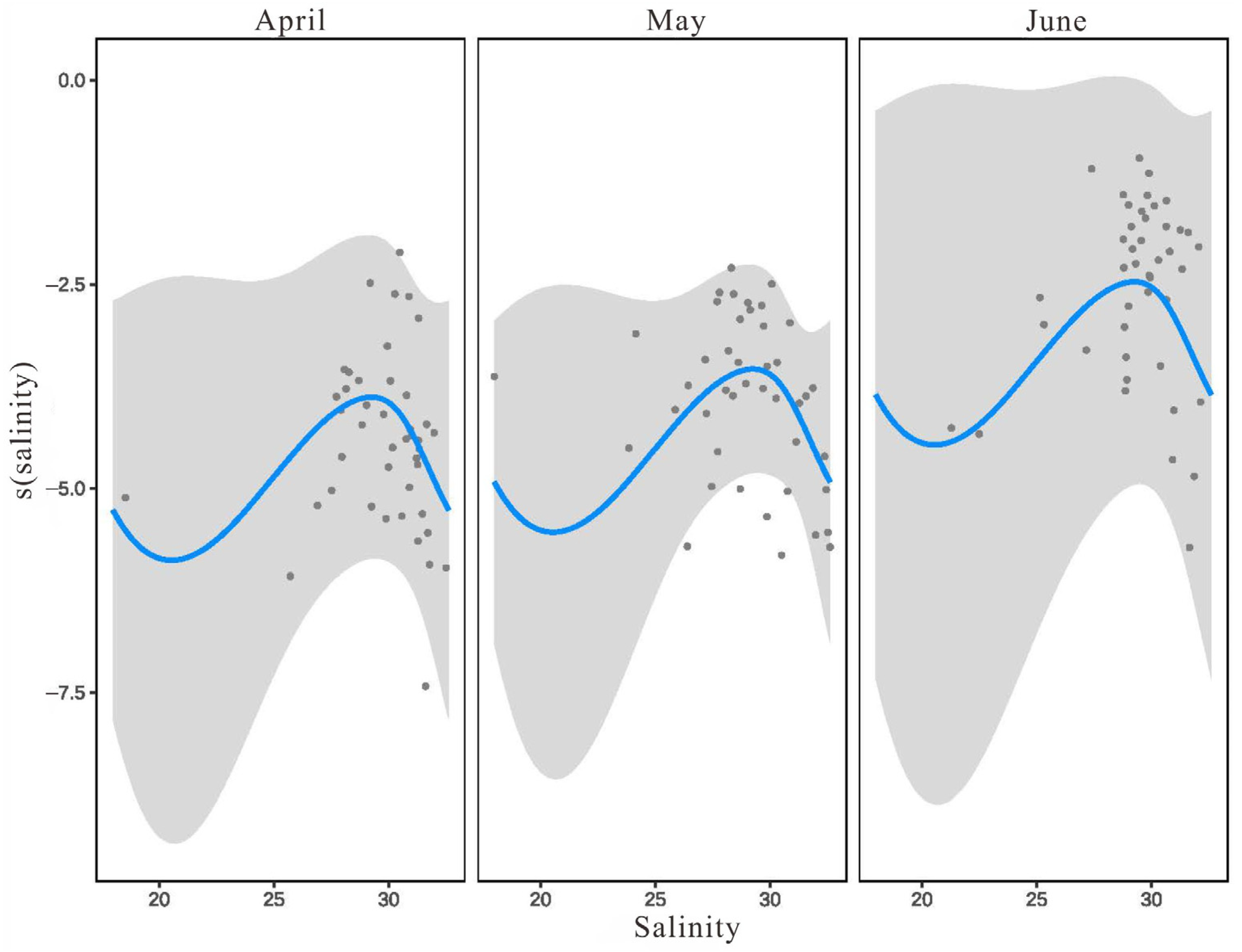
3.3.2. The Relationship between L. polyactis Density and Environmental Factors
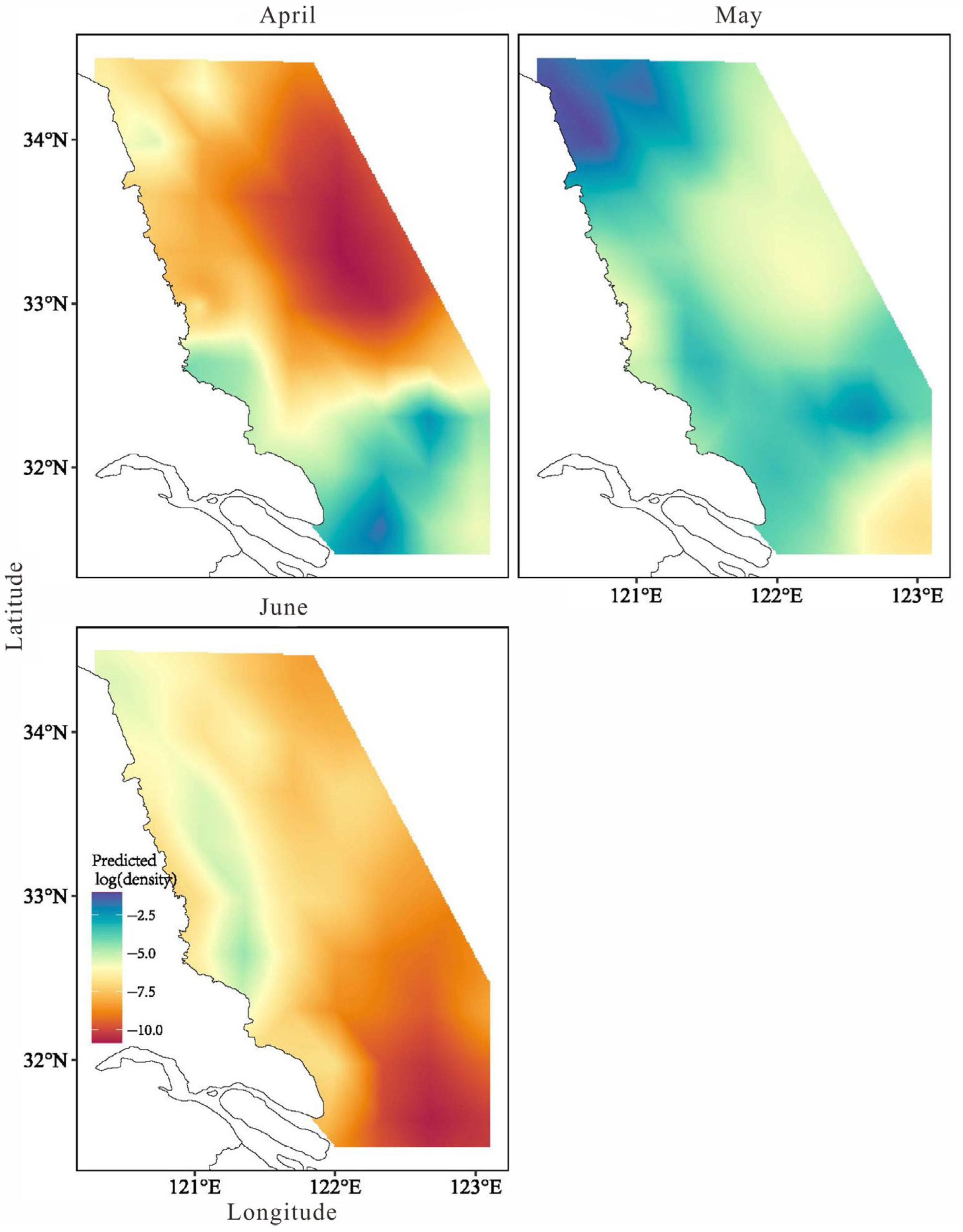
3.3.3. Model Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takatsu, T.; Toyonaga, T.; Hirao, S.; Ooka, E.; Kobayashi, N.; Nakaya, M. Spawning ground selection and larval feeding habits of Arabesque greenling Pleurogrammus azonus around the Matsumae Peninsula, Japan. Fish. Sci. 2024, 90, 435–452. [Google Scholar] [CrossRef]
- Arevalo, E.; Cabral, H.N.; Villeneuve, B.; Possémé, C.; Lepage, M. Fish larvae dynamics in temperate estuaries: A review on processes, patterns and factors that determine recruitment. Fish Fish. 2023, 24, 466–487. [Google Scholar] [CrossRef]
- Tyler, K.J.; Wedd, D.; Crook, D.; Kennard, M.; King, A.J. Hydrology drives variation in spawning phenologies and diversity of larval assemblages of Australian wet–dry tropical fish. Freshw. Biol. 2021, 66, 1949–1967. [Google Scholar] [CrossRef]
- Brosset, P.; Smith, A.D.; Plourde, S.; Castonguay, M.; Lehoux, C.; Van Beveren, E. A fine-scale multi-step approach to understand fish recruitment variability. Sci. Rep. 2020, 10, 16064. [Google Scholar] [CrossRef]
- Liu, Z.L.; Jin, Y.; Yang, L.L.; Yuan, X.W.; Yan, L.P.; Zhang, Y.; Zhang, H.; Xu, M.; Song, X.J.; Tang, J.H.; et al. Improving prediction for potential spawning areas from a two-step perspective: A comparison of multi-model approaches for sparse egg distribution. J. Sea Res. 2023, 197, 102460. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Martinho, F.; Baptista, J.; Costa, F.; Pardal, M.A.; Primo, A.L. Function of estuaries and coastal areas as nursery grounds for marine fish early life stages. Mar. Environ. Res. 2021, 170, 105408. [Google Scholar] [CrossRef] [PubMed]
- Acha, E.M.; Piola, A.; Iribarne, O.; Mianzan, H. Biology of Fronts. In Ecological Processes at Marine Fronts; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Zheng, J.; Gao, T.X.; Yan, Y.R.; Song, N. Genetic variation of the small yellow croaker (Larimichthys polyactis) inferred from mitochondrial DNA provides novel insight into the fluctuation of resources. Acta Oceanol. Sin. 2022, 41, 88–95. [Google Scholar] [CrossRef]
- Lin, L.S.; Liu, Z.L.; Jiang, Y.Z.; Huang, W.; Gao, T.X. Current status of small yellow croaker resources in the southern Yellow Sea and the East China Sea. Chin. J. Oceanol. Limnol. 2011, 29, 547–555. [Google Scholar] [CrossRef]
- Zhang, C.; Ye, Z.J.; Wan, R.; Ma, Q.Y.; Li, Z.G. Investigating the population structure of small yellow croaker (Larimichthys polyactis) using internal and external features of otoliths. Fish. Res. 2014, 153, 41–47. [Google Scholar] [CrossRef]
- Li, Z.L.; Shan, X.J.; Jin, X.S.; Dai, F.Q. Long-term variations in body length and age at maturity of the small yellow croaker (Larimichthys polyactis Bleeker, 1877) in the Bohai Sea and the Yellow Sea, China. Fish. Res. 2011, 110, 67–74. [Google Scholar] [CrossRef]
- Liang, Z.L.; Sun, P.; Yan, W.; Huang, L.Y.; Tang, Y.L. Significant effects of fishing gear selectivity on fish life history. J. Ocean Univ. China 2014, 13, 467–471. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.H.; Liu, Z.L.; Liu, Y.; Zhang, Y.; Yang, L.L.; Wang, F.; Wu, H.; Cheng, J.H. Seasonal distribution of the early life stages of the small yellow croaker (Larimichthys polyactis) and its dynamic controls adjacent to the Changjiang River Estuary. Fish. Oceanogr. 2023, 32, 390–404. [Google Scholar] [CrossRef]
- Koenigbauer, S.T.; Höök, T.O. Increased offspring provisioning by large female fish and consequences for reproductive efficiency. Ecol. Evol. 2023, 13, e10555. [Google Scholar] [CrossRef]
- Grüss, A.; Biggs, C.; Heyman, W.D.; Erisman, B. Prioritizing monitoring and conservation efforts for fish spawning aggregations in the US Gulf of Mexico. Sci. Rep. 2018, 8, 8473. [Google Scholar]
- Dambrine, C.; Woillez, M.; Huret, M.; de Pontual, H. Characterising Essential Fish Habitat using spatio-temporal analysis of fishery data: A case study of the European seabass spawning areas. Fish. Oceanogr. 2021, 30, 413–428. [Google Scholar] [CrossRef]
- Laman, E.A.; Rooper, C.N.; Turner, K.; Rooney, S.; Cooper, D.W.; Zimmermann, M. Using species distribution models to describe essential fish habitat in Alaska. Can. J. Fish. Aquat. Sci. 2018, 75, 1230–1255. [Google Scholar] [CrossRef]
- Zhong, X.M.; Zhang, H.; Tang, J.H.; Zhong, F.; Zhong, J.S.; Xiong, Y.; Gao, Y.S.; Ge, K.K.; Yu, W.W. Temporal and spatial distribution of Larimichthys polyactis Bleeker resources in offshore areas of Jiangsu Province. J. Fish. China 2011, 235, 238–246. [Google Scholar]
- Lin, N.; Chen, Y.G.; Jin, Y.; Yuan, X.W.; Ling, J.Z.; Jiang, Y.Z. Distribution of the early life stages of small yellow croaker in the Yangtze River estuary and adjacent waters. Fish. Sci. 2018, 84, 357–363. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, C.L.; Xu, K.D.; Zhou, Y.D.; Jiang, R.J.; Li, Z.H.; Li, X.F. Reproductive Status of Larimichthys polyactis in Zhoushan Fishing Ground and Adjacent Waters from 2020 to 2021. Ocean Dev. Manag. 2022, 39, 53–57. [Google Scholar]
- Zhan, W.; Lou, B.; Chen, R.Y.; Mao, G.M.; Liu, F.; Xu, D.D.; Wang, L.G.; Ma, T.; Xu, Q.X. Observation of embryonic, larva and juvenile development of small yellow croaker, Larimichthys polyactis. Oceanol. Limnol. Sin. 2016, 47, 1033–1039. [Google Scholar]
- Yin, J.; Wang, J.; Zhang, C.L.; Xu, B.D.; Xue, Y.; Ren, Y.P. Spatial and temporal distribution characteristics of Larimichthys polyactis eggs in Haizhou Bay and adjacent regions based on two-stage GAM. J. Fish. Sci. China 2019, 26, 1164–1174. [Google Scholar]
- Nakazawa, T. Ontogenetic niche shifts matter in community ecology: A review and future perspectives. Popul. Ecol. 2015, 57, 347–354. [Google Scholar] [CrossRef]
- Rao, W.B.; Mao, C.P.; Wang, Y.G.; Su, J.B.; Balsam, W.; Ji, J.F. Geochemical constraints on the provenance of surface sediments of radial sand ridges off the Jiangsu coastal zone, East China. Mar. Geol. 2015, 359, 35–49. [Google Scholar] [CrossRef]
- Zhou, F.; Su, J.L.; Huang, D.J. Study on the intrusion of coastal low salinity water in the west of southern Huanghai Sea during spring and summer. Haiyang Xuebao 2004, 26, 34–44. [Google Scholar]
- Liu, Z.L.; Jin, Y.; Yang, L.L.; Yan, L.P.; Zhang, Y.; Xu, M.; Tang, J.H.; Zhou, Y.D.; Hu, F.; Cheng, J.H. Incorporating egg-transporting pathways into conservation plans of spawning areas: An example of small yellow croaker (Larimichthys polyactis) in the East China Sea zone. Front. Mar. Sci. 2022, 9, 941411. [Google Scholar] [CrossRef]
- Ahlstrom, E.H.; Moser, H.G. Eggs and larvae of fishes and their role in systematic investigations and in fisheries. Rev. Trav. L’institut Peches Marit. 1976, 40, 379–398. [Google Scholar]
- Ahlstrom, E.H.; Moser, H.G. Systematics and development of early life history stages of marine fishes: Achievements during the past century, present status and suggestions for the future. Rapp. P.-V. Réun. Cons. Int. Explor. Mer. 1981, 178, 541–546. [Google Scholar]
- Miller, B.S.; Kendall, A.W.J. Early Life History of Marine Fishes; University of California Press: London, UK, 2009. [Google Scholar]
- Zhang, R.Z.; Lu, H.F.; Zhao, C.Y.; Chen, L.F.; Zang, Z.J.; Jiang, W.Y. Fish Eggs and Larvae in the Offshore Waters of China; Shanghai Scientific & Technical Publishers: Shanghai, China, 1985. [Google Scholar]
- Anderson, S.C.; Ward, E.J.; English, P.A.; Barnett, L.A.K. sdmTMB: An R package for fast, flexible, and user-friendly generalized linear mixed effects models with spatial and spatiotemporal random fields. bioRxiv 2022, 485545. [Google Scholar] [CrossRef]
- Lindgren, F.; Rue, H. Bayesian Spatial Modelling with R-INLA. J. Stat. Softw. 2015, 63, 1–25. [Google Scholar] [CrossRef]
- Kristensen, K.; Nielsen, A.; Berg, C.W.; Skaug, H.; Bell, B.M. TMB: Automatic Differentiation and Laplace Approximation. J. Stat. Softw. 2016, 70, 1–21. [Google Scholar] [CrossRef]
- Commander, C.J.C.; Barnett, L.A.K.; Ward, E.J.; Anderson, S.C.; Essington, T.E. The Shadow Model: How and Why Small Choices in Spatially Explicit Species Distribution Models Affect Predictions. Peerj 2022, 10, e12783. [Google Scholar] [CrossRef]
- Grüss, A.; Drexler, M.; Ainsworth, C.H. Using Delta Generalized Additive Models to Produce Distribution Maps for Spatially Explicit Ecosystem Models. Fish. Res. 2014, 159, 11–24. [Google Scholar] [CrossRef]
- Thorson, J.T.; Cunningham, C.J.; Jorgensen, E.; Havron, A.; Hulson, P.J.F.; Monnahan, C.C.; von Szalay, P. The surprising sensitivity of index scale to delta-model assumptions: Recommendations for model-based index standardization. Fish. Res. 2021, 233, 105–745. [Google Scholar] [CrossRef]
- Thorson, J.T.; Shelton, A.O.; Ward, E.J.; Skaug, H.J. Geostatistical Delta-Generalized Linear Mixed Models Improve Precision for Estimated Abundance Indices for West Coast Groundfishes. Ices J. Mar. Sci. 2015, 72, 1297–1310. [Google Scholar] [CrossRef]
- Post, S.; Fock, H.O.; Jansen, T. Blue whiting distribution and migration in Greenland waters. Fish. Res. 2019, 212, 123–135. [Google Scholar] [CrossRef]
- Barnett, L.A.K.; Ward, E.J.; Anderson, S.C. Improving estimates of species distribution change by incorporating local trends. Ecography 2020, 44, 427–439. [Google Scholar] [CrossRef]
- Evans, A.L.; Bulla, A.J.; Kieta, A.R. The precision teaching system: A synthesized definition, concept analysis, and process. Behav. Anal. Pract. 2021, 14, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Deroba, J.J.; Miller, T.J.; Legault, C.M.; Perretti, C.T. An evaluation of common stock assessment diagnostic tools for choosing among state-space models with multiple random effects processes. Fish. Res. 2024, 273, 106968. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package version 0.4.6. 2022. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 6 April 2023).
- Chen, M.Y.; Zeng, C.; Zeng, X.; Liu, Y.; Wang, Z.H.; Shi, X.J.; Cao, L. Assessment of marine protected areas in the East China Sea using a management effectiveness tracking tool. Front. Mar. Sci. 2023, 10, 1081036. [Google Scholar] [CrossRef]
- Wu, H.; Gu, J.H.; Zhu, P. Winter Counter-Wind Transport in the Inner Southwestern Yellow Sea. J. Geophys. Res.-Ocean. 2018, 123, 411–436. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, H. Origins and transports of the low-salinity coastal water in the southwestern Yellow Sea. Acta Oceanol. Sin. 2018, 37, 1–11. [Google Scholar] [CrossRef]
- Lasker, R. The relation between oceanographic conditions and larval anchovy food in the California Current: Identification of factors contributing to recruitment failure. Rapp. P.-V. Réun. Cons. Int. Explor. Mer. 1978, 173, 212–230. [Google Scholar]
- Cushing, D. Plankton production and year-class strength in fish populations: An update of the match/mismatch hypothesis. Adv. Mar. Biol. 1990, 26, 249–293. [Google Scholar]
- Tao, J.F.; Wang, Z.B.; Zhou, Z.; Xu, F.; Zhang, C.K.; Stive, M.J.F. A Morphodynamic Modeling Study on the Formation of the Large-Scale Radial Sand Ridges in the Southern Yellow Sea. J. Geophys. Res.-Earth 2019, 124, 1742–1761. [Google Scholar] [CrossRef]
- Xu, F.; Tao, J.F.; Zhou, Z.; Coco, G.; Zhang, C.K. Mechanisms underlying the regional morphological differences between the northern and southern radial sand ridges along the Jiangsu Coast, China. Mar. Geol. 2016, 371, 1–17. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, J.; Jiang, T.; Liu, H.B.; Zhong, X.M.; Tang, J.H. Early life history of the small yellow croaker (Larimichthys polyactis) in sandy ridges of the South Yellow Sea. Mar. Biol. Res. 2017, 13, 993–1002. [Google Scholar] [CrossRef]
- Wu, W.F.; Zhai, F.G.; Liu, Z.Z.; Liu, C.; Gu, Y.Z.; Li, P.L. The spatial and seasonal variability of nutrient status in the seaward rivers of China shaped by the human activities. Ecol. Indic. 2023, 157, 111223. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hajima, T.; Yamazaki, D.; Aita, M.N.; Ito, A.; Kawamiya, M. Competing and accelerating effects of anthropogenic nutrient inputs on climate-driven changes in ocean carbon and oxygen cycles. Sci. Adv. 2022, 8, eabl9207. [Google Scholar] [CrossRef]
- Santos, I.R.; Chen, X.G.; Lecher, A.L.; Sawyer, A.H.; Moosdorf, N.; Rodellas, V.; Tamborski, J.; Cho, H.M.; Dimova, N.; Sugimoto, R.; et al. Submarine groundwater discharge impacts on coastal nutrient biogeochemistry. Nat. Rev. Earth Environ. 2021, 2, 307–323. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Yang, X.; Wang, Y.; Li, F.F.; Wang, J.T.; Tan, L.J. Exogenous nutrient inputs restructure phytoplankton community and ecological stoichiometry of Eastern Indian Ocean. Ecol. Indic. 2021, 127, 107801. [Google Scholar] [CrossRef]
- Polte, P.; Kotterba, P.; Moll, D.; von Nordheim, L. Ontogenetic loops in habitat use highlight the importance of littoral habitats for early life-stages of oceanic fishes in temperate waters. Sci. Rep. 2017, 7, 42709. [Google Scholar] [CrossRef] [PubMed]
- Galaiduk, R.; Radford, B.T.; Saunders, B.J.; Newman, S.J.; Harvey, E.S. Characterizing ontogenetic habitat shifts in marine fishes: Advancing nascent methods for marine spatial management. Ecol. Appl. 2017, 27, 1776–1788. [Google Scholar] [CrossRef]
- Komyakova, V.; Munday, E.L.; Jones, G.P. Comparative analysis of habitat use and ontogenetic habitat-shifts among coral reef damselfishes. Environ. Biol. Fish. 2019, 102, 1201–1218. [Google Scholar] [CrossRef]
- Félix-Hackradt, F.C.; Hackradt, C.W.; Treviño-Otón, J.; Pérez-Ruzafa, A.; García-Charton, J.A. Habitat use and ontogenetic shifts of fish life stages at rocky reefs in South-western Mediterranean Sea. J. Sea Res. 2014, 88, 67–77. [Google Scholar] [CrossRef]
- Drake, P.; Arias, A.M. Composition and seasonal fluctuations of the ichthyoplankton community in a shallow tidal channel of Cadiz Bay (S.W. Spain). J. Fish Biol. 1991, 39, 245–263. [Google Scholar] [CrossRef]
- Leggett, W.C. Fish Migrations in Coastal and Estuarine Environments: A Call for New Approaches to the Study of an Old Problem. In Mechanisms of Migration in Fishes; NATO Conference Series; Springer: Boston, MA, USA, 1984; Volume 14. [Google Scholar]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Asch, R. Climate change and decadal shifts in the phenology of larval fishes in the California Current ecosystem. Proc. Natl. Acad. Sci. USA 2015, 112, E4065–E4074. [Google Scholar] [CrossRef]
- Kumar, P.; Babita, M.; Kailasam, M.; Muralidhar, M.; Hussain, T.; Behera, A.; Jithendran, K.P. Effect of Changing Environmental Factors on Reproductive Cycle and Endocrinology of Fishes. In Outlook of Climate Change and Fish Nutrition; Springer: Singapore, 2022. [Google Scholar]
- Fincham, J.I.; Rijnsdorp, A.D.; Engelhard, G.H. Shifts in the timing of spawning in sole linked to warming sea temperatures. J. Sea Res. 2013, 75, 69–76. [Google Scholar] [CrossRef]
- Sparks, M.M.; Westley, P.A.H.; Falke, J.A.; Quinn, T.P. Thermal adaptation and phenotypic plasticity in a warming world: Insights from common garden experiments on Alaskan sockeye salmon. Glob. Chang. Biol. 2017, 23, 5203–5217. [Google Scholar] [CrossRef]
- Beer, W.N.; Anderson, J.J. Effect of spawning day and temperature on salmon emergence: Interpretations of a growth model for Methow River chinook. Can. J. Fish. Aquat. Sci. 2001, 58, 943–949. [Google Scholar] [CrossRef]
- Wilson, S.M.; Moore, J.W.; Ward, E.J.; Kinsel, C.W.; Anderson, J.H.; Buehrens, T.W.; Carr-Harris, C.N.; Cochran, P.C.; Davies, T.D.; Downen, M.R.; et al. Phenological shifts and mismatch with marine productivity vary among Pacific salmon species and populations. Nat. Ecol. Evol. 2023, 7, 852–861. [Google Scholar] [CrossRef]
- Nati, J.J.H.; Svendsen, M.B.S.; Marras, S.; Killen, S.S.; Steffensen, J.F.; McKenzie, D.J.; Domenici, P. Intraspecific variation in thermal tolerance differs between tropical and temperate fishes. Sci. Rep. 2021, 11, 21272. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shen, S.F.; Chen, Y.S.; Kiang, Y.K.; Heino, M. Life histories determine divergent population trends for fishes under climate warming. Nat. Commun. 2020, 11, 4088. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Richards, S.A.; Stuart-Smith, R.D.; Pecl, G.; Edgar, G.J.; Barrett, N.S.; Payne, N.; Blanchard, J.L. Fish body sizes change with temperature but not all species shrink with warming. Nat. Ecol. Evol. 2020, 4, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Geburzi, J.C.; McCarthy, M.L. How Do They Do It?–Understanding the Success of Marine Invasive Species. In YOUMARES 8–Oceans Across Boundaries: Learning from Each Other; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Ye, G.Q.; Lin, Y.; Feng, C.C.; Chou, L.M.; Jiang, Q.T.; Ma, P.P.; Yang, S.Y.; Shi, X.F.; Chen, M.R.; Yang, X.C.; et al. Could the wild population of Large Yellow Croaker Larimichthys crocea (Richardson) in China be restored? A case study in Guanjingyang, Fujian, China. Aquat. Living Resour. 2020, 33, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.J.; Chen, J.J.; Xie, B.; Huang, L.F. Climate-induced habitat suitability changes intensify fishing impacts on the life history of large yellow croaker (Larimichthys crocea). Ecol. Evol. 2022, 12, e9342. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.C.; Cheng, B.S. Populations adapt more to temperature in the ocean than on land. Nat. Clim. Chang. 2022, 12, 1098–1099. [Google Scholar]
- Swadling, D.S.; Knott, N.A.; Taylor, M.D.; Rees, M.J.; Cadiou, G.; Davis, A.R. Consequences of Juvenile Fish Movement and Seascape Connectivity: Does the Concept of Nursery Habitat Need a Rethink? Estuaries Coast. 2024, 47, 607–621. [Google Scholar] [CrossRef]
- Sheaves, M.; Baker, R.; Nagelkerken, I.; Connolly, R.M. True Value of Estuarine and Coastal Nurseries for Fish: Incorporating Complexity and Dynamics. Estuaries Coast. 2015, 38, 401–414. [Google Scholar] [CrossRef]
- Takahashi, I.; Azuma, K.; Fujita, S.; Kinoshita, I. Habitat shift of ayu Plecoglossus altivelis altivelis in early stages from waters adjacent to the bank to the center of flow in the Shimanto Estuary. Fish. Sci. 2002, 68, 554–559. [Google Scholar] [CrossRef]
- Hogan, J.D.; Kozdon, R.; Blum, M.J.; Gilliam, J.F.; Valley, J.W.; McIntyre, P.B. Reconstructing larval growth and habitat use in an amphidromous goby using otolith increments and microchemistry. J. Fish Biol. 2017, 90, 1338–1355. [Google Scholar] [CrossRef]
- Sagoe, A.A.; Aheto, D.W.; Okyere, I.; Adade, R.; Odoi, J. Community participation in assessment of fisheries related ecosystem services towards the establishment of marine protected area in the Greater Cape Three Points area in Ghana. Mar. Policy 2020, 124, 104336. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.H.; Ding, Y.F. Jiangsu Haizhou Bay Marine Farm Construction Present Situation and Development Countermeasures and Suggestions. China Resour. Compr. Util. 2016, 34, 43–45. [Google Scholar]
- Fu, X.M.; Wu, W.Q.; Zhang, S. Evaluation of ecological restoration performance in Haizhou Bay, Lianyungang. J. Dalian Ocean Univ. 2017, 32, 93–98. [Google Scholar]
- Neilson, J.D.; Perry, R.I. Diel Vertical Migrations of Marine Fishes: An Obligate or Facultative Process? In Advances in Marine Biology; Academic Press: Pittsburgh, PA, USA, 1990. [Google Scholar]
- Hawes, S.; Miskiewicz, T.; Garcia, V.; Figueira, W. Size and stage-dependent vertical migration patterns in reef-associated fish larvae off the eastern coast of Australia. Deep-Sea Res. Part I 2020, 164, 103362. [Google Scholar] [CrossRef]
- Liu, Y.B.; Song, C.L.; Yang, X.; Zhuo, H.H.; Zhou, Z.; Cao, L.; Cao, X.Y.; Zhou, Y.Y.; Xu, J.; Wan, L.L. Hydrological regimes and water quality variations in the Yangtze River basin from 1998 to 2018. Water Res. 2023, 249, 120910. [Google Scholar] [CrossRef]
- Yang, S.L.; Xu, K.H.; Milliman, J.D.; Yang, H.F.; Wu, C.S. Decline of Yangtze River water and sediment discharge: Impact from natural and anthropogenic changes. Sci. Rep. 2015, 5, 12581. [Google Scholar] [CrossRef]

| Model | Covariate | Anisotropy | Spatiotemporal |
|---|---|---|---|
| M1 | Month + s(depth, cs = “cc”, k =4) + s(temperature, cs = “cc”, k = 4) + s(salinity, cs = “cc”, k = 4) | FALSE | “off” |
| M2 | Month + s(depth, cs = “cc”, k =4) + s(temperature, cs = “cc”, k = 4) + s(salinity, cs = “cc”, k = 4) | TRUE | “off” |
| M3 | Month + s(depth, cs = “cc”, k =4) + s(temperature, cs = “cc”, k = 4) + s(salinity, cs = “cc”, k = 4) | FALSE | “iid” |
| M4 | Month + s(depth, cs = “cc”, k =4) + s(temperature, cs = “cc”, k = 4) + s(salinity, cs = “cc”, k = 4) | TRUE | “iid” |
| Model | Statistics | p Value |
|---|---|---|
| M1 | 0.0946 | 0.1987 |
| M2 | 0.0972 | 0.1743 |
| M3 | 0.1531 | 0.0047 |
| M4 | 0.1413 | 0.0116 |
| Model | AIC |
|---|---|
| M1 | −76.8110 |
| M2 | −74.1120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Hu, F.; Xu, M.; Zhang, Y.; Jin, Y.; Gao, X.; Liu, Z.; Ling, J.; Li, S.; Cheng, J. Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys Polyactis) in the Southern Yellow Sea. Diversity 2024, 16, 521. https://doi.org/10.3390/d16090521
Song X, Hu F, Xu M, Zhang Y, Jin Y, Gao X, Liu Z, Ling J, Li S, Cheng J. Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys Polyactis) in the Southern Yellow Sea. Diversity. 2024; 16(9):521. https://doi.org/10.3390/d16090521
Chicago/Turabian StyleSong, Xiaojing, Fen Hu, Min Xu, Yi Zhang, Yan Jin, Xiaodi Gao, Zunlei Liu, Jianzhong Ling, Shengfa Li, and Jiahua Cheng. 2024. "Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys Polyactis) in the Southern Yellow Sea" Diversity 16, no. 9: 521. https://doi.org/10.3390/d16090521
APA StyleSong, X., Hu, F., Xu, M., Zhang, Y., Jin, Y., Gao, X., Liu, Z., Ling, J., Li, S., & Cheng, J. (2024). Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys Polyactis) in the Southern Yellow Sea. Diversity, 16(9), 521. https://doi.org/10.3390/d16090521







