Age and Growth of Mitre Squid (Uroteuthis chinensis) in the Northwestern South China Sea Based on Statolith Microstructure Analysis
Abstract
1. Introduction
2. Materials and Methods
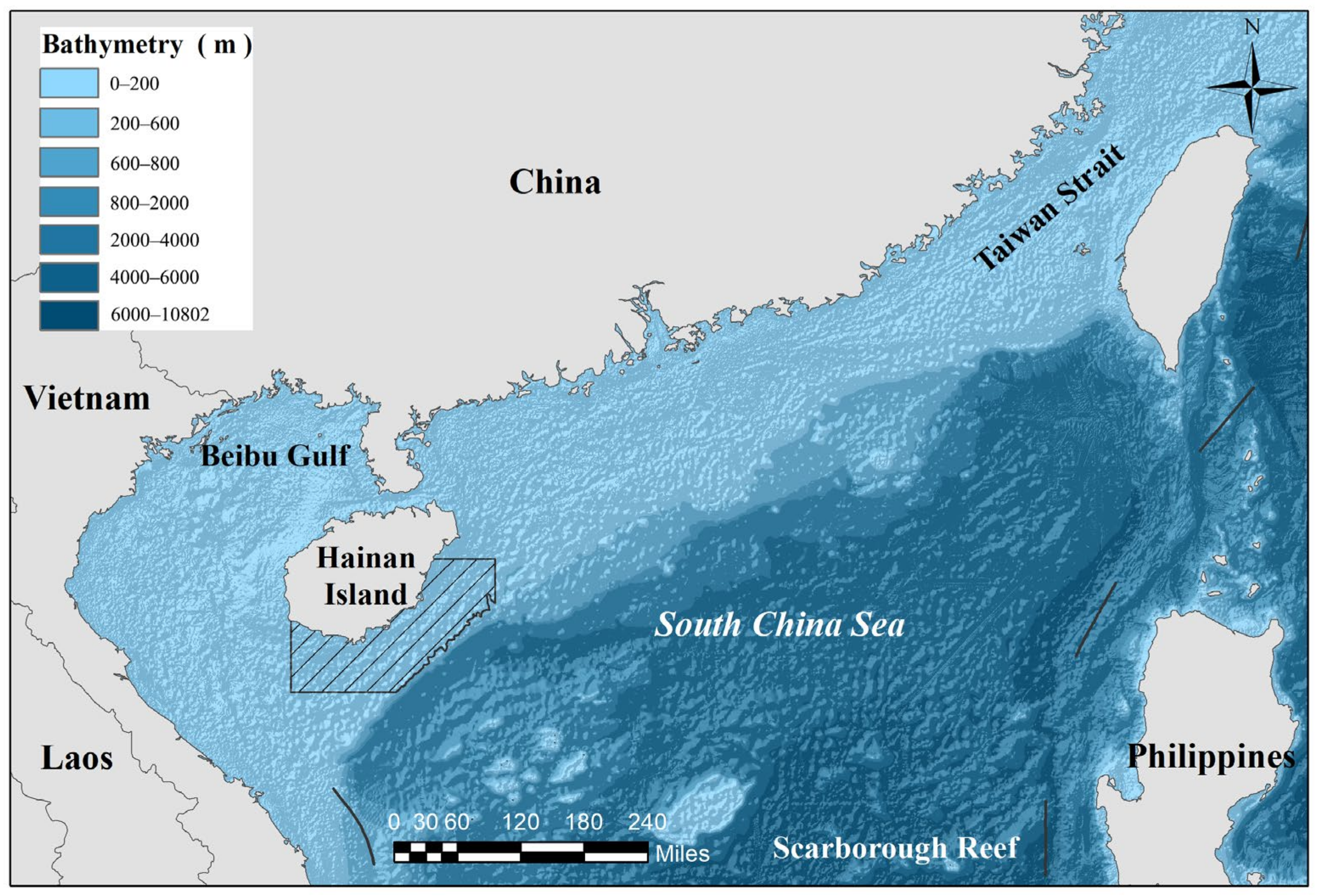
2.1. Sample Collection
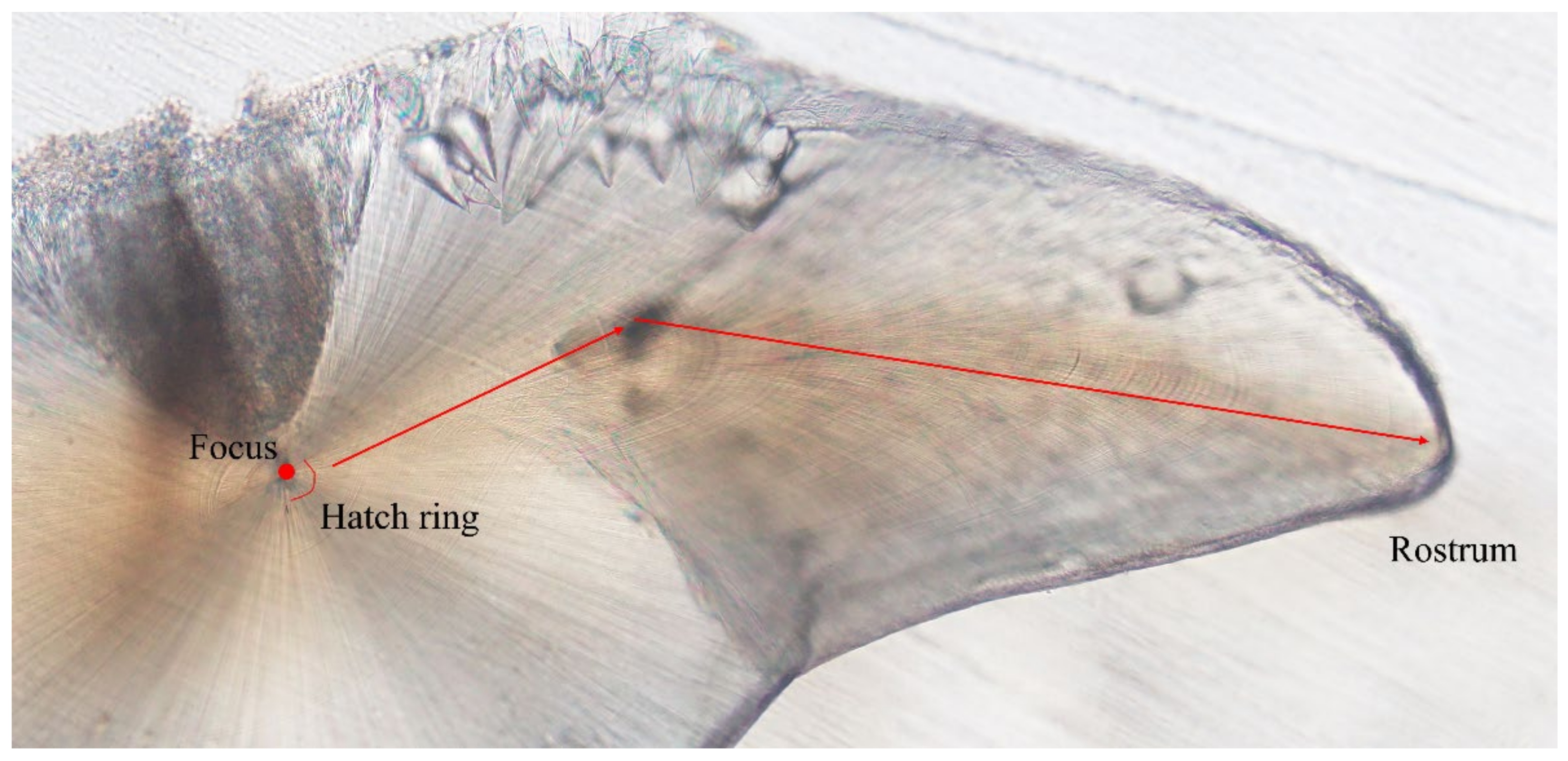
2.2. Statoliths Processing and Reading
2.3. Data Analysis
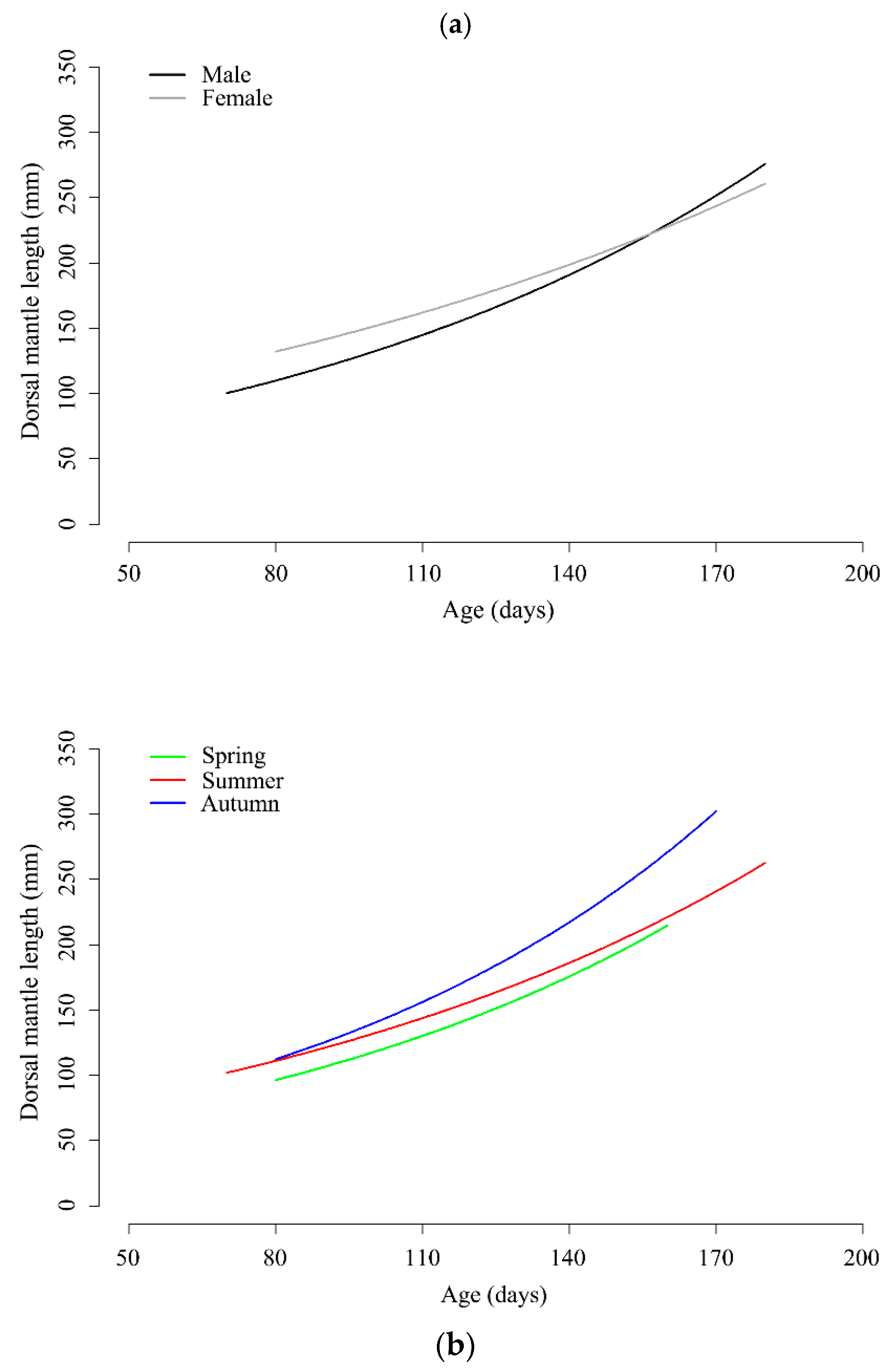
3. Results
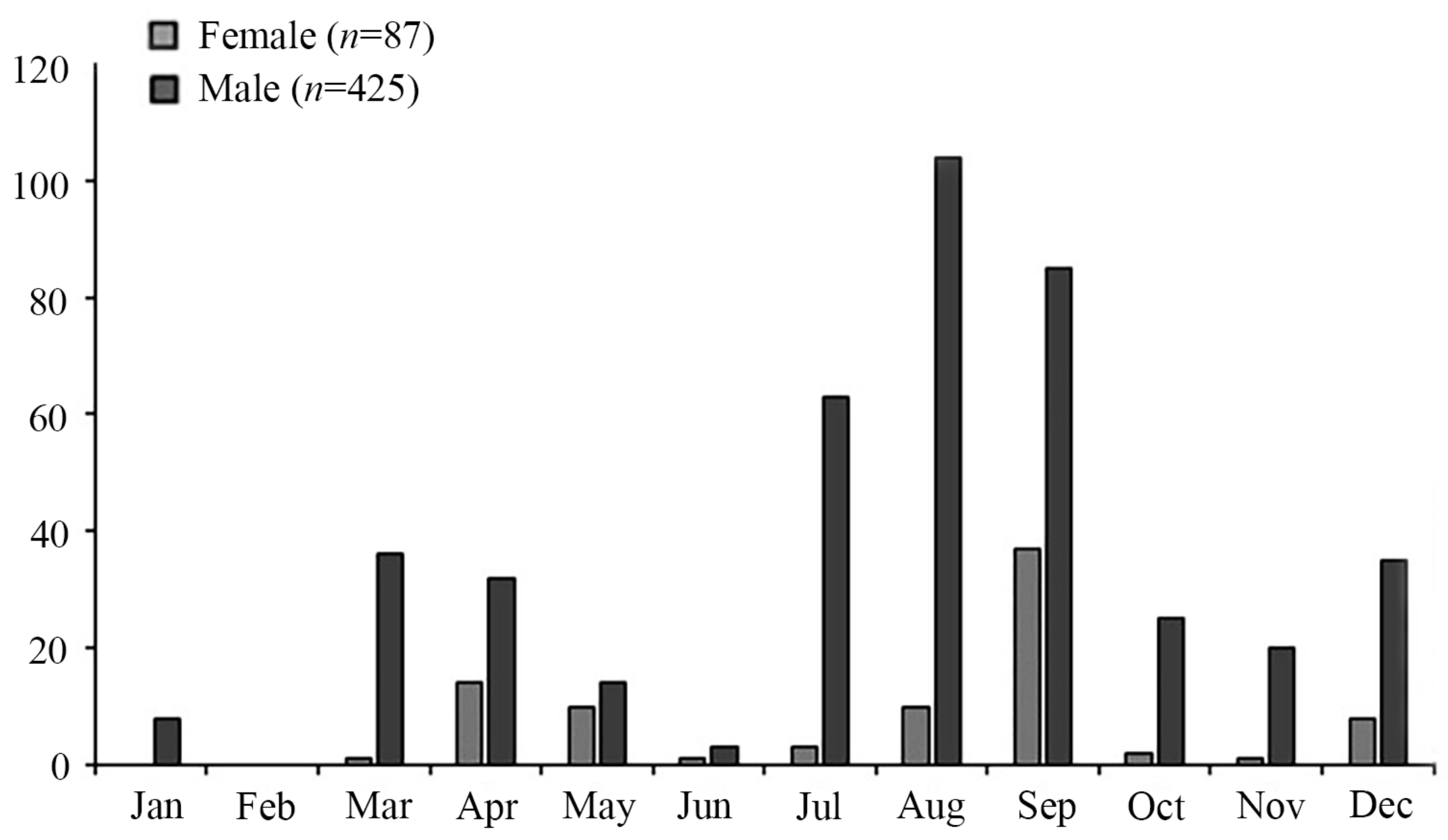
3.1. Dorsal Mantle Length, Weight and Age Distributions
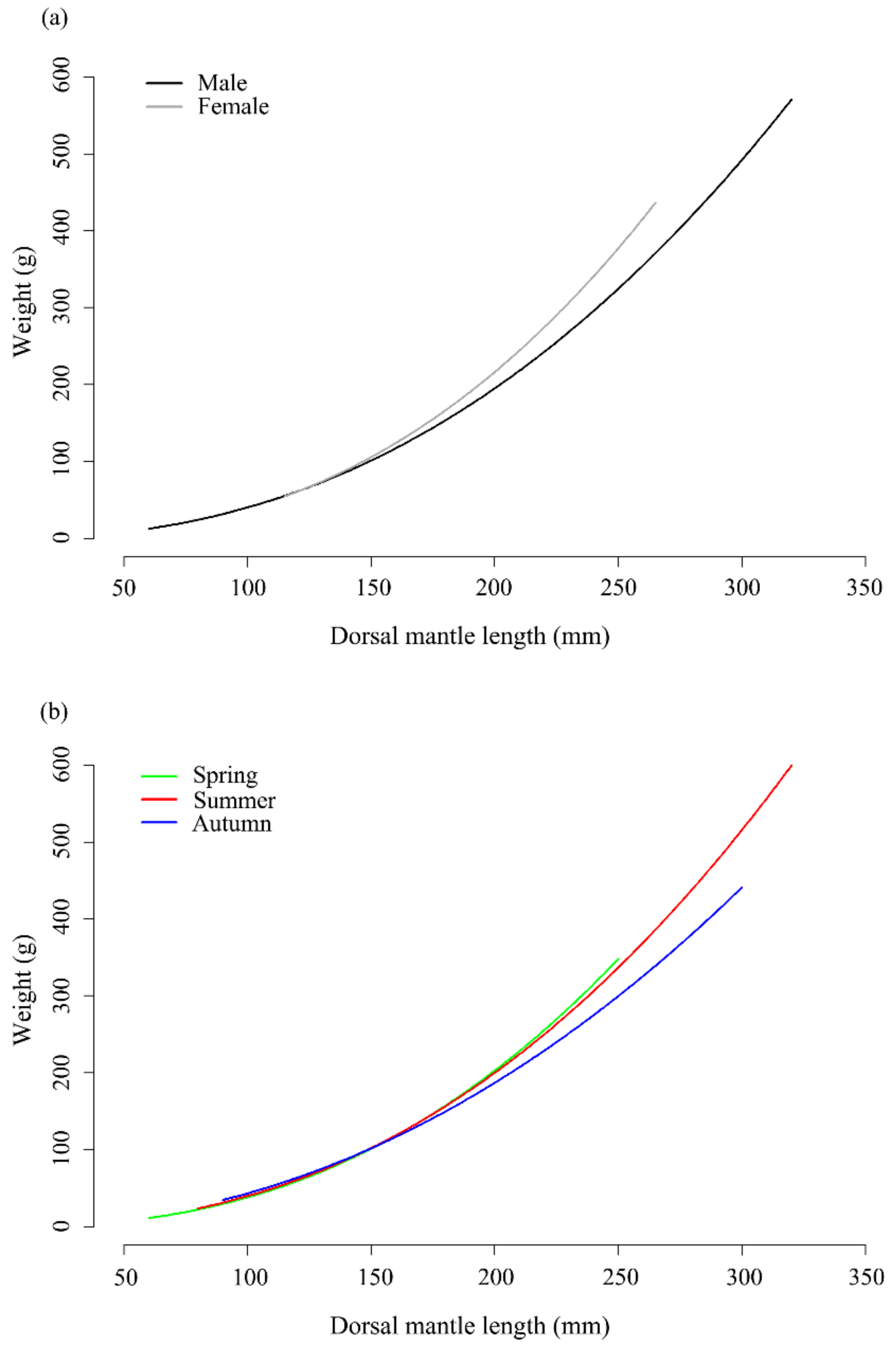
3.2. Length–Weight Relationship
3.3. Growth Model
3.4. DML at First Maturation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jereb, P.; Roper, C.F.E. Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date; Myopsid and Oegopsid Squids; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; Volume 2. [Google Scholar]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World Squid Fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics 2019; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Rosa, R.; Pierce, G.; O’dor, R. (Eds.) Advances in Squid Biology, Ecology and Fisheries, 1st ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013. [Google Scholar]
- Luna, A.; Sánchez, P.; Chicote, C.; Gazo, M. Cephalopods in the diet of Risso’s dolphin (Grampus griseus) from the Mediterranean Sea: A review. Mar. Mammal Sci. 2022, 38, 725–741. [Google Scholar] [CrossRef]
- Xavier, J.; Wood, A.; Rodhouse, P.; Croxall, J. Interannual variations in cephalopod consumption by albatrosses at South Georgia: Implications for future commercial exploitation of cephalopods. Mar. Freshw. Res. 2007, 58, 1136–1143. [Google Scholar] [CrossRef]
- Santos, R.A.; Haimovici, M. Cephalopods in the trophic relations off southern Brazil. Bull. Mar. Sci. 2002, 71, 753–770. [Google Scholar]
- Clarke, M.; MacLeod, N.; Paliza, O. Cephalopod remains from the stomachs of Sperm whales caught off Peru and Chile. J. Zool. 2009, 180, 477–493. [Google Scholar] [CrossRef]
- Staudinger, M.D. Seasonal and size-based predation on two species of squid by four fish predators on the Northwest Atlantic continental shelf. Fish. Bull. 2006, 104, 605–615. [Google Scholar]
- Doubleday, Z.A.; Prowse, T.A.; Arkhipkin, A.; Pierce, G.J.; Semmens, J.; Steer, M.; Leporati, S.C.; Lourenco, S.; Quetglas, A.; Sauer, W.; et al. Global proliferation of cephalopods. Curr. Biol. 2016, 26, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.D.; O’Dor, R.K. Time, space and the ecophysiology of squid growth, life in the fast lane. Vie et Milieu 2001, 51, 205–215. [Google Scholar]
- Pecl, G.T.; Jackson, G.D. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Rev. Fish Biol. Fish. 2008, 18, 373–385. [Google Scholar] [CrossRef]
- Pecl, G.T.; Moltschaniwskyj, N.A.; Tracey, S.R.; Jordan, A. Inter-annual plasticity of squid life history and population structure: Ecological and management implications. Oecologia 2004, 139, 515–524. [Google Scholar] [CrossRef]
- Anderson, C.; Rodhouse, P. Life cycles, oceanography and variability: Ommastrephid squid invariable oceanographic environments. Fish. Res. 2001, 54, 133–143. [Google Scholar] [CrossRef]
- Croxall, J.P.; Prince, P.A. Cephalopods as prey. I. Seabirds. Philos. Trans. R. Soc. Lond. B 1996, 351, 1023–1043. [Google Scholar] [CrossRef]
- O’Dor, R.K.; Webber, D.M. The constraints on cephalopods: Why squid aren’t fish. Can. J. Zool. 1986, 64, 1591–1605. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Hendrickson, L.C.; Payá, I.; Pierce, G.J.; Roa-Ureta, R.H.; Robin, J.P.; Winter, A. Stock assessment and management of cephalopods: Advances and challenges for short-lived fishery resources. ICES J. Mar. Sci. 2021, 78, 714–730. [Google Scholar] [CrossRef]
- Rodhouse, P.; Griffiths, H.; Xavier, J. Southern Ocean Squid, 1st ed.; The Scientific Committee on Antarctic Research, Scott Polar Research Institute: Cambridge, UK, 2014; pp. 284–289. [Google Scholar]
- Watson, R.; Pauly, D. Systematic distortions in world fisheries catch trends. Nature 2001, 414, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Tian, Y.; Fu, C.; Wang, B.; Li, J.; Ren, Y.; Wan, R. Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 2018, 208, 22–33. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, R.G.; Qian, W.G. Important Economic Cephalopod Resources and Fisheries in the Coastal Waters of China; Science Press: Beijing, China, 2013; pp. 64–65. [Google Scholar]
- Lu, H.; Chen, X.J. Age, growth and population structure of Illex argentinus based on statolith microstructure in Southwest Atlantic Ocean. J. Fish. China 2012, 36, 1049–1056. [Google Scholar] [CrossRef]
- Song, W.; Yixuan, F.; Dongming, L.; Chen, X. Stable isotope evidence for a shift in diet with increasing energy demand for reproduction in squid Illex argentinus. Hydrobiologia 2023, 850, 489–502. [Google Scholar] [CrossRef]
- Han, Q.; Shan, X.; Guan, L.; Jin, X.; Wan, R.; Chen, Y. Application of SPiCT for data-limited stock assessment of short-lived Illex argentinus. J. Fish. Sci. China 2018, 25, 169–177. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Chen, Y. Projecting distributions of Argentine shortfin squid (Illex argentinus) in the Southwest Atlantic using a complex integrated model. Acta Oceanol. Sin. 2018, 37, 31–37. [Google Scholar] [CrossRef]
- Yu, W.; Feng, Z.P.; Li, N.; Chen, B.J.; Chen, X.J. Identifying Summer/Autumn Habitat Hotspots of Jumbo Flying Squid (Dosidicus gigas) off Chile. J. Ocean Univ. China 2022, 21, 1669–1681. [Google Scholar] [CrossRef]
- Gao, X.; Gong, Y.; Chen, X.; Li, Y. Trophic niche and gut microbiota of Dosidicus gigas in the eastern equatorial Pacific Ocean. J. Appl. Ecol. 2021, 32, 1087–1095. [Google Scholar] [CrossRef]
- Mu, H.; Weng, P.; Wu, Z. Effect of Inoculation with Lacticaseibacillus casei and Staphylococcus carnosus on the Quality of Squid (Dosidicus gigas) Surimi Sausage. Fermentation 2023, 9, 794. [Google Scholar] [CrossRef]
- Hu, G.; Boenish, R.; Zhao, Z.; Li, J.; Chen, X. Ontogenetic and Spatiotemporal Changes in Isotopic Niche of Jumbo Squid (Dosidicus gigas) in the Southeastern Pacific. Front. Mar. Sci. 2022, 9, 806847. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Liu, Y.; Liu, B.; Li, G. Development and characterization of 101 SNP markers in jumbo flying squid, Dosidicus gigas. Conserv. Genet. Resour. 2020, 13, 13–20. [Google Scholar] [CrossRef]
- Jin, Y.; Li, N.; Chen, X.J.; Liu, B.L.; Li, J.H. Comparative age and growth of Uroteuthis chinensis and Uroteuthis edulis from China Seas based on statolith. Aquac. Fish. 2019, 4, 166–172. [Google Scholar] [CrossRef]
- Liao, C.T. Age, growth and reproduction of Uroteuthis (Photololigo) chinensis in Taiwan Strait. Master’s Thesis, National Taiwan Ocean University, Taiwan, China, 2018. [Google Scholar]
- Wang, X.; He, Y.; Du, F.; Liu, M.; Bei, W.; Cai, Y.; Qiu, Y. Using LBB Tools to Assess Miter Squid Stock in the Northeastern South China Sea. Front. Mar. Sci. 2021, 7, 518627. [Google Scholar] [CrossRef]
- Sukramongkol, N.; Tsuchiya, K.; Segawa, S. Age and maturation of Loligo duvauceli and L. chinensis from Andaman Sea of Thailand. Rev. Fish Biol. Fish. 2007, 17, 237–246. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Bizikov, V.A.; Doubleday, Z.A.; Laptikhovsky, V.V.; Lishchenko, F.V.; Perales-Raya, C.; Hollyman, P.R. Techniques for estimating the age and growth of Molluscs: Cephalopoda. J. Shellfish Res. 2018, 37, 783–793. [Google Scholar] [CrossRef]
- Guo, J.; Liu, D.; Zhang, C.; Tian, Y.; Li, Z. Using statolith shape analysis to identify five commercial Loliginidae squid species in Chinese waters. J. Oceanol. Limnol. 2021, 39, 1160–1168. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Y.; Liu, Z.; Lin, N.; Li, S.; Cheng, J. Analysis of beak morphology of Loligo beka in the East China Sea. J. Fish. Sci. China 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Statoliths as ‘black boxes’ (life recorders) in squid. Mar. Freshw. Res. 2005, 56, 573–583. [Google Scholar] [CrossRef]
- Jackson, G.D. Advances in defining the life histories of myopsid squid. Mar. Freshw. Res. 2004, 55, 357–365. [Google Scholar] [CrossRef]
- Chang, K.Y.; Liao, C.H.; Huang, H.T.; Wu, C.L.; Wang, K.Y. Age and Growth of Uroteuthis (Photololigo) chinensis, U. (P.) duvauceli and U. (P.) edulis from the Waters around Taiwan. J. Taiwan Fish. Res. 2014, 22, 1–13. [Google Scholar]
- Dong, Z.Z. Chinese Fauna, Mollusca, Cephalopods; Science Press: Beijing, China, 1988. (In Chinese) [Google Scholar]
- Lipiński, M.R.; Underhill, L.G. Sexual maturation in squid: Quantum or continuum? S. Afr. J. Mar. Sci. 1995, 15, 207–223. [Google Scholar] [CrossRef]
- Markaida, U. Reproductive biology of jumbo squid Dosidicus gigas in the Gulf of California, 1995–1997. Fish. Res. 2021, 54, 63–82. [Google Scholar] [CrossRef]
- Olyott, L.J.H.; Sauer, W.H.H.; Booth, A.J. Spatio-temporal patterns in maturation of the chokka squid (Loligo vulgaris reynaudii) off the coast of South Africa. ICES J. Mar. Sci. 2006, 63, 1649–1664. [Google Scholar] [CrossRef]
- Moustahfid, H. Age and growth of arrow squid Todarodes sagittatus (Cephalopoda: Ommastrephidae) sampled in summer in Atlantic Moroccan waters. Bull. Mar. Sci. 2002, 71, 535–543. [Google Scholar]
- Natsukari, Y.; Nakanose, T.; Oda, K. Age and growth of loliginid squid Photololigo edulis (Hoyle, 1885). J. Exp. Mar. Biol. Ecol. 1988, 116, 177–190. [Google Scholar] [CrossRef]
- Steer, M.; Pecl, G.; Moltschaniwskyj, N. Are bigger calamary Sepioteuthis australis hatchlings more likely to survive? A study based on statolith dimensions. Mar. Ecol. Prog. Ser. 2003, 261, 175–182. [Google Scholar] [CrossRef][Green Version]
- Jackson, G.D. The use of tetracycline staining techniques to determine statolith growth ring periodicity in the tropical loliginid squids Loliolus noctiluca and Loligo chinensis. Veliger 1990, 33, 389–393. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 5 July 2019).
- Mangiafico, S.S. An R Companion for the Handbook of Biological Statistics, Version1.09 i; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2015. [Google Scholar]
- Dongliang, W.; Yao, L.; Yu, J.; Chen, P. The Role of Environmental Factors on the Fishery Catch of the Squid Uroteuthis chinensis in the Pearl River Estuary, China. J. Mar. Sci. Eng. 2021, 9, 131. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Yang, S.; Wu, G.; Tao, Y.; Feng, Q.; Lu, H. Biological Characteristics and Spatial—Temporal Distribution of Mitre Squid, Uroteuthis chinensis, in the Beibu Gulf, South China Sea. J. Shellfish Res. 2013, 32, 835–844. [Google Scholar] [CrossRef]
- Islam, R.; Hajisamae, S.; Pradit, S.; Perngmak, P.; Paul, M. Feeding habits of two sympatric loliginid squids, Uroteuthis (Photololigo) chinensis (Gray, 1849) and Uroteuthis (Photololigo) duvaucelii (d’Orbigny, 1835), in the lower part of the South China Sea. Molluscan Res. 2018, 38, 155–162. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, B.; Chen, X.; Staples, K. Morphological beak differences of loliginid squid, Uroteuthis chinensis and Uroteuthis edulis, in the northern South China Sea. Chin. J. Oceanol. Limnol. 2017, 36, 559–571. [Google Scholar] [CrossRef]
- Naud, M.J.; Havenhand, J.N. Sperm motility and longevity in the giant cuttlefish, Sepia apama (Mollusca: Cephalopoda). Mar. Biol. 2006, 148, 559–566. [Google Scholar] [CrossRef]
- Jackson, G.D. Seasonal variation in reproductive investment in the tropical loliginid squid Loligo chinensis and the small tropical sepioid Idiosepius pygmaeus. Fish. Bull. 1993, 91, 260–270. [Google Scholar]
- Shashar, N.; Hanlon, R.T. Spawning behavior dynamics at communal egg beds in the squid Doryteuthis (Loligo) pealeii. J. Exp. Mar. Biol. Ecol. 2013, 447, 65–74. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, C.-S.; Iwata, Y. Variations in female swordtip squid Uroteuthis edulis life history traits between southern Japan and northern Taiwan (Northwestern Pacific). Fish. Sci. 2020, 86, 1005–1017. [Google Scholar] [CrossRef]
- Jackson, G.D.; Moltschaniwskyj, N.A. Spatial and temporal variation in growth rates and maturity in the Indo-Pacific squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae). Mar. Biol. 2002, 140, 747–754. [Google Scholar] [CrossRef]
- Arkhipkin, A.; Jereb, P.; Ragonese, S. Growth and maturation in two successive seasonal groups of the short-finned squid, Illex coindetii from the Strait of Sicily (central Mediterranean). ICES J. Mar. Sci. 2000, 57, 31–41. [Google Scholar] [CrossRef]
- Jackson, G.D.; Moltschaniwskyj, N.A. Temporal variation in growth rates and reproductive parameters in the small near-shore tropical squid Loliolus noctiluca; is cooler better? Mar. Ecol. Prog. Ser. 2001, 218, 167–177. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chang, K.Y.; Liao, C.H.; Lee, M.A.; Lee, K.T. Growth strategies of the Swordtip Squid, Uroteuthis edulis, in response to environmental changes in the Southern East China Sea—A cohort analysis. Bull. Mar. Sci. 2013, 89, 677–698. [Google Scholar] [CrossRef]
- Forsythe, J.W.; Walsh, L.S.; Turk, P.E.; Lee, P.G. Impact of temperature on juvenile growth and age at first egg-laying of the Pacific reef squid Sepioteuthis lessoniana reared in captivity. Mar. Biol. 2001, 138, 103–112. [Google Scholar] [CrossRef]
- Moreno, A.; Azevedo, M.; Pereira, J.; Pierce, G. Growth strategies in the squid Loligo vulgaris from Portuguese waters. Mar. Biol. Res. 2007, 3, 49–59. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takayama, K.; Hirose, N. Influence of migratory route on early maturation of swordtip squid, Uroteuthis edulis, caught off western Kyushu Island, Japan. Fish. Res. 2022, 249, 106233. [Google Scholar] [CrossRef]


| Year | Month | Number of Individuals | DML Range (mm) | Weight Range (g) | |||
|---|---|---|---|---|---|---|---|
| F | M | F (Mean) | M (Mean) | F (Mean) | M (Mean) | ||
| 2019 | |||||||
| December | 23 | 23 | 125–168 (141) | 113–183 (139) | 55.1–117.4 (80.9) | 44.0–136.0 (73.7) | |
| 2020 | |||||||
| January | 13 | 35 | 135–192 (165) | 122–195 (158) | 90.7–188.4 (134.9) | 55.4–151.1 (103.0) | |
| April | 8 | 41 | 194–259 (214) | 179–297 (213) | 180.3–360.2 (242.5) | 148.3–410.9 (221.4) | |
| July | 1 | 59 | 126 | 70–170 (126) | 82.3 | 19.3–166.7 (71.0) | |
| September | 24 | 23 | 169–215 (191) | 151–229 (177) | 150.0–279.3 (200.5) | 118.8–291.8 (158.6) | |
| November | 2 | 87 | 182–190 (186) | 94–218 (140) | 181.2–198.2 (189.7) | 29.9–203.9 (94.6) | |
| December | 3 | 66 | 140–176 (153) | 137–173 (155) | 105.5–140.2 (121.7) | 81.2–163.0 (115.4) | |
| 2021 | |||||||
| January | 12 | 69 | 117–253 (197) | 110–312 (159) | 62.0–480.5 (259.2) | 49.2–560.2 (141.2) | |
| March | 1 | 22 | 125 | 100–144 (121) | 95.8 | 40.8–93.9 (67.1) | |
| Cohort | Number | DML Range (mm) | Weight Range (g) | Age Range (Days) | ||||
|---|---|---|---|---|---|---|---|---|
| F | M | F (Mean) | M (Mean) | F (Mean) | M (Mean) | F (Mean) | M (Mean) | |
| Spring | 25 | 82 | 126–215 (188) | 70–229 (140) | 82.3–279.3 (195.8) | 19.3–291.8 (95.6) | 107–159 (132) | 88–159 (116) |
| Summer | 50 | 252 | 117–253 (113) | 94–312 (151) | 55.1–480.5 (141.7) | 29.9–560.2 (112.5) | 85–154 (113) | 76–175 (115) |
| Autumn | 11 | 80 | 125–259 (192) | 100–297 (165) | 69.5–360.2 (202.2) | 40.8–410.9 (136.3) | 82–173 (122) | 85–161 (113) |
| Cohort | Number of Mature Individuals | DML Range (mm) | Age Range (Days) | |||
|---|---|---|---|---|---|---|
| F | M | F | M | F | M | |
| Spring | 17 | 58 | 169–215 (193 ± 14) | 90–229 (157 ± 32) | 107–159 (126 ± 14) | 88–159 (120 ± 14) |
| Summer | 25 | 187 | 132–253 (182 ± 28) | 108–312 (162 ± 35) | 90–154 (120 ± 15) | 76–175 (116 ± 15) |
| Autumn | 7 | 44 | 125–235 (198 ± 27) | 122–297 (198 ± 31) | 94–138 (126 ± 15) | 86–161 (122 ± 15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, C.; Guo, J.; Wang, H.; Pang, Y.; Tian, Y. Age and Growth of Mitre Squid (Uroteuthis chinensis) in the Northwestern South China Sea Based on Statolith Microstructure Analysis. Diversity 2024, 16, 395. https://doi.org/10.3390/d16070395
Liu D, Zhang C, Guo J, Wang H, Pang Y, Tian Y. Age and Growth of Mitre Squid (Uroteuthis chinensis) in the Northwestern South China Sea Based on Statolith Microstructure Analysis. Diversity. 2024; 16(7):395. https://doi.org/10.3390/d16070395
Chicago/Turabian StyleLiu, Dan, Chi Zhang, Jianzhong Guo, Haozhan Wang, Yumeng Pang, and Yongjun Tian. 2024. "Age and Growth of Mitre Squid (Uroteuthis chinensis) in the Northwestern South China Sea Based on Statolith Microstructure Analysis" Diversity 16, no. 7: 395. https://doi.org/10.3390/d16070395
APA StyleLiu, D., Zhang, C., Guo, J., Wang, H., Pang, Y., & Tian, Y. (2024). Age and Growth of Mitre Squid (Uroteuthis chinensis) in the Northwestern South China Sea Based on Statolith Microstructure Analysis. Diversity, 16(7), 395. https://doi.org/10.3390/d16070395







