Climate-Change-Driven Shifts in Aegilops tauschii Species Distribution: Implications for Food Security and Ecological Conservation
Abstract
1. Introduction
2. Materials and Methods
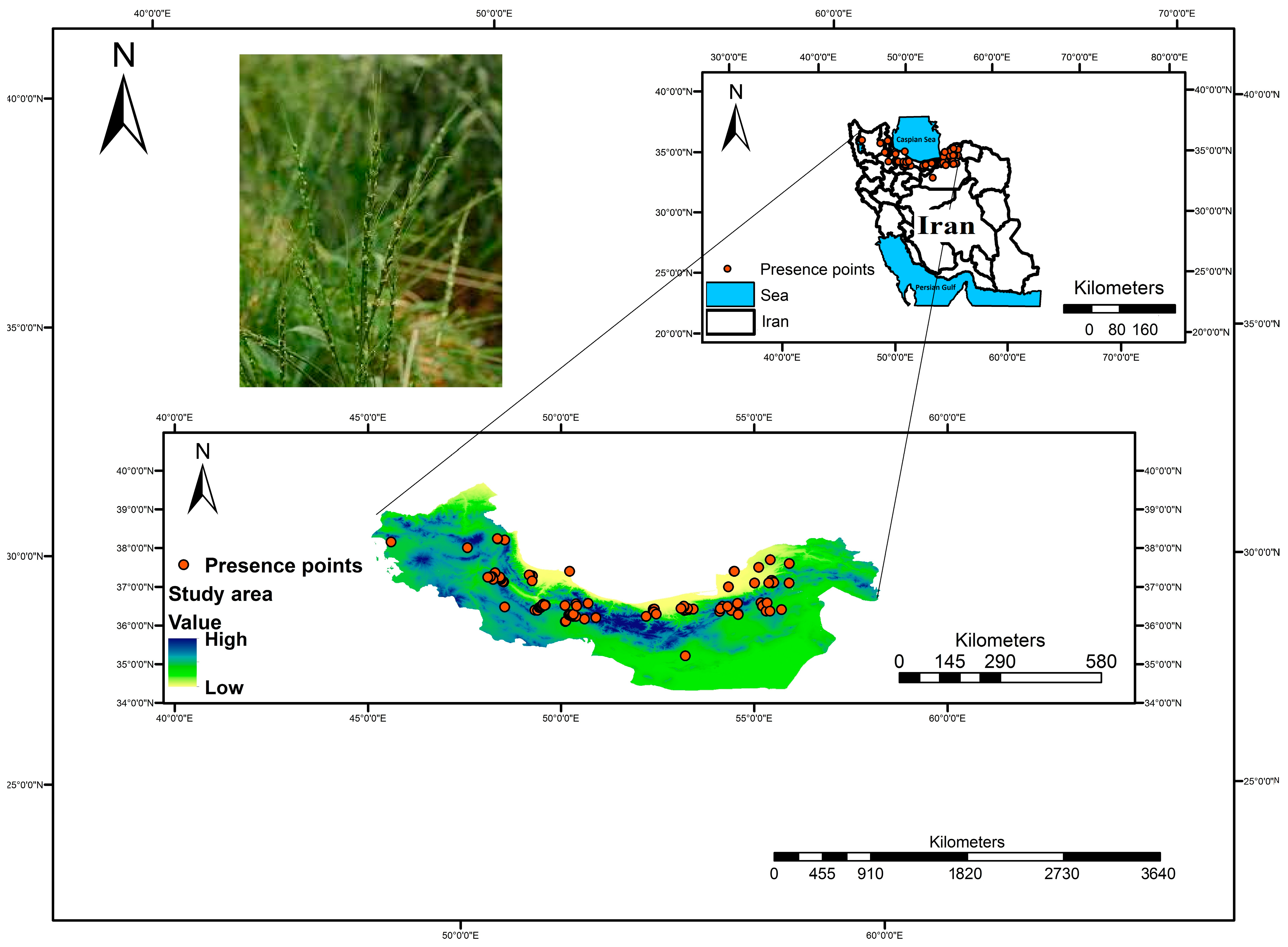
2.1. Study Area and Sampling Method
2.2. Environmental Variables
2.3. Species Distribution Models and Evaluation Criteria
3. Results
3.1. Evaluation of Species Distribution Models
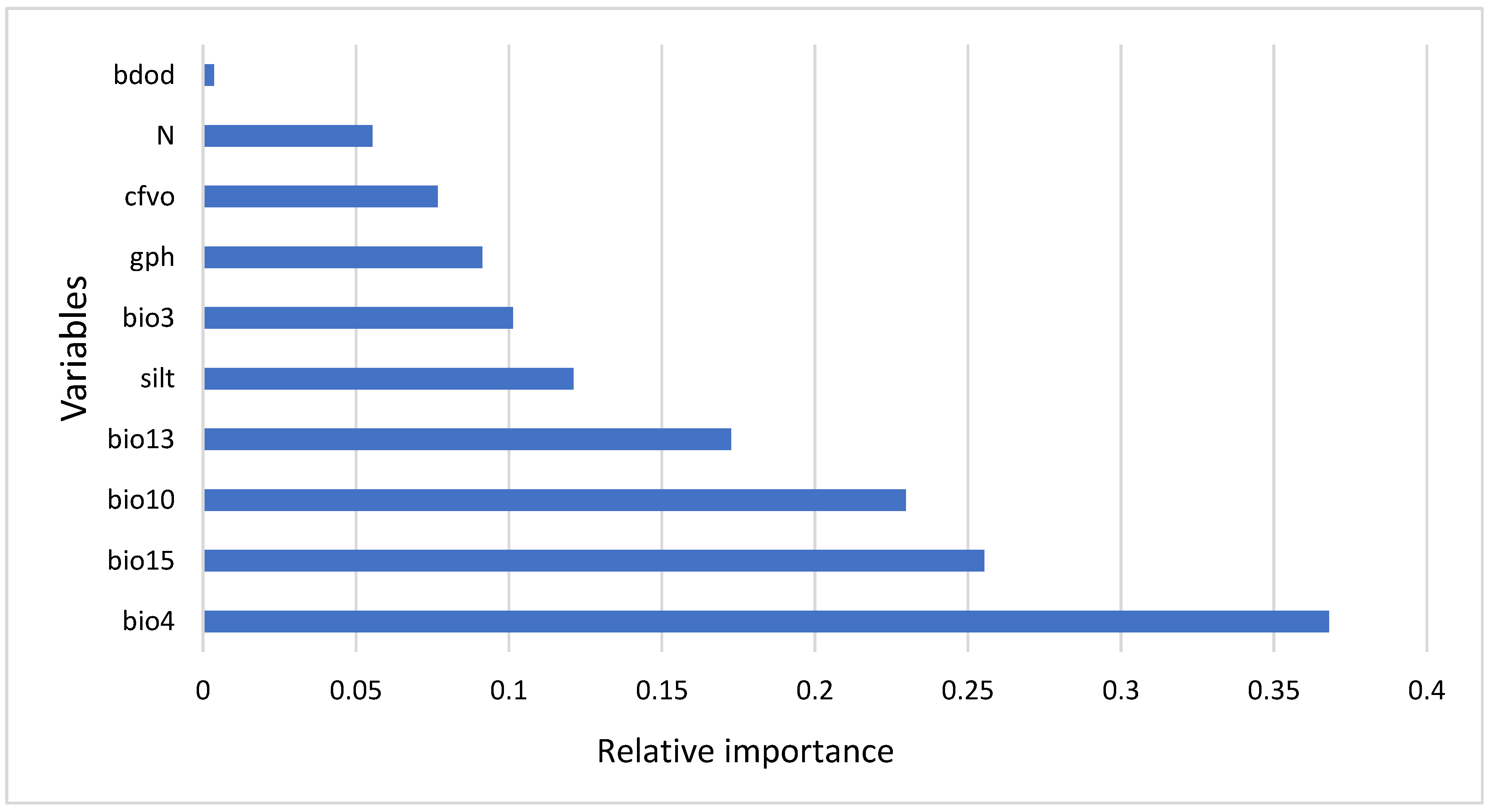
3.2. Variable Importance
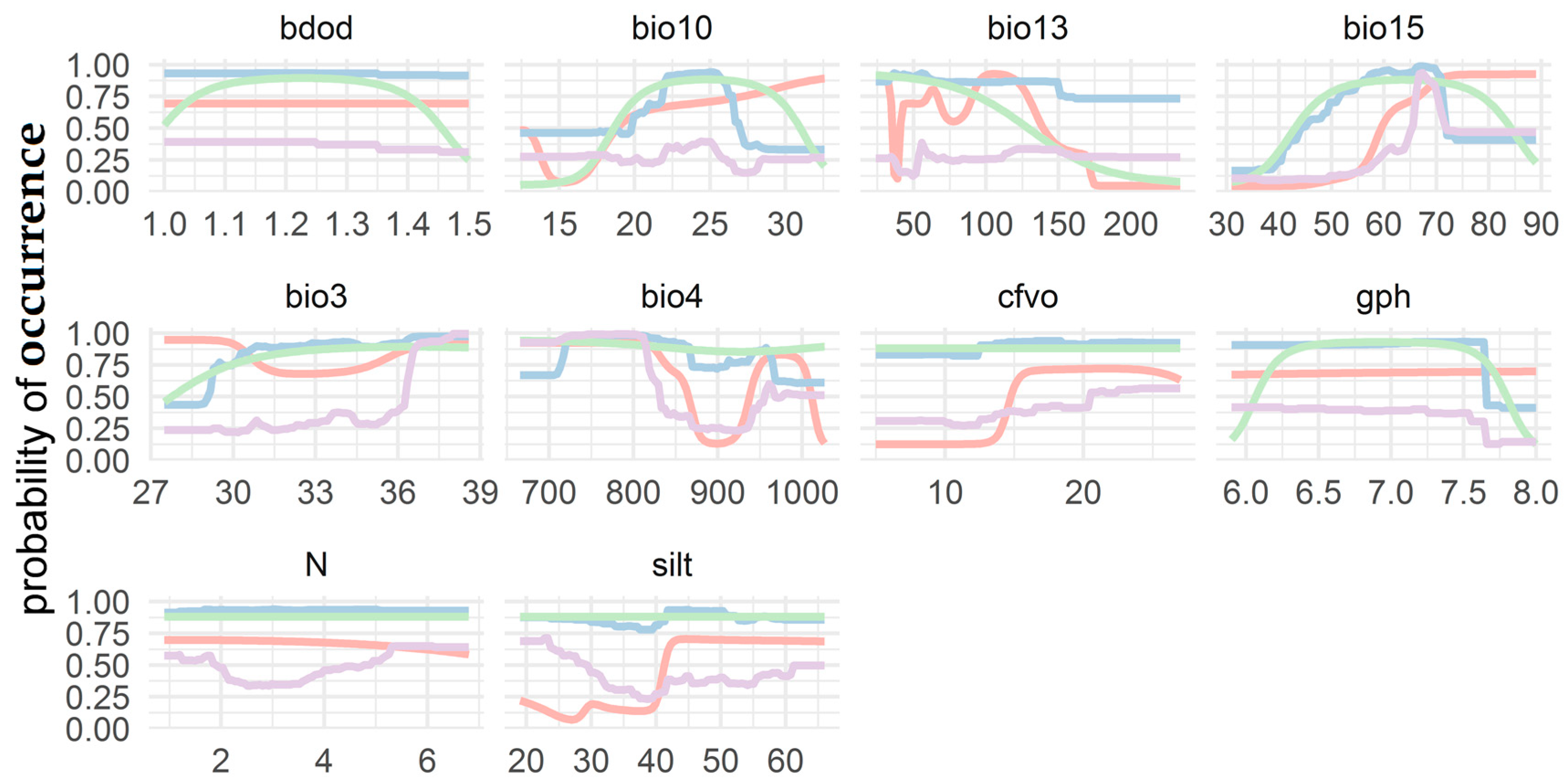
3.3. Response Curve
3.4. Current and Future Distribution of Ae. tauschii
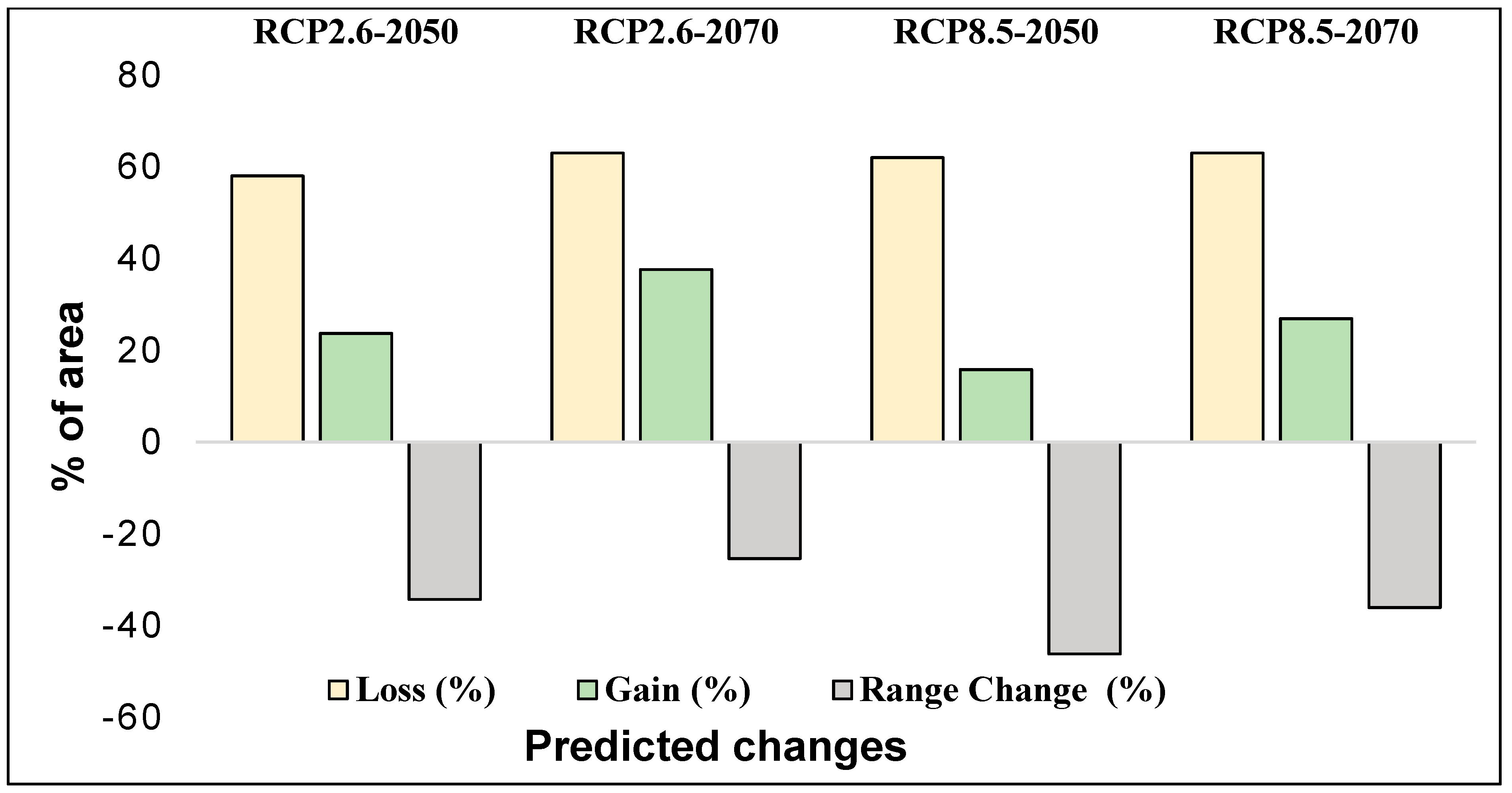
3.5. Range Size of Ae. tauschii
4. Discussion
- Protected areas and habitat restoration: Establishing and managing protected areas, as well as restoring degraded habitats, can help conserve Ae. tauschii and its habitats currently and in the future.
- Assisted migration and translocation: Moving Ae. tauschii to new locations that are more suitable under future climatic conditions can help conserve this species.
- In situ conservation: Maintaining populations of Ae. tauschii in its current location through methods such as controlled breeding and seed banking can help conserve this valuable species.
- Climate-smart land use planning: Integrating climate change considerations into land use planning and management can help ensure that future land use decisions conserve and protect not only Ae. tauschii and its habitats but also other plant species in the study area.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, S.; Qin, D.; Manning, M. Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Diffenbaugh, N.S.; Ashfaq, M. Intensification of hot extremes in the United States. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. ECOGEG 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Thuiller, W. Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Halbritter, A.H.; Alexander, J.M.; Edwards, P.J.; Billeter, R. How comparable are species distributions along elevational and latitudinal climate gradients? Glob. Ecol. Biogeogr. 2013, 22, 1228–1237. [Google Scholar] [CrossRef]
- Azizi, G.H.; Roshani, M. Study of climate change on the southern coast of the Caspian Sea with the Mann-Kendall method. Phys. Geogr. 2008, 64, 13–28. [Google Scholar]
- Franklin, J. Moving beyond static species distribution models in support of conservation biogeography. Divers. Distrib. 2010, 16, 321–330. [Google Scholar] [CrossRef]
- Jones, C.C. Challenges in predicting the future distributions of invasive plant species. For. Ecol. Manag. 2012, 284, 69–77. [Google Scholar] [CrossRef]
- Lemke, A.; Buchholz, S.; Kowarik, I.; Starfinger, U.; von der Lippe, M. Interaction of traffic intensity and habitat features shape invasion dynamics of an invasive alien species (Ambrosia artemisiifolia) in a regional road network. NeoBiota 2021, 64, 155–175. [Google Scholar] [CrossRef]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Taylor, S.; Kumar, L. Potential distribution of an invasive species under climate change scenarios using CLIMEX and soil drainage: A case study of Lantana camara L. in Queensland, Australia. J. Environ. Manag. 2013, 114, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Vardien, W.; Richardson, D.; Foxcroft, L.; Thompson, G.; Wilson, J.; Le Roux, J. Invasion dynamics of Lantana camara L. (sensu lato) in South Africa. South Afr. J. Bot. 2012, 81, 81–94. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Ahmadi, K.; Heydari, M.; Karami, O.; Esmailzadeh, O.; Heung, B. Elevational shift of endangered European yew under climate change in Hyrcanian mountain forests: Rethinking conservation-restoration strategies and management. For. Ecol. Mana. 2023, 529, 120693. [Google Scholar] [CrossRef]
- Ahmadi, K.; Mahmoodi, S.; Pal, S.C.; Saha, A.; Chowdhuri, I.; Nguyen, T.T.; Jarvie, S.; Szostak, M.; Socha, J.; Thai, V.N. Improving species distribution models for dominant trees in climate data-poor forests using high-resolution remote sensing. Ecol. Model. 2023, 475, 110190. [Google Scholar] [CrossRef]
- Ahmadi, K.; Alavi, S.J.; Amiri, G.Z.; Hosseini, S.M.; Serra-Diaz, J.M.; Svenning, J.-C. The potential impact of future climate on the distribution of European yew (Taxus baccata L.) in the Hyrcanian Forest region (Iran). Int. J. Biometeorol. 2020, 64, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Mandal, A.; Bandyopadhyay, S. Short jute fiber reinforced polypropylene composites: Effect of compatibiliser, impact modifier and fiber loading. Compos. Sci. Technol. 2003, 63, 801–806. [Google Scholar] [CrossRef]
- Neupane, D.; Adhikari, P.; Bhattarai, D.; Rana, B.; Ahmed, Z.; Sharma, U.; Adhikari, D. Does Climate Change Affect the Yield of the Top Three Cereals and Food Security in the World? Earth 2022, 3, 45–71. [Google Scholar] [CrossRef]
- Hovhannisyan, V.; Gould, B.W. Quantifying the structure of food demand in China: An econometric approach. Agric. Econ. 2011, 42, 1–18. [Google Scholar] [CrossRef]
- Rana, A.; Moradkhani, H.; Qin, Y. Understanding the joint behavior of temperature and precipitation for climate change impact studies. Theor. Appl. Clim. 2017, 129, 321–339. [Google Scholar] [CrossRef]
- Aghaei, M.J.; Mozafari, J.; Taleei, A.R.; Naghavi, M.R.; Omidi, M. Distribution and diversity of Aegilops tauschii in Iran. Genet. Resour. Crop. Evol. 2008, 55, 341–349. [Google Scholar] [CrossRef]
- Schneider, T.R. Evaluations of stressful transactions: What’s in an appraisal? Stress Health ISIS 2008, 24, 151–158. [Google Scholar] [CrossRef]
- Wang, J.; Luo, M.C.; Chen, Z.; You, F.M.; Wei, Y.; Zheng, Y.; Dvorak, J. Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol. 2013, 198, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Van Slageren, M.W. Wild Wheats: A Monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae); Agricultural University: Wageningen, The Netherlands, 1994. [Google Scholar]
- Babaeian, E.; Nagafineik, Z.; Zabolabasi, F.; Habeibei, M.; Adab, H.; Malbisei, S. Climate change assessment over Iran during 2010–2039 by using statistical downscaling of ECHO-G model. Geogr. Dev. 2009, 7, 135–152. [Google Scholar]
- Pressey, R.L.; Cabeza, M.; Watts, M.E.; Cowling, R.M.; Wilson, K.A. Conservation planning in a changing world. Trends Ecol. Evol. 2007, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, H.; Rahiminejad, M.R.; Vallian, S.; Heslop-Harrison, J.S. Biodiversity of Diploid D-Genome Aegilops Tauschii Coss. in Iran Measured Using Microsatellites. Genet. Resour. Crop. Evol. 2006, 53, 1477–1484. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F.; Georges, M.D.; Thuiller, C.W. Package ‘biomod2’. Species distribution modeling within an ensemble forecasting framework. Ecography 2016, 32, 369–373. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Jarvis, A.; Lane, A.; Hijmans, R.J. The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ. 2008, 126, 13–23. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Williams, S.E. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Schwinning, S.; Wilsey, B.J. Mechanisms controlling biomass partitioning in grasses. Oecologia 1998, 115, 259–266. [Google Scholar]
- Pereira, O.A.; Gómez-García, M.Á.; Arroyo, J. Aegilops in the Iberian Peninsula: A taxonomic, phytogeographical and evolutionary approach. Plant Syst. Evol. 2012, 298, 1023–1039. [Google Scholar]
- Givnish, T.J. Adaptive Significance of Leaf Size and Shapes in Heteroblasty. Annu. Rev. Ecol. Evol. 2002, 33, 579–609. [Google Scholar]
- Chala, D.; Roos, C.; Svenning, J.C.; Zinner, D. Species-specific effects of climate change on the distribution of suitable baboon habitats–Ecological niche modeling of current and Last Glacial Maximum conditions. J. Hum. Evol. 2019, 132, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ghamkhar, K.; Croser, J.; Aryamanesh, N.; Campbell, M.; Kon’kova, N.; Francis, C. Camelina (Camelina sativa (L.) Crantz) as an alternative oilseed: Molecular and ecogeographic analyses. Genome 2010, 53, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Kaul, M.; Hill, R.L.; Walthall, C. Artificial neural networks for corn and soybean yield prediction. Agric. Syst. 2005, 85, 1–18. [Google Scholar] [CrossRef]
- Chen, J.; Keddie, B. Soil texture effects on soil water and solute transport in an agricultural field. Eur. J. Soil Sci. 2008, 59, 567–576. [Google Scholar]
- Haefele, S. Soil Erosion, Soil Formation, and Soil Structure. In Agroecology; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–20. [Google Scholar]
- Ostrowski, M.F.; Prosperi, J.M.; David, J. Potential implications of climate change on Aegilops species distribution: Sympatry of these crop wild relatives with the major European crop Triticum aestivum and conservation issues. PLoS ONE 2016, 11, e0153974. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.; Mehrabian, A.; Mostafavi, H. Modeling climate change effects on spatial distribution of wild Aegilops L. (Poaceae) toward food security management and biodiversity conservation in Iran. Integr. Environ. Assess. Manag. 2022, 18, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant, Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Suneja, Y.; Gupta, A.K.; Bains, N.S. Stress adaptive plasticity: Aegilops tauschii and Triticum dicoccoides as potential donors of drought associated morpho-physiological traits in wheat. Front. Plant Sci. 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.P.; Prasad, P.V.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. Response of Aegilops species to drought stress during reproductive stages of development. Funct. Plant Biol. 2011, 39, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; van den Boogaard, R.I.K.I.; Veneklaas, E.J.; Villar, R. Effects of global environmental change on carbon partitioning in vegetative plants of Triticum aestivum and closely related Aegilops species. Glob. Chang. Biol. 1995, 1, 397–406. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWRs) Threatened and Endemic to Italy: Urgent Actions for Protection and Use. Biology 2022, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) Priority in Italy: Distribution, Ecology, In Situ and Ex Situ Conservation and Expected Actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P.; Medagli, P. Aegilops(Poaceae) in Italy: Taxonomy, geographical distribution, ecology, vulnerability and conservation. Syst. Biodivers. 2014, 12, 331–349. [Google Scholar] [CrossRef]
- Maxted, N.; White, K.; Valkoun, J.; Konopka, J.; Hargreaves, S. Towards a conservation strategy for Aegilops species. Plant Genet. Resour. Charact. Util. 2008, 6, 126–141. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]

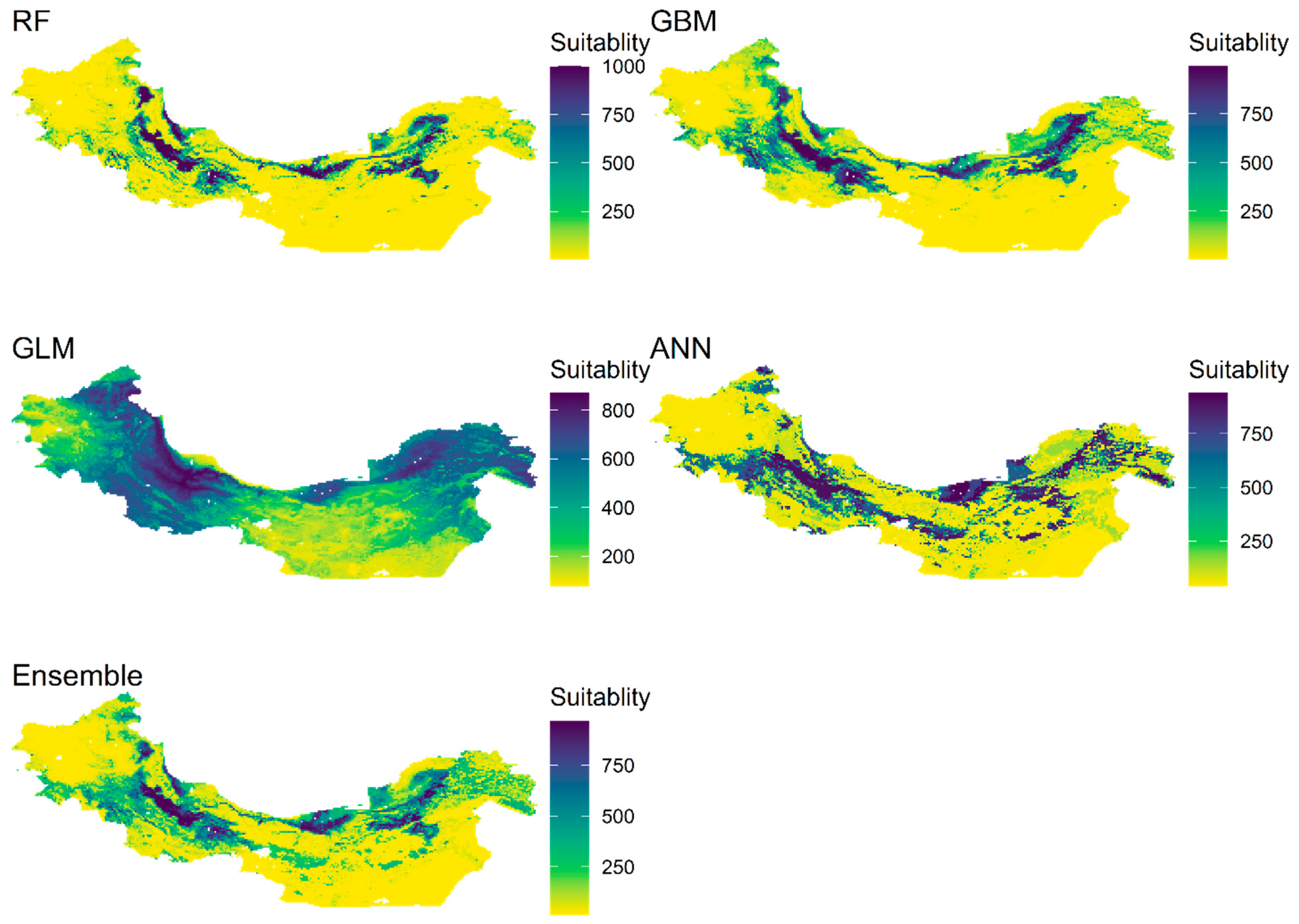

| Evaluation Criteria | Species Distribution Models | |||
|---|---|---|---|---|
| RF | GBM | GLM | ANN | |
| TSS | 0.55 | 0.545 | 0.165 | 0.395 |
| ROC | 0.82 | 0.803 | 0.562 | 0.689 |
| Kappa | 0.532 | 0.498 | 0.119 | 0.289 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoodi, S.; Jaffar Aghaei, M.; Ahmadi, K.; Naghibi, A. Climate-Change-Driven Shifts in Aegilops tauschii Species Distribution: Implications for Food Security and Ecological Conservation. Diversity 2024, 16, 241. https://doi.org/10.3390/d16040241
Mahmoodi S, Jaffar Aghaei M, Ahmadi K, Naghibi A. Climate-Change-Driven Shifts in Aegilops tauschii Species Distribution: Implications for Food Security and Ecological Conservation. Diversity. 2024; 16(4):241. https://doi.org/10.3390/d16040241
Chicago/Turabian StyleMahmoodi, Shirin, Mohammad Jaffar Aghaei, Kourosh Ahmadi, and Amir Naghibi. 2024. "Climate-Change-Driven Shifts in Aegilops tauschii Species Distribution: Implications for Food Security and Ecological Conservation" Diversity 16, no. 4: 241. https://doi.org/10.3390/d16040241
APA StyleMahmoodi, S., Jaffar Aghaei, M., Ahmadi, K., & Naghibi, A. (2024). Climate-Change-Driven Shifts in Aegilops tauschii Species Distribution: Implications for Food Security and Ecological Conservation. Diversity, 16(4), 241. https://doi.org/10.3390/d16040241








