Abstract
The high diversity and biomass of organisms associated with coral communities depend directly on the maintenance or changes in the benthic composition. Over a decade, we evaluated the spatiotemporal variation in the benthic structure and composition of an insular coral community in the Northeastern Tropical Pacific. Our results show that local conditions drive spatiotemporal differences, and benthic organisms such as sponges, crustose coralline algae, octocorals, and hydrocorals all increased in abundance (cover) in response to negative thermal anomalies caused by the 2010–2011 La Niña event. In contrast, abnormally high temperatures, such as those recorded during the 2015–2016 El Niño Southern Oscillation (ENSO) event, explain the loss of scleractinian corals and crustose coralline algae coverage, which reduced the benthic groups’ richness (BGR), diversity (H’BG), and evenness (J’BG), with evidence of a consequent decrease in ecosystem function recorded the following year. Our analysis also showed that sites with high habitat heterogeneity harbored higher average BRG and H’BG values and were less affected by environmental fluctuations than sites with high live scleractinian coral cover and lower BRG and H’BG values. Therefore, the benthic structure was impacted differently by the same perturbation, and changes in the benthic community composition affected the groups associated with the community and ecological functions. More importantly, regional stressors such as the ENSO event caused only temporary changes in the benthic community structure, demonstrating the high resilience of the community to annual and interannual stressors.
1. Introduction
Coral communities are complex and dynamic habitats, with a structural base composed of rocky substrate where calcifying organisms such as hermatypic corals and crustose coralline algae (CCA) reside [1,2,3]. These calcifiers build the biogenic base for other benthic organisms, such as macroalgae, turf algae, sponges, hydrocorals, and octocorals [4,5]. The presence of various sessile groups contributes to the reef system’s functionality, as they provide shelter, accommodation, and food resources for high biodiversity and biomass among mobile species [6]. In addition, the presence of heterotrophic sessile organisms can influence the water column conditions, such as by altering turbidity through actively feeding on suspended organic matter [7,8]. Therefore, a benthic community’s composition impacts habitat diversity and functional richness [9].
The composition and structure of the benthos and its associated functions depend on the reproduction and recruitment of their representative taxa. These processes are limited or enhanced initially by the available settlement substrate and afterward by the ability of the organisms to cope with the synergic effects of seasonal and annual environmental conditions [10,11]. Sea surface temperature (SST) is considered to be the environmental condition with the most significant effect on the presence and survival of invertebrates, and it can directly affect biochemical and physiological processes such as growth and reproduction [12]. Additionally, light and nutrient concentrations are also crucial, especially for photosynthetic autotrophs such as algae and algal turf [13]. These factors are similarly important to organisms that rely on the efficiency of their photosynthetically active endosymbiotic microalgae [14], including octocorals [15], hydrocorals [16], and scleractinian corals [17,18,19].
Also, the influence of interannual climatic fluctuations, such as those driven by El Niño Southern Oscillation (ENSO) events, can trigger ephemeral [20] or permanent changes in the presence or abundance of benthic communities [21]. Abnormally high temperatures elicit a stress response in all the organisms. However, they are particularly threatening to scleractinian corals, which can bleach if the high-temperature event is prolonged (generally for days to weeks), promoting massive mortality rates depending on the intensity of the thermal anomaly [22]. In contrast, abnormally low temperatures associated with La Niña events can also trigger a bleaching response, albeit generally with lower incidences of coral mortality [20]. Since there is variation in the thermotolerances of various organisms [20,21], moderate stress levels can promote colonization by new, more thermotolerant groups, while intense or frequent stress events can drive permanent changes in the functional dynamics of coral communities [23,24].
The Eastern Tropical Pacific (ETP) is considered suboptimal for coral reef development [25]. Despite this, it indeed hosts important, rich, and diverse hermatypic coral communities, including 45 reef-building coral species (of which eight are endemic) [3,25]. The Northern ETP, which includes the Revillagigedo Islands, the Gulf of California, and tropical mainland Mexico, constitutes the northern limit of the distribution of scleractinian corals in the ETP [2]. Northern ETP marine ecosystems shelter coral patches and small frameworks, with an average coral coverage of 20–50% composed of 24 scleractinian coral species, mainly represented by the genera Pocillopora, Pavona, Porites, and Psammocora though with a few representatives from Cycloseris, Gardineroseris, and Leptoseris [2,3,25,26]. ETP coral communities, like reefs elsewhere, have historically been negatively affected by ENSO events, which have caused mass mortality and, consequently, changes in coral richness and abundance. Nevertheless, the region has shown slow recovery from these events over the last few decades [27]. However, it has not been determined whether they have permanently changed the benthic composition of the coral communities. A shift in community dynamics has far-reaching implications. Aside from loss or change in key groups such as scleractinian corals and other associated organisms such as echinoderms and fish, more widespread changes in ecological processes and services can transpire over extended periods.
Therefore, a comprehensive understanding of the short- and long-term changes in benthic composition is crucial for elucidating a coral community’s function and its ability to persist or recover from a disturbance. The present study represents the first long-term study of spatiotemporal variation in a coral community’s structure and function in the northernmost region of the ETP. We were particularly interested in understanding to what extent temporal variation in the structure and function of a community is caused by annual sea temperature fluctuations or interannual stress events such as the thermal positive and negative anomalies caused by ENSO events over a decadal timescale. The results could have implications for our overall assessment of the ability of these coral ecosystems to resist future climate change. Finally, the results of our temporal analysis could potentially be extrapolated to other coral ecosystems located throughout the ETP or other areas with conditions similar to those in this study, as the response or change of the benthic community in any coralline area will affect the abundance and health of the resident organisms.
2. Materials and Methods
2.1. Study Area
Islas Marietas National Park (IMNP) is an insular natural protected area (NPA) located in the Central Mexican Pacific (20°41′56″–20°41′53″ N, 105°35′02″–105°34′59″ W; Figure 1). The NPA consists of two islands of volcanic origin—Isla Redonda (IR) and Isla Larga (IL)—which differ in benthic structure. Each harbors a diverse coral community where Pocillopora is the most abundant coral genus across a depth range of 1–10 m [28]. IL features a higher cover of pocilloporids with a lower cover of massive and encrusting Porites and Pavona spp. The latter are more common on IR across a depth range of 2–18 m [29,30].
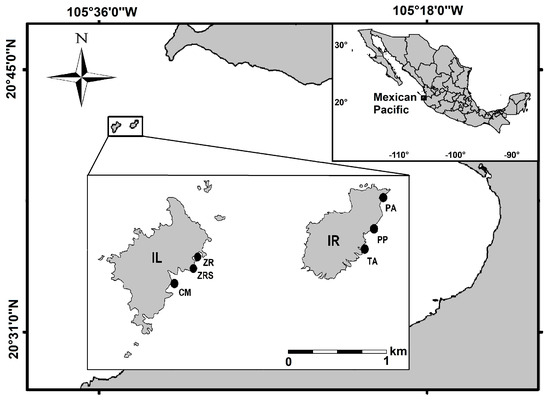
Figure 1.
Study area and sampling sites at Islas Marietas National Park in the Mexican Pacific. Codes: IL = Isla Larga, CM = Cueva del Muerto, ZR = Zona de Restauración, ZRS = Zona de Restauración Sur, IR = Isla Redonda, TA = Túnel-Amarradero, PP = Plataforma Pavonas, and PA = Playa del Amor.
Three water masses influence the region, resulting in two hydroclimatic periods. The first, the California Current, carries cold, low-salinity waters from January to May, leading to a cold period (18–21 °C), followed by the Mexican Coastal Current and the water mass of the Gulf of California, characterized by high-temperature (27–30 °C) and high-salinity waters from July to November [31,32]. The area is also influenced by seasonal events such as upwelling, storms, hurricanes, and abnormal interannual conditions such as positive and negative thermal anomalies derived from ENSO events [32,33,34]. IMNP possesses high ecological, economic, and social relevance, as it represents a tourist hotspot where activities such as snorkeling, diving, and boating are allowed [29]. Despite the protection of the area, the touristic use of the site has historically exceeded the ecosystem’s capacity which, in addition to the aforementioned natural stressors, has negatively affected the coral cover [29,35]. As a mitigation response, a restoration program was implemented in 2014 to reverse the loss of cover of Pocillopora spp. corals [36].
2.2. Data Collection and Analyses
The sampling work was conducted from 2012 to 2021 as part of a long-term monitoring program. Surveys were conducted over two hydroclimatic periods (warm and cold) in each year at the two islands (IL and IR). Three representative sites were surveyed at IL—Cueva del Muerto (CM), Zona de Restauración (ZR), and Zona de Restauración Sur (ZRS)—and at IR: Plataforma Pavonas (PP), Túnel Amarradero (TA), and Playa del Amor (PA). Surveys consisted of visual censuses of five 25 m belt transects laid parallel to the coast per site, resulting in 600 surveyed transects (5/site/season/year × 6 sites × 2 seasons/year × 10 years). In each transect, benthic group coverage (%) was estimated in situ and recorded using six quadrats (1 m2) distributed equidistantly along the 25 m linear transect. Benthic groups were classified as scleractinian corals (Pocillopora, Pavona, or Porites), sponges, hydrocorals, octocorals, algal turf, macroalgae, crustose coralline algae (CCA), and articulated calcareous algae (ACA; Table S1). Additionally, the mean annual values of the sea surface temperature (SST), photosynthetically available radiation (PAR), and diffuse attenuation coefficient (Kd 490) over the study period were calculated using monthly satellite images (4 × 4 km) from the AQUA/MODIS Oceancolor platform (https://oceancolor.gsfc.nasa.gov/l3/ accessed on 3 November 2023).
The variations in the benthic groups’ compositions and coverage were analyzed using permutational multidimensional analysis of variance (PERMANOVA). The data underwent a fourth-root transformation before constructing the Bray–Curtis similarity matrix. The contribution of benthic groups to average dissimilarity among each factor level was estimated using similarity percentage analysis (SIMPER) with a 65% cutoff, based on the same data pretreatment and resemblance coefficient used in PERMANOVA. For each transect, the benthic groups’ structural changes were evaluated using the following metrics with PRIMER v.6: (1) the benthic group richness (BGR), (2) Shannon entropy (H’BG nats), (3) Pielou evenness (J’BG), and Simpson dominance (D BG) [37]. In most studies, these community metrics have been estimated at the species level. However, they can also be applied to a group of organisms [38,39,40,41,42,43,44,45] and, for our particular purpose, benthic groups. This numerical approximation allows for evaluation of the changes in the heterogeneity of their richness (number of groups) and abundance (percentage of cover) in different spatiotemporal scales.
The benthic group’s functional changes were assessed from a multidimensional approach based on five functional transversal traits for marine benthic groups: shape, size, type of nutrition, consumption/biomass rate (Q/B), and production/biomass rate (P/B). This allowed for assessment of the group’s qualitative and quantitative characteristics [46,47,48]. Additionally, a new trait, “framework builders”, was included to classify organisms based on their contributions to calcification and reef consolidation: “reef-building” or “non-reef-building” (Table 1 and Table 2). The benthic groups’ functions were estimated for each transect using the following multi-functional metrics: (1) benthic functional richness (FRicBG), (2) functional divergence (FDivBG), and (3) functional dispersion (FDisBG). These functional metrics were calculated with the “FD” package in RStudio [49].

Table 1.
Description of benthic group functional traits used to build the functional entities matrix.

Table 2.
Functional entities matrix of the benthic groups recorded at Islas Marietas National Park and coverage of each group per island (mean ± std. error). Abbreviations: consumption/biomass rate (Q/B), production/biomass rate (P/B), Isla Larga (IL), Isla Redonda (IR), crustose coralline algae (CCA), and articulated calcareous algae (ACA).
Spatiotemporal variation in the benthic groups’ structural and functional metrics was analyzed using permutational covariance analyses (ANCOVAs), with the total live coral cover used as a covariate to eliminate bias due to its influence on the cover of other benthic groups and the changes generated by the coral restoration project. The permutational ANCOVAs were constructed with matrices of the Euclidean distance following the criteria described previously [37]. In the case of J’BG and FdivBG, the covariate was not significant, and spatiotemporal variation was evaluated with permutational analyses of variance (ANOVA) using an Euclidean distance matrix. PERMANOVAs (Equation (1)), permutational ANOVAs (Equation (1)), and permutational ANCOVAs (Equation (2)) were run as two-way analyses, with the crossed- and fixed-effect factors (model type I) of the year (Yei; 10 levels: 2012–2021), island (Isj; two levels: IL or IR), and, in the case of ANCOVAs, the covariate (Co). These models could be expressed as follows:
where Y is the predicted variable or matrix, μ is the mean, and εij is the cumulative error.
Y = μ + Yei + Isj + Yei × Isj + εij
Y = μ + Co + Yei + Isj + Yei × Isj + εij
The statistical significances of the PERMANOVA, permutational ANOVA, and permutational ANCOVA models were tested using 10,000 permutations under reduced models, but the former two used sum of squares (SS) type III (partial), while the third used SS type I (sequential). PRIMER v.6 with PERMANOVA+ software [37] was used to perform the ANOVAs, ANCOVAs, PERMANOVAs, and SIMPERs. Next, multiple linear regressions were calculated to explore the relationships among the benthic groups’ structural and functional metrics with environmental variables, selecting models without multicollinearity. Therefore, we selected models that included predictor variables with Pearson correlations <90% and variance inflation factor (VIF) values <3. Each regression factor passed the homogeneity of variance and normality tests (Shapiro–Wilk p < 0.05). Simple linear regressions were performed using SigmaPlot V. 11.0. 3 for the single-variable models.
3. Results
The benthic groups’ compositions exhibited spatiotemporal differences, with the spatial factor explaining the highest percentage of the variation (Table 3). Over the 10 years, significant differences were observed between the islands (Table S2), and the highest contribution to dissimilarity was attributed to the cover of scleractinian corals, macroalgae, articulated calcareous algae (ACA), and CCA (Table S3). IL exhibited greater coverage of scleractinian corals and macroalgae (Figure 2 and Table 2). Meanwhile, IR harbored greater cover of CCA and ACA (Figure 2 and Table 2). The remaining benthic groups (algal turf, hydrocorals, and octocorals) were primarily represented at IR (Figure 2 and Table 2).

Table 3.
Results of PERMANOVA, permutational ANCOVA 1, and permutational ANOVA 2. Abbreviations: Co = covariate (live coral cover), BGR = benthic group richness, H’BG = Shannon entropy, DBG = Simpson dominance, J’BG = Pielou evenness, FRicBG = functional richness, FDisBG = functional dispersion, FDivBG = functional divergence, P-F = Pseudo-F, p = p-value, and C.V.% = component of variation in percentage (%). The p-values in bold indicate statistical differences (p ≤ 0.05).
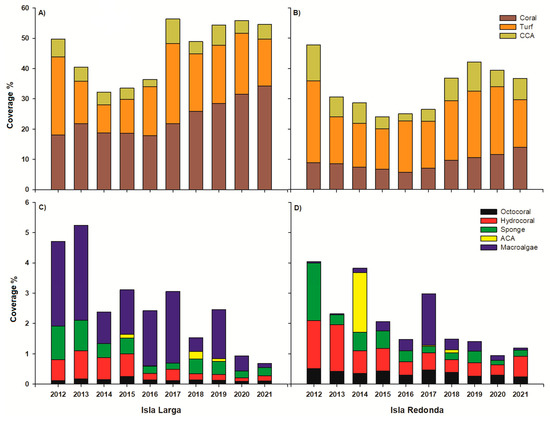
Figure 2.
Spatiotemporal variation in the benthic communities at Isla Larga (A,C) and Isla Redonda (B,D).
Temporal variation was evident over the 10 year period for each island. Differences in IR (Table S2) could be attributed to a decrease in algal turf (Figure 2B and Table S4) and sponges (Figure 2D and Table S4) in 2013 and an increase in macroalgae and ACA in 2014 (Figure 2D and Table S4). Meanwhile, differences at IL were observed from 2015 to 2018 (Table S2) and were linked to variation in the cover of algal turf (Figure 2A and Table S4), CCA, and ACA (Figure 2C and Table S4). In general, there was a gradual increase in live coral cover (LCC) over time, with the highest values recorded in 2021: 34.2% on Isla Larga and 13.9% on Isla Redonda (Figure 2A,B). In contrast, the lowest values were observed in 2016 (17.8 and 5.6%, respectively). The CCA and algal turf reached peak values at IR in 2012 (11.9% and 27.0%, respectively; Figure 2B), while the levels at IL instead peaked in 2017 (8.1% and 26.5%, respectively; Figure 2A). The minimum CCA abundance (2.3%) was documented for both islands in 2016 (Figure 2A,B).
The highest sponge cover was recorded in 2012, with values of 1.1% for IL and 1.9% for IR (Figure 2C,D). In the same year, the highest octocoral (0.5%) and hydrocoral (1.5%) covers were observed at IR (Figure 2D). In 2020, the lowest cover of octocorals was observed at IL (0.08%; Figure 2C), and the lowest hydrocoral abundances were documented at IL and IR at this time (0.02% and 0.3%, respectively; Figure 2C,D). Regarding macroalgae, the greatest cover was noted in 2013 for IL (3.1%; Figure 2C). Macroalgae were, in contrast, least abundant in 2013 at IR (0.03%; Figure 2D). ACA’s abundance was highest at IR in 2014 (1.9%) and at IL in 2018 (0.2%; Figure 2C).
The permutational ANCOVA outputs show that the covariate (LCC) was significant for all structural and functional metrics, except J’BG and FDivBG. Likewise, the spatial or temporal variation observed in the benthic groups’ structures was related to the fluctuation in LCC over time (Table 3). However, when considering the benthic structural metrics, the richness and Shannon entropy varied significantly among islands (Table 3). The highest mean BGR (5.51 ± 0.06; Figure 3A) and Shannon entropy (H’BG = 1.06 ± 0.01; Figure 3B) were documented at IR. Also, differences in the benthic structure diversity over the 10 years were evident (Table 3 and Table S5), and the maximum BGR (5.75 ± 0.15; Figure 3A) was observed in 2015, followed by a subsequent decline in H’BG (0.95 ± 0.02; Figure 3B) and J’BG (0.58 ± 0.01; Figure 3B) in 2016. During the same year (2016), there was an increase in DBG (0.48 ± 0.01; Figure 3B). In 2020, both H’BG (0.92 ± 0.02) and J’BG (0.60 ± 0.01) declined (Figure 3B).
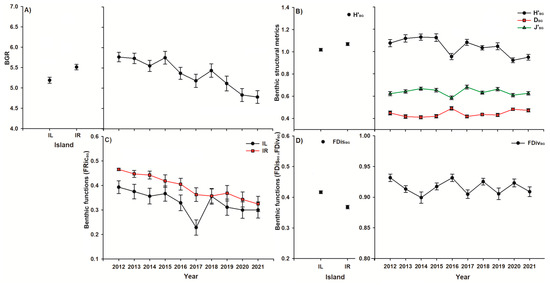
Figure 3.
Spatiotemporal comparison of benthic structural and functional metrics (A–D; mean ± std. error). Abbreviations: BGR = benthic group richness, H’BG = Shannon entropy, J’BG = Pielou evenness, DBG = Simpson dominance, FRicBG = functional richness, FDisBG = functional dispersion, FDivBG = functional divergence, IL = Isla Larga, and IR = Isla Redonda.
The benthic groups’ functions also showed variations as the functional dispersion varied significantly between islands (Table 3), and the highest functional dispersion (FDisBG = 0.41 ± 0.004; Figure 3D) was reported in IL. The functional richness showed significant differences in spatiotemporal interaction (Table 3). The decrease in the functional richness (Figure 3C) over time resulted in significant differences between 2012 (FRicBG = 0.46 ± 0.004) and 2021 (FRicBG = 0.32 ± 0.03) for Isla Redonda (Figure 3C, Table S6). Isla Larga showed differences between 2017 (FRicBG = 0.22 ± 0.03) and 2018 (FRicBG = 0.35 ± 0.03) after the increase in FRicBG (Figure 3C, Table S6). The spatial variation of the benthic groups’ functional richness was similar, but the temporal change showed a loss of functional space loss over time (Table S6). Additionally, the functional divergence varied temporally (Table 3), with a significant change in 2012 (FDivBG = 0.93 ± 0.006) versus 2013 (FDivBG = 0.91 ± 0.005) and later in 2017 (FDivBG = 0.90 ± 0.006) versus 2018 (FDivBG = 0.92 ± 0.005) (Figure 3D, Table S7).
When considering the functional traits of each benthic group, Isla Redonda displayed higher heterogeneity in its benthic organisms and average production/biomass, which is related to the coverage of benthic autotrophs such as algal turf, ACA, CCA, and macroalgae (Table 2). Also, the temporal variation of benthic groups caused a higher average coverage of framework builders and organisms with various shapes and sizes at Isla Redonda during 2012. In 2016, the coverage of framework builders with a high consumption/biomass rate and large size decreased in both islands. The following year (2017), small autotrophic organisms with a high production/biomass rate and a single shape surpassed them in coverage on Isla Larga.
The data on environmental conditions show that the highest sea surface temperature was recorded in 2015 (27.94 ± 0.67 °C), while the lowest one was observed in 2021 (26.31 ± 0.99 °C) (Figure 4). Photosynthetically available radiation increased from 2013 (45.78 ± 2.54 Einstein m−2 day−1) to 2020 (48.62 ± 2.58 Einstein m−2 day−1) (Figure 4). The lowest values for Kd 490 (0.08 ± 0.006 m−1) were observed in 2016, while the highest values (0.26 ± 0.07 m−1) were observed in 2021 (Figure 4). The regression models between the structural and functional metrics and environmental variables indicated a negative relationship between the Shannon entropy and PAR (Table 4, Figure 5) and a relationship between the benthic group richness and benthic functional richness with the PAR, SST and Kd 490 (Table 4). The rest of the indexes were not significantly related to the environmental variables.
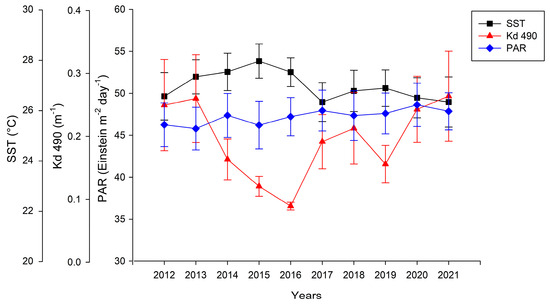
Figure 4.
Temporal variability (±std. error) in diffuse attenuation coefficient (Kd 490), sea surface temperature (SST), and photosynthetically active radiation (PAR) at Islas Marietas National Park.

Table 4.
Simple and multiple linear regression outputs for the relationships among the benthic groups’ structural and functional metrics versus environmental variables. Abbreviations: BGR = benthic group richness, H’BG = Shannon entropy, DBG = Simpson dominance, J’BG = Pielou evenness, FRicBG = functional richness, FDivBG = functional divergence, PAR = photosynthetically active radiation, SST = sea surface temperature, Kd 490 = diffuse attenuation coefficient, and p = p-value. Values in bold are significant (p-value ≤ 0.05).
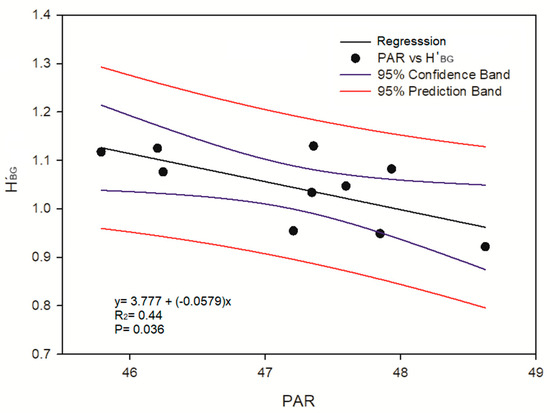
Figure 5.
Linear regression between photosynthetically active radiation (PAR) and Shannon entropy (H’BG).
4. Discussion
As a region, the ETP shares conditions and, therefore, a homogeneous ecological process and similar life history trails. However, the benthic composition will also be affected by seasonal local conditions (e.g., the presence of upwellings or runoffs), the degree of wave exposure, light penetration, and depth [42,43,44,50,51,52]. Within the benthic groups of any coral ecosystem, the response and sensitivity to local conditions and the role that life history has in the long term have been primarily studied in scleractinian corals [53,54]. However, the maintenance of the coral ecosystem depends on the whole benthic composition and their tolerance and response to spatial (local vs. regional) and temporal (seasonal vs. interannual fluctuations) conditions, as the changes will be evident not only in the structure (i.e., benthic composition) but in the ecosystem’s functions when considered long term.
Although Isla Redonda and Isla Larga are close to each other, each has a particular structure and local conditions. Isla Larga is a shallow area affected by high wave exposure and sedimentation rates [55], both of which are parameters considered detrimental to coral development. However, the site harbors the highest scleractinian coral coverage, where branching Pocillopora corals are the most abundant forming reef patches, but there is also the presence of encrusting Porites corals distributed as separated colonies contributing to the benthic composition [30]. The high abundance of Pocillopora can be attributed to an acclimatization response and ability to cope with daily environmental fluctuations [27], and instead of being considered stressful conditions, it promotes other processes such as fragmentation, which is the primary mode of reproduction of this genus [48,56], increasing their self-recruitment process, maintaining high coral coverage, and decreasing the competition by limiting the habitable space for other benthic groups and resulting in low richness and diversity but high functional dispersion.
Meanwhile, Isla Redonda’s physical structure has a steep slope. Therefore, the wave exposure is lower, and the coral is distributed along a 2–18 m depth, with low coverage of Pocillopora in the shallower areas and a high presence of encrusting and submassive colonies such as Porites and Pavona, which are associated with rocky slopes with sandy bottoms [29,30]. The abrupt slope limits the available substrata for Pocillopora, and the presence of extensive coral patches favors other groups, such as octocorals, sponges, and algae [57]. In addition, with the available substrata, the high level of inorganic nutrients, such as nitrates and nitrites, recorded at Isla Redonda [53] promotes the growth of other groups, such as algal turf, crustose coralline algae, and articulated calcareous algae [58,59], increasing both the benthic groups’ richness and the benthic functional richness of the site, as observed for the other studied benthic (J’BG) and functional metrics. This demonstrates that a high heterogeneity of benthic organisms, which usually differ in shape and size, could provide greater availability of refuge areas for associated fauna [60]. In addition, due to its greater coverage of autotrophic organisms compared with Isla Larga, the production/biomass rates and the amount of energy available to sustain the different trophic levels were higher. Sites with such characteristics usually harbor a greater biomass of organisms such as fish and marine invertebrates [46,61], increasing the site’s relevance as an NPA and refuge.
Changes in the cover and diversity of benthic groups can reveal the effects of regional changes in conditions over time. Over the study period, both La Niña and El Niño events occurred [62]. During 2010–2011, an intense La Niña event affected the region, causing abnormally low temperatures and the presence of high-nutrient and acidic seawater [54]. This led to coral bleaching and partial coral mortality [20,63] alongside a peak in the abundance of sponges, CCA, octocorals, and hydrocorals in 2012. These organisms presumably benefited from both the availability of new substrate (i.e., dead corals) and higher nutrient levels [7,10,12]. As a cascade effect, herbivorous organisms such as the sea urchin Toxopneustes roseus and the omnivorous sea urchin Centrostephanus coronatus also increased in density [64]. However, this trend was reversed in 2013, possibly due to top-down control imposed by echinoderms and the recovery of non-lethally bleached coral colonies.
Later, the 2015–2016 ENSO event which affected the region was the first “extreme El Niño” of the 21st century [62] and caused an abnormal increase in SST alongside the lowest recorded values of Kd 490. The effect of this event was also reflected in a decrease in cover of coral and CCA, though the former was only 1.3%. The recovery in subsequent years was due to both natural and human efforts in the area [36], and this reflects the high thermotolerance of the community [27]. Also, abnormally high temperatures led to a slight decline in CCA abundance, and it has indeed been suggested that such conditions could elicit chronic photoinactivation in these autotrophs [65].
Although the strong 2015–2016 El Niño event led to only rather small decreases in the cover of scleractinian corals, CCA, ACA, sponges, hydrocorals, and octocorals, there was nevertheless a change in the benthic structure and notable decreases in the benthic groups’ richness (BGR), Shanon entropy (H’BG), Pielou evenness (J’BG), benthic functional richness, and functional divergence. This may suggest a reduction in ecosystem function and production and a higher functional resemblance of the functional roles of dominant benthic groups [66]. During 2016, a reduction in the cover of heterotrophic, reef-building organisms with a high rate of consumption/biomass and a high diversity of shapes and sizes was evident. In 2017, the highest turf algal, CCA, and macroalgal cover were recorded, possibly from having capitalized on newly available settlement space [20,67,68,69]. The following year, the IMNP study sites showed rapid recovery in BGR, functional richness, and functional divergence. In 2020–2021, a decrease in octocoral coverage coincided with the highest PAR and Kd 490 values recorded. We hypothesize that the co-occurring high nutrient levels led to greater primary production, chlorophyll concentrations, and turbidity [70,71], all conditions under which octocorals fair poorly [72,73]. ENSO events will become ever more intense and frequent [62], which could reduce coral ecosystem richness, diversity, structural complexity, and ecosystem function. The latter could decrease food availability and shelter for myriad organisms, especially as framework builders decline in abundance.
Over a decade, changes in the benthic community of IMNP were evident. Such changes were driven not only by fluctuations in the local conditions of each site but also by regional-scale events, such as thermal anomalies caused by ENSO events. Even a strong ENSO in 2015–2016 affected some benthic groups, but it did not cause a permanent shift in the composition or structure of the community. Notably, significant recovery was noted within two years, indicating the resilience of the ecosystem. Maintenance of the coral ecosystem depends directly on the abundance and presence of diverse benthic groups, and at IMNP, differences in the benthic structures across islands led to differing ecological responses to the same environmental disturbance. Moreover, on a long-term basis, these study reefs can evidently cope with both short- and long-term environmental changes and support the associated biomass of reef flora and fauna. This resilience highlights the value of these reefs and provides hope for the permanence of coral communities in the region.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16070372/s1, Table S1. List and/or description of the benthic groups recorded in Islas Marietas National Park. Only the scleractinian corals included at species level; Table S2. PERMANOVA’s pairwise comparisons of benthic composition and benthic coverage. Codes: t = t-test, p = p-value. p-values in bold indicate statistical differences (p ≤ 0.05); Table S3. Spatio-temporal SIMPER results of the significant factors in the pairwise comparisons for benthic composition and coverage. Codes: Average Cov. = average coverage, contribution = Contrib%, cumulative contribution = Cum.%; Table S4. Spatio-temporal SIMPER results of the significant factors in the pairwise test for composition and benthic coverage. Codes: Average Cov. = average coverage, contribution = Contrib%, cumulative contribution = Cum.%; Table S5. Pairwise comparisons of the benthic groups’ structural metrics. Codes: BGR = benthic group richness, H’BG = Shannon entropy, DBG = Simpson dominance, J’BG = Pielou evenness, t = Student’s t-test, p = p-value. p-values in bold indicate statistical differences (p ≤ 0.05),1 = permutational ANCOVA, 2 = permutational ANOVA; Table S6. Permutational ANCOVA’s Pairwise comparisons of FRicBG. Codes: t = t-test, p = p-value. p-values in bold indicate statistical differences (p ≤ 0.05); Table S7. Permutational ANOVA’s pairwise comparisons of FDivBG. Codes: t = t-test, p = p-value, 1 = p-values in bold indicate statistical differences (p ≤ 0.05).
Author Contributions
Conceptualization, C.d.A.-G., A.L.C.-M. and A.P.R.-T.; methodology, C.d.A.-G., R.A.C.-T. and F.A.R.-Z.; validation, C.d.A.-G., A.L.C.-M., J.d.J.A.T.-L. and A.P.R.-T.; formal analysis, C.d.A.-G., R.A.C.-T., J.d.J.A.T.-L. and F.A.R.-Z.; investigation, C.d.A.-G., J.d.J.A.T.-L., A.L.C.-M. and A.P.R.-T.; resources, A.L.C.-M., J.d.J.A.T.-L. and A.P.R.-T.; writing—original draft preparation, C.d.A.-G. and A.P.R.-T.; writing—review and editing, C.d.A.-G., A.L.C.-M., R.A.C.-T., J.d.J.A.T.-L., F.A.R.-Z. and A.P.R.-T.; visualization, C.d.A.-G. and F.A.R.-Z.; funding acquisition, A.P.R.-T. and A.L.C.-M. All authors have read and agreed to the published version of the manuscript.
Funding
C.A.G. received a Ph.D. scholarship from the Centro Nacional de Ciencia y Tecnología (CONACYT) while conducting this study and writing the manuscript (ID. 806622). The present research project was funded by two National Geographic Society (NGS) grants (NGS-55349R-19 and NGS-100354C-23 to A.P.R.T.) and by the project CONANP (PROCER/CCER/DROPC/09/2016 to A.L.C.M.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data have been included in the manuscript or as part of the Supplementary Materials.
Acknowledgments
The authors thank Islas Marietas National Park’s authorities for their logistic assistance. They also thank Anderson Mayfield (CORDAP) for carefully proofreading the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Benfield, S.; Baxter, L.; Guzman, H.M.; Mair, J.M. A comparison of coral reef and coral community fish assemblages in Pacific Panama and environmental factors governing their structure. J. Mar. Biol. Assoc. UK 2008, 88, 1331–1341. [Google Scholar] [CrossRef]
- Glynn, P.W.; Alvarado, J.J.; Banks, S.; Cortés, J.; Feingold, J.S.; Jiménez, C.; Zapata, F.A. Eastern Pacific coral reef provinces, coral community structure and composition: An overview. In Coral Reefs of the Eastern Tropical Pacific: Persistence and Loss in a Dynamic Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 107–176. [Google Scholar]
- Reyes-Bonilla, H.; López-Pérez, R.A. Corals Coral Reef Communities in the Gulf of California Atlas of Coastal Ecosystems in the Western Gulf of California; Johnson, A., Ledezma-Vázquez, J., Eds.; The University of Arizona Press: Tucson, AZ, USA, 2009; pp. 45–57. [Google Scholar]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, N.E.; Morrow, K.M. Competition among sessile organisms on coral reefs. In Coral Reefs: An Ecosystem in Transition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 347–371. [Google Scholar]
- Wilson, S.K.; Graham, N.A.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Change Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Lira, A.K.; Naud, J.P.; Gomes, P.B.; Santos, A.M.; Perez, C.D. Trophic ecology of the octocoral Carijoa riisei from littoral of Pernambuco, Brazil. I. Composition and spatio-temporal variation of the diet. J. Mar. Biol. Assoc. UK 2009, 89, 89–99. [Google Scholar] [CrossRef]
- Jiménez, C. Arrecifes y ambientes coralinos de Bahía Culebra, Pacífico de Costa Rica: Aspectos biológicos, económico-recreativos y de manejo. Rev. Biol. Trop. 2001, 49, 215–231. [Google Scholar]
- Pineda, J.; Reyns, N.B.; Starczak, V.R. Complexity and simplification in understanding recruitment in benthic populations. Popul. Ecol. 2009, 51, 17–32. [Google Scholar] [CrossRef]
- Robinson, J.P.; Williams, I.D.; Yeager, L.A.; McPherson, J.M.; Clark, J.; Oliver, T.A.; Baum, J.K. Environmental conditions and herbivore biomass determine coral reef benthic community composition: Implications for quantitative baselines. Coral Reefs 2018, 37, 1157–1168. [Google Scholar] [CrossRef]
- Brierley, A.S.; Kingsford, M.J. Impacts of climate change on marine organisms and ecosystems. Curr. Biol. 2009, 19, R602–R614. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Roth, M.S. The engine of the reef: Photobiology of the coral–algal symbiosis. Front. Microbiol. 2014, 5, 422. [Google Scholar] [CrossRef]
- Rossi, S.; Schubert, N.; Brown, D.; Soares, M.D.O.; Grosso, V.; Rangel-Huerta, E.; Maldonado, E. Linking host morphology and symbiont performance in octocorals. Sci. Rep. 2018, 8, 12823. [Google Scholar] [CrossRef]
- Olguín-López, N.; Hernández-Elizárraga, V.H.; Hernández-Matehuala, R.; Rojas-Molina, J.I.; Guevara-Gonzalez, R.; Ibarra-Alvarado, C.; Molina, A.R. Effects of thermal stress caused by the 2015–2016 El Niño on the biochemical composition, exoskeleton structure, and symbiont density of the fire coral Millepora alcicornis. Cienc. Mar. 2023, 49, e3296. [Google Scholar] [CrossRef]
- Abrego, D.; Ulstrup, K.E.; Willis, B.L.; van Oppen, M.J. Species–specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. R. Soc. B Biol. Sci. 2008, 275, 2273–2282. [Google Scholar] [CrossRef]
- Leggat, W.; Seneca, F.; Wasmund, K.; Ukani, L.; Yellowlees, D.; Ainsworth, T.D. Differential responses of the coral host and their algal symbiont to thermal stress. PLoS ONE 2011, 6, e26687. [Google Scholar] [CrossRef]
- Morris, L.A.; Voolstra, C.R.; Quigley, K.M.; Bourne, D.G.; Bay, L.K. Nutrient availability and metabolism affect the stability of coral–Symbiodiniaceae symbioses. Trends Microbiol. 2019, 27, 678–689. [Google Scholar] [CrossRef]
- Cruz-García, R.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A.; Mayfield, A.; Cupul-Magaña, A.L. Ephemeral effects of El Niño–Southern Oscillation events on an eastern tropical Pacific coral community. Mar. Freshw. Res. 2020, 71, 1259–1268. [Google Scholar] [CrossRef]
- Glynn, P.W.; Mones, A.B.; Podestá, G.P.; Colbert, A.; Colgan, M.W. El Niño-Southern Oscillation: Effects on Eastern Pacific coral reefs and associated biota. In Coral Reefs of the Eastern Tropical Pacific: Persistence and Loss in a Dynamic Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 251–290. [Google Scholar]
- Baird, A.H.; Bhagooli, R.; Ralph, P.J.; Takahashi, S. Coral bleaching: The role of the host. Trends Ecol. Evol. 2009, 24, 16–20. [Google Scholar] [CrossRef]
- Richardson, L.E.; Graham, N.A.; Pratchett, M.S.; Eurich, J.G.; Hoey, A.S. Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob. Change Biol. 2018, 24, 3117–3129. [Google Scholar] [CrossRef]
- Sotelo-Casas, R.C.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A.; Solís-Marín, F.A.; Godínez-Domínguez, E.; Cupul-Magaña, A.L. Spatial-temporal variations in echinoderm diversity within coral communities in a transitional region of the northeast of the eastern pacific. Estuar. Coast. Shelf Sci. 2019, 227, 106346. [Google Scholar] [CrossRef]
- Glynn, P.W.; Ault, J.S. A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 2000, 19, 1–23. [Google Scholar]
- Cortés, J.; Enochs, I.C.; Sibaja-Cordero, J.; Hernández, L.; Alvarado, J.J.; Breedy, O.; Cruz-Barraza, J.A.; Esquivel-Garrote, O.; Fernández-García, C.; Hermosillo, A.; et al. Marine Biodiversity of Eastern Tropical Pacific Coral Reefs. In Coral Reefs of the Eastern Tropical Pacific. Coral Reefs of the World; Glynn, P., Manzello, D., Enochs, I., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 8. [Google Scholar]
- Romero-Torres, M.; Acosta, A.; Palacio-Castro, A.M.; Treml, E.A.; Zapata, F.A.; Paz-García, D.A.; Porter, J.W. Coral reef resilience to thermal stress in the Eastern Tropical Pacific. Glob. Change Biol. 2020, 26, 3880–3890. [Google Scholar] [CrossRef]
- Velarde, E.; Cartron, J.L.E.; Drummond, H.U.G.H.; Anderson, D.W.; Gallardo, F.R.; Palacios, E.; Rodríguez, C. Nesting seabirds of the Gulf of California’s offshore islands: Diversity, ecology, and conservation. In Biodiversity, Ecosystems, and Conservation in Northern Mexico; Oxford University Press: Oxford, UK, 2005; pp. 452–470. [Google Scholar]
- CONANP (Comisión Nacional de Áreas Naturales Protegidas). Programa de Conservación Y Manejo, Parque Nacional Islas Marietas; Secretaria de Medio Ambiente y Recursos Naturales (SEMARENAT): Mexico City, Mexico, 2007; pp. 21–57. [Google Scholar]
- Hernández-Zulueta, J.; Rodríguez-Zaragoza, F.A.; Araya, R.; Vargas-Ponce, O.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, A.L.; Díaz-Pérez, L.; Ríos-Jara, E.; Ortiz, M. Multi-scale analysis of hermatypic coral assemblages at Mexican Central Pacific. Sci. Mar. 2017, 81, 91–102. [Google Scholar]
- Palacios-Hernández, E.; Carrillo, L.E.; Filonov, A.; Brito-Castillo, L.; Cabrera-Ramos, C.E. Seasonality and anomalies of sea surface temperature off the coast of Nayarit, Mexico. Ocean. Dyn. 2010, 60, 81–91. [Google Scholar]
- Pantoja, D.A.; Marinone, S.G.; Parés-Sierra, A.; Gómez-Valdivia, F. Modelación numérica de la hidrografía y circulación estacional y de mesoescala en el Pacífico central mexicano. Cienc. Mar. 2012, 38, 363–379. [Google Scholar]
- García-Oliva, F.; Camou, A.; Maass, J.M. El clima de la región central de la costa del Pacífico mexicano. In Historia Natural de Chamela; Instituto de Biología de la UNAM: CDMX, Mexico, 2002; pp. 3–10. [Google Scholar]
- Wang, C.; Fiedler, P.C. ENSO variability and the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 239–266. [Google Scholar] [CrossRef]
- Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P. Tourist carrying capacity at Islas Marietas National Park: An essential tool to protect the coral community. Appl. Geogr. 2017, 88, 15–23. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.A.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, A.L.; Alarcón-Ortega, L.C.; Santiago-Valentín, J.D. Accelerated recovery of calcium carbonate production in coral reefs using low-tech ecological restoration. Ecol. Eng. 2019, 128, 89–97. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, R.K. PERMANOVA+ para PRIMER: Guía para el Programa y Métodos Estadísticos; PRIMER-E, Massey University: Plymouth, UK, 2008; p. 264. [Google Scholar]
- Usseglio-Polatera, P.; Bournaud, M.; Richoux, P.; Tachet, H. Biological and ecological traits of benthic freshwater macroinvertebrates: Relationship and definition of groups with similar traits. Freshw. Biol. 2000, 43, 175–205. [Google Scholar] [CrossRef]
- Haybach, A.; Schöll, F.; König, B.; Kohmann, F. Use of biological traits for interpreting functional relationships in large rivers. Limnologica 2004, 34, 451–459. [Google Scholar] [CrossRef]
- Devin, S.; Beisel, J.N.; Usseglio-Polatera, P.; Moreteau, J.C. Changes in functional diversity in an invaded freshwater ecosystem: The Moselle River. Hydrobiologia 2005, 542, 113–120. [Google Scholar] [CrossRef]
- Heino, J. Functional diversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshw. Biol. 2005, 50, 1578–1587. [Google Scholar] [CrossRef]
- Heino, J. Patterns of functional biodiversity and function-environment relationships in lake littoral macroinvertebrates. Limnol. Oceanogr. 2008, 53, 1446–1455. [Google Scholar] [CrossRef]
- Bazzanti, M.; Bella, V.D.; Grezzi, F. Functional characteristics of macroinvertebrate communities in Mediterranean ponds (Central Italy): Influence of water permanence and microhabitat type. Annales de Limnologie—International. J. Limnol. 2009, 45, 29–39. [Google Scholar] [CrossRef]
- Göthe, E.; Sandin, L.; Allen, C.R.; Angeler, D.G. Quantifying spatial scaling patterns and their local and regional correlates in headwater streams: Implications for resilience. Ecol. Soc. 2014, 19, 15. [Google Scholar] [CrossRef]
- Rodríguez-Zaragoza, F.A.; Arias-González, J.E. Coral biodiversity and bio-construction in the northern sector of the mesoamerican reef system. Front. Mar. Sci. 2015, 2, 13. [Google Scholar] [CrossRef]
- Cáceres, I.; Ibarra-García, E.C.; Ortiz, M.; Ayón-Parente, M.; Rodríguez-Zaragoza, F.A. Effect of fisheries and benthic habitat on the ecological and functional diversity of fish at the Cayos Cochinos coral reefs (Honduras). Mar. Biodivers. 2020, 50, 9. [Google Scholar] [CrossRef]
- Figueroa, N.N.; Brante, A.; Viard, F.; Leclerc, J.C. Greater functional similarity in mobile compared to sessile assemblages colonizing artificial coastal habitats. Mar. Pollut. Bull. 2021, 172, 112844. [Google Scholar] [CrossRef]
- Van Son, T.C.; Oug, E.; Halvorsen, R.; Melsom, F. Gradients in traits composition and their relation to environmental complex-gradients and structuring processes: A study of marine sediment species communities. Open Mar. Biol. J. 2013, 7, 14–27. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Keith, S.A.; Woolsey, E.S.; Madin, J.S.; Byrne, M.; Baird, A.H. Differential establishment potential of species predicts a shift in coral assemblage structure across a biogeographic barrier. Ecography 2015, 38, 1225–1234. [Google Scholar] [CrossRef]
- Lange, I.D.; Benkwitt, C.E.; McDevitt-Irwin, J.M.; Tietjen, K.L.; Taylor, B.; Chinkin, M.; Gunn, R.L.; Palmisciano, M.; Steyaert, M.; Wilson, B.; et al. Wave exposure shapes reef community composition and recovery trajectories at a remote coral atoll. Coral Reefs 2021, 40, 1819–1829. [Google Scholar] [CrossRef]
- Yasuhara, M.; Danovaro, R. Temperature impacts on deep-sea biodiversity. Biol. Rev. 2016, 91, 275–287. [Google Scholar] [CrossRef]
- Martínez-Castillo, V.; Rodríguez-Troncoso, A.P.; Santiago-Valentín, J.D.; Cupul-Magaña, A.L. The influence of urban pressures on coral physiology on marginal coral reefs of the Mexican Pacific. Coral Reefs 2020, 39, 625–637. [Google Scholar] [CrossRef]
- Gilmour, J.P.; Cook, K.L.; Ryan, N.M.; Puotinen, M.L.; Green, R.H.; Heyward, A.J. A tale of two reef systems: Local conditions, disturbances, coral life histories, and the climate catastrophe. Ecol. Appl. 2022, 32, e2509. [Google Scholar] [CrossRef]
- Martínez-Castillo, V.; Rodríguez-Troncoso, A.P.; de Jesús Adolfo Tortolero-Langarica, J.; Bautista-Guerrero, E.; Padilla-Gamiño, J.; Cupul-Magaña, A.L. Coral performance and bioerosion in Central Mexican Pacific reef communities. Hydrobiologia 2022, 849, 2395–2412. [Google Scholar] [CrossRef]
- Chávez-Romo, H.E.; Paz-García, D.A.; Correa-Sandoval, F.; Reyes-Bonilla, H.; López-Pérez, R.A.; Medina-Rosas, P. Difference in reproductive strategies of two scleractinian corals (branching vs. massive) along the west coast of Mexico. Cienc. Mar. 2013, 39, 387–400. [Google Scholar] [CrossRef][Green Version]
- Sánchez, J.A.; Ardila, N.E.; Andrade, J.; Dueñas, L.F.; Navas, R.; Ballesteros, D. Octocoral densities and mortalities in Gorgona Island, Colombia, Tropical Eastern Pacific. Rev. Biol. Trop. 2014, 62, 209–219. [Google Scholar] [CrossRef]
- Minnery, G.A. Crustose coralline algae from the Flower Garden Banks, Northwestern Gulf of Mexico; controls on distribution and growth morphology. J. Sediment. Res. 1990, 60, 992–1007. [Google Scholar]
- Miller, R.J.; Juska, C.; Hocevar, J. Submarine canyons as coral and sponge habitat on the eastern Bering Sea slope. Glob. Ecol. Conserv. 2015, 4, 85–94. [Google Scholar] [CrossRef]
- Dominici-Arosemena, A.; Wolff, M. Reef fish community structure in the Tropical Eastern Pacific (Panamá): Living on a relatively stable rocky reef environment. Helgol. Mar. Res. 2006, 60, 287–305. [Google Scholar] [CrossRef]
- Russ, G.R. Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral reefs 2003, 22, 63–67. [Google Scholar] [CrossRef]
- Santoso, A.; Mcphaden, M.J.; Cai, W. The defining characteristics of ENSO extremes and the strong 2015/2016 El Niño. Rev. Geophys. 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Nava, H.; Carballo, J.L. Environmental factors shaping boring sponge assemblages at Mexican Pacific coral reefs. Mar. Ecol. 2013, 34, 269–279. [Google Scholar] [CrossRef]
- Sotelo-Casas, R.C.; Cupul-Magaña, A.L.; Rodríguez-Zaragoza, F.A.; Solís-Marín, F.A.; Rodríguez-Troncoso, A.P. Structural and environmental effects on an assemblage of echinoderms associated with a coral community. Mar. Biodivers. 2018, 48, 1401–1411. [Google Scholar] [CrossRef]
- Webster, N.S.; Soo, R.; Cobb, R.; Negri, A.P. Elevated seawater temperature causes a microbial shift on crustose coralline algae with implications for the recruitment of coral larvae. ISME J. 2011, 5, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.L.; Edwards, C.B.; Fox, M.D.; Amir, C.G.; Eynaud, Y.; Johnson, M.D.; Smith, J.E. Changes in benthic community composition associated with the outbreak of the corallimorph, Rhodactis howesii, at Palmyra Atoll. Coral Reefs 2019, 38, 1267–1279. [Google Scholar] [CrossRef]
- Fox, M.D.; Carter, A.L.; Edwards, C.B.; Takeshita, Y.; Johnson, M.D.; Petrovic, V.; Smith, J.E. Limited coral mortality following acute thermal stress and widespread bleaching on Palmyra Atoll, central Pacific. Coral Reefs 2019, 38, 701–712. [Google Scholar] [CrossRef]
- Glynn, P.W.; Riegl, B.; Correa, A.; Baums, I.B. Rapid recovery of a coral reef at Darwin Island, Galapagos Islands. Galapagos Res. 2009, 66, 6. [Google Scholar]
- Barth, J.A.; Menge, B.A.; Lubchenco, J.; Chan, F.; Bane, J.M.; Kirincich, A.R.; McManus, M.A.; Nielsen, K.J.; Pierce, S.D.; Washburn, L. Delayed upwelling alters nearshore coastal ocean ecosystems in the northern California current. Proc. Natl. Acad. Sci. USA 2007, 104, 3719–3724. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.M.; Moynihan, M.A.; Sanwlani, N.; Switzer, A.D. Light limitation and depth-variable sedimentation drives vertical reef compression on turbid coral reefs. Front. Mar. Sci. 2020, 7, 571256. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 2010, 20, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Abad, R.; Jaramillo, K.B.; Castro, D.; Sánchez, J.A.; Rodríguez, J. Octocoral Distribution Patterns at the Equatorial Front (Tropical Eastern Pacific): Muricea and Leptogorgia. Oceans 2022, 3, 218–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).